Back to Journals » ClinicoEconomics and Outcomes Research » Volume 12
Budget Impact Analysis of the Introduction of Injectable Prolonged-Release Buprenorphine on Opioid Use Disorder Care Resource Requirements
Authors Phillips-Jackson H , Hallam C, Cullen N, Pearson T, Gilman M, Li L, Musgrave P
Received 19 December 2019
Accepted for publication 29 March 2020
Published 6 May 2020 Volume 2020:12 Pages 233—240
DOI https://doi.org/10.2147/CEOR.S242984
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samer Hamidi
Helen Phillips-Jackson,1 Clive Hallam,2 Niamh Cullen,3 Terry Pearson,4 Mark Gilman,5 Li Li,6 Paul Musgrave7
1Working Group on Innovation Assessment, Expert Faculty on Commissioning, London, UK; 2Richmond and Wandsworth Council, London, UK; 3Public Health, Calderdale, UK; 4Northamptonshire County Council, Northamptonshire, UK; 5Discovering Health, Manchester, UK; 6Applied Strategic, London, UK; 7Public Health, Cumbria, UK
Correspondence: Paul Musgrave
Public Health, Cumbria, UK
Email [email protected]
Objective: To assess budget impact of the introduction of prolonged-release buprenorphine (PRB) for care of opioid use disorder (OUD) over 1 year in a defined population.
Materials and Methods: A healthcare perspective, decision-tree model analysis of the cost of OUD care for a standard population was prepared to compare two scenarios: treatment of a population under the existing standard of care, or with the addition of PRB. The model assessed OUD-related direct costs (medication, delivery, psychosocial treatment), other services costs (harm reduction, general healthcare, social and justice services) and the impact of behaviors such as engaging with treatment and electing to use additional opioids “on top” of treatment regimens, and “dropping out” from treatment.
Results: Standard population definition (persons offered OUD care services) is based on a typical administrative region in England with general population of 400,000 citizens, 1,777 high-risk opioid users requiring treatment and 909 patients initiating treatment in a year. The cost to provide OUD care for 1 year under the current scenario (70% treated with methadone, 30% sublingual buprenorphine) is £ 19.7M. In scenarios with increased PRB adoption/reduced sublingual buprenorphine or oral methadone use, the cost reduction ranges from £ 0.2M to 0.7M.
Conclusion: The assessment showed a reduction of overall costs after introduction of PRB.
Keywords: opioid use disorder, budget impact, pharmacotherapy, buprenorphine, methadone, injectable prolonged-release buprenorphine
Introduction
Opioid use disorder (OUD) is an important individual and public health issue.1 Adverse health outcomes include risk of death due to overdose, infectious diseases, comorbidities, trauma, and suicide;2 negative social impacts include unemployment, homelessness, family disruption, loss of economic productivity, social instability, criminal activities, and economic burden.3–5
Integrated treatment with pharmacotherapy and psychosocial support is effective and well-evidenced.6 Standard care commonly includes medication choices of oral methadone or sublingual buprenorphine. OUD care programs are effective but associated with significant burdens and risks. Obligatory daily attendance at a clinic or pharmacy for supervised consumption of medication is common, especially at the start of therapy as provision of oral medication has a serious risk of diversion.7 Daily attendance for supervised therapy can limit the ability to work, lead to discrimination, and perceived loss of social equity or agency. Therapy is marginalizing for some people. Engaging with therapy whether collecting medication regularly at a pharmacy or visiting a treatment center – may be associated with its own limits and create stigma, which can make adherence difficult, leading to sub-optimal dosing, “on top” use of illicitly sourced opioids and other drugs.8,9 Innovation can address limitations of OUD treatment.
Prolonged-release buprenorphine (PRB)10,11 is approved for management of opioid dependence. Different doses of the PRB product are given by weekly or monthly subcutaneous injections. Evidence including comparison to sublingual buprenorphine treatment12–15 demonstrates efficacy and safety in treating patients with OUD.16 The product has the potential to overcome the limits, burdens and risks of daily observed medication administration.17–19
In England, there are an estimated 250–300,000 people with a history of OUD who may require treatment;20 approximately 140,000 engaged with treatment services.21 OUD care is planned and commissioned by Public Health departments responsible for drug and alcohol services within 152 administrative regions/municipal “Local Authorities” (LA) in England.22,23 This work assessed the budget impact of including PRB therapy in the standard of care.
Materials and Methods
Budget impact was assessed using a decision-tree model from a healthcare system perspective based on previous work.24–26 The model was prepared to compare direct costs and indirect costs of OUD care for a standard population in two scenarios: existing standard of care, or with the introduction of PRB.
Direct costs were modelled for the provision of OUD care, including medication, delivery, and psychosocial treatment (Table 1). Medication cost was estimated based on daily treatment dose recommended in national guidelines.27,28 Distribution costs included item fees, applicable for each methadone prescription, and fees charged for each patient interaction at pharmacy visits29,30 for dispensing and controlled drug handling. Supervised consumption payment was based on the normal agreement with pharmacies. Costs of clinical interventions included monthly counselling services often led by key workers or other healthcare professionals.31
 |
Table 1 Direct Costs Associated with Delivering OUD Care |
Indirect costs were assessed for the subpopulations: 1) engaged in treatment but electing to use additional opioids “on top” of the recommended treatment regimen; and 2) those electing to cease or “drop out” of the recommended treatment regimen, or never engaging with such during the period of assessment. Indirect costs include harm reduction, general healthcare, criminal justice and child safeguarding (Table 2). Evidence describing costs was identified from published sources or local records.24,26
 |
Table 2 Indirect Healthcare and Non-healthcare Costs Associated with Delivering OUD Care |
Costs to provide care at weekly intervals were calculated and summed for the year based on distributions of subpopulations. Subpopulations were defined according to behavior, persons who were: 1) engaged with treatment, no additional opioid use; 2) engaged with treatment, additional opioid use present; and 3) never in or no longer engaged with treatment, other opioid use continues. The relative changes over the year of the distribution between subpopulations were simulated in the model, based on clinical outcomes and subgroup analysis of a Phase III trial.16,32,33
One-way sensitivity analyses assessed the impact of variance in: 1) the proportion of population living rurally; 2) the percentage of estimated high-risk opioid users in treatment; 3) the percentage of people on buprenorphine at baseline; 4) the unit costs for supervised consumption of oral methadone and sublingual buprenorphine; and 5) the level of adoption of PRB.
Results
A theoretical standard region of 400,000 citizens (85% urban residents; 15% living in rural areas) was defined for the purpose of this analysis, based on the average of six identified regions in England (population range for the six regions: 195,700–741,209). The estimated number of people using opioids in the region was 1,777 (based on an average number in the six identified regions; value range, 628–3,245), with an estimated 909 engaged in OUD care (based on the average number in the six regions; value range 238–1,958).
The overall costs to provide OUD care and associated services under each scenario are summarized in Table 3. Costs to provide OUD care for 1 year in the current scenario (scenario 1, 70% treated with oral methadone, 30% sublingual buprenorphine): £19.7M. For a future scenario (scenario 2) in which 10% receive injectable PRB, 20% sublingual buprenorphine, 70% methadone, costs were £19.4M (Figure 1), a reduction of £0.2M in costs (direct (£89,420), indirect healthcare (£24,220) and indirect non-healthcare (£93,915)) (Table 3).
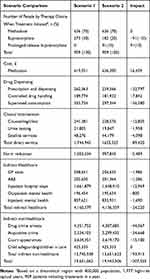 |
Table 3 Budget Impact of Prolonged-Release Buprenorphine Adoption |
One-way sensitivity analyses completed show further reduction in costs of care of £0.3–0.7M (assuming higher levels of treatment engagement, higher rates of supervised consumption frequency, greater fraction of rural population (Table 4), higher level of adoption for PRB (Table 5).
 |
Table 4 Sensitivity Analysis on Parameters with Local Variations |
 |
Table 5 Sensitivity Test of Level of Adoption of Prolonged-Release Buprenorphine |
Discussion
Introduction of injectable PRB directly addresses limitations7 of current medication choices in OUD care. This analysis assessed the cost impact of introducing PRB for a standard population. For a scenario with 10% patients on PRB, the total resources for direct OUD care and other related health, social and justice services were lower: 43.1% (£89,420) of the reduction comes from direct costs associated with frequent drug dispensing (prescription and dispensing, controlled drug handling, and supervised consumption), and requirement of clinical interventions (counselling/clinic, urine testing, satellite services); 45.2% (£93,915) from reduced indirect non-healthcare costs associated with drug and acquisitive crimes and court appearances; 11.6% (£24,220) from indirect healthcare costs (harm reduction GP, A&E, inpatient care, and mental health care). The medication costs increased by £16,459. Results are consistent with other work.34,35 An analysis in the UK using a 5-state Markov model suggested that PRB accrued lower annual total per-patient costs compared to sublingual buprenorphine/naloxone.34 Cost savings were attributed to lower crime rate, reduced supervised self-administration, prescription/controlled drug fees, avoided HIV/HCV infections. One study in Sweden highlighted reduced criminality/victimization costs and lower direct medical costs driven by reduced emergency and hospital services.35
This analytical method was consistent with a previously validated approach24,26 based on two subpopulations (engaged in treatment, never in or no longer engaged with treatment). This study included a subdivision of the “engaged in treatment” subpopulation, based on choice to use additional opioids “on top” of treatment regimens (as defined by positive urine drug results). The decision-tree model did not include a scenario in which a population discontinued any form of treatment and also did not revert to additional opioid use (for example, injected heroin use).
Important assumptions determine the results; it was assumed, scenarios including both the use of additional opioids “on-top” while engaged in treatment and also “dropping out from treatment” (often measured by “retention”) increased the need for additional resources in care. Retention was determined from different sources. For patients treated with methadone, retention was estimated from a previous study.32 Evidence for retention with PRB and sublingual buprenorphine was estimated from a subgroup analysis of a phase III clinical study using data on file;16,33 this subgroup represented subjects with recorded use of primarily illicit drugs, mainly injected heroin, and accounted for 71% of the total study population, consistent with the profile of patients with OUD in England.36 These sources of evidence describing retention are different (observational vs phase III study): they represent the best known evidence for assumptions.
It was assumed in this work that no additional incremental cost (indirect and non-healthcare costs) are required for the group in treatment with no additional opioid use. Direct costs to provide OUD treatment services were considered for this group only. This work assumed that PRB is administered in the normal course of contact with healthcare services, and that this does not incur additional cost. For the subpopulation that is engaged with treatment with additional opioid use present, it was assumed that additional costs are needed to provide full supervision, based on clinical experience. Treatment dose in the standard-of-care arm of the analysis determined cost; assumptions were based on a typical dose in national guidance (oral methadone 80 mg, guidance 60–120 mg; sublingual buprenorphine 16 mg (12–32 mg).31 PRB listed cost does not vary with dose.
This work identified the budget impact and reduction in cost following introduction of PRB over 1 year: it does not attempt to capture all possible benefits and does not count future benefits beyond 1 year. This analysis was based on current approach to services build up around daily, observed oral medication; weekly or monthly treatment may potentially change the current model of treatment delivery significantly and allow for further reallocation of current resources.
It is likely that the realization of benefits from improved treatment in family status and reduction in resources needed for child safeguarding are not fully captured in this analysis. Benefit to families and children could be greater than stated because analysis linked potential benefit to engagement in treatment which was unchanged for the subpopulations treated on PRB/sublingual buprenorphine. This is likely to lead to an underestimate of benefit: analysis shows that as novel product adoption level increases, reduced costs associated with a reduction in need for child safeguarding are observed. In the situation where collection of medications or attendance for daily observed therapy is not possible or is not desirable because of association with major limiting risk, the benefits of PRB are likely significantly greater.
Conclusion
This analysis shows that introduction of PRB to treatment choices was associated with a decrease in costs required for care of a population with OUD.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
This work was funded by Camurus AB. Camurus had no influence on the research nor content of this work. The authors declare no conflicts of interest in this work.
References
1. Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. doi:10.1016/S0140-6736(12)61689-4
2. Dematteis M, Auriacombe M, D’Agnone O, et al. Recommendations for buprenorphine and methadone therapy in opioid use disorder: a European consensus. Expert Opin Pharmacother. 2017;18(18):1987–1989. doi:10.1080/14656566.2017.1409722
3. Callaham S, LoSasso A, Olson B. Income generation in recovering heroin users: a comparative analysis of legal and illegal earning. J Offender Rehabil. 2015;54(5):338–349. doi:10.1080/10509674.2015.1043479
4. National Alliance to End Homelessness. Opioid abuse and homelessness. National Alliance to End Homelessness; 2016. Available from: https://endhomelessness.org/resource/opioid-abuse-and-homelessness/.
5. Daley DC. Family and social aspects of substance use disorders and treatment. J Food Drug Anal. 2013;21(4):S73–S76. doi:10.1016/j.jfda.2013.09.038
6. Nielsen S, Degenhardt L, Larance B, Gowing L, Kehler C, Lintzeris N. Opioid agonist treatment for pharmaceutical opioid dependent people (Protocol). Cochrane Database Syst Rev. 2016. doi:10.1002/14651858.CD011117
7. Stöver H. Barriers to opioid substitution treatment access, entry and retention: survey of opioid users, patients in treatment, and treating and non-treating physicians. Eur Addict Res. 2010;17(1):44–54. doi:10.1159/000320576
8. Anstice S, Strike CJ, Brands B. Supervised methadone consumption: client issues and stigma. Subst Use Misuse. 2009;44(6):794–808. doi:10.1080/10826080802483936
9. Vigilant LG. The stigma paradox in methadone maintenance: naïve and positive consequences of a “treatment punishment” approach to opiate addiction. Sage. 2004;28(4):403–418.
10. European Medicines Agency. Buvidal Buprenorphine, summary of opinion (initial authorisation). 2018.
11. European Medicines Agency. Buvidal - summary of product characteristics; 2018. Available from: https://www.ema.europa.eu/en/documents/product-information/buvidal-epar-product-information_en.pdf.
12. European Medicines Agency. Assessment Report - Buvidal - International Non-Proprietary Name: buprenorphine; 2018. Available from: https://www.ema.europa.eu/en/documents/assessment-report/buvidal-epar-public-assessment-report_en.pdf.
13. Albayaty M, Linden M, Olsson H, Johnsson M, Strandgården K, Tiberg F. Pharmacokinetic evaluation of once-weekly and once-monthly buprenorphine subcutaneous injection depots (CAM2038) versus intravenous and sublingual buprenorphine in healthy volunteers under naltrexone blockade: an open-label phase 1 study. Adv Ther. 2017;34(2):560–575. doi:10.1007/s12325-016-0472-9
14. Walsh SL, Comer SD, Lofwall MR, et al. Effect of buprenorphine weekly depot (CAM2038) and hydromorphone blockade in individuals with opioid use disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(9):894–902. doi:10.1001/jamapsychiatry.2017.1874
15. Haasen C, Linden M, Tiberg F. Pharmacokinetics and pharmacodynamics of a buprenorphine subcutaneous depot formulation (CAM2038) for once-weekly dosing in patients with opioid use disorder. J Subst Abuse Treat. 2017;78:22–29. doi:10.1016/j.jsat.2017.04.008
16. Lofwall MR, Walsh SL, Nunes EV, et al. Weekly and monthly subcutaneous buprenorphine depot formulations vs daily sublingual buprenorphine with naloxone for treatment of opioid use disorder a randomized clinical trial. JAMA Intern Med. 2018;178(6):764–773. doi:10.1001/jamainternmed.2018.1052
17. Institute for Clinical and Economic Review. Extended-release opioid agonists and antagonists for Medication- Assisted Treatment (MAT) of opioid use disorder: effectiveness and value. 2018.
18. Frost M, Bailey GL, Lintzeris N, et al. Long-term safety of a weekly and monthly subcutaneous buprenorphine depot (CAM2038) in the treatment of adult outpatients with opioid use disorder. Addiction. 2019;114(8):1416–1426. doi:10.1111/add.14636
19. Gilman M, Li L, Hudson K, et al. Current and future options for opioid use disorder: a survey assessing real-world opinion of service users on novel therapies including depot formulations of buprenorphine. Patient Prefer Adherence. 2018;12:2123–2129. doi:10.2147/PPA.S180641
20. England Public Health. United Kingdom drug situation: focal point annual report 2017. 2017. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/713101/Focal_Point_Annual_Report.pdf.
21. England Public Health. Alcohol and drug treatment for adults: statistics summary 2017 to 2018. 2018.
22. National Treatment Agency for Substance Misuse. JSNA support pack for commissioners of recovery in communities 2013 JSNA support pack for commissioners. 2013;(April).
23. National Audit Office. Public Health England’s Grant to Local Authorities; 2014.
24. Connock M, Juarez-Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–37.
25. Gibbons C, Jensen R Modelling the budgetary impact of treating opioid dependence in the English municipal setting. 2018.
26. Kenworthy J, Yi Y, Wright A, Brown J, Maria Madrigal A, Dunlop WCN. Use of opioid substitution therapies in the treatment of opioid use disorder: results of a UK cost-effectiveness modelling study. J Med Econ. 2017;20(7):740–748. doi:10.1080/13696998.2017.1325744
27. NHS. Amendments to the drug tariff April 2019; April 2019. Available from: https://www.nhsbsa.nhs.uk/sites/default/files/2019-03/. Drug Tariff April 2019.pdf.
28. Department of Health (England). Clinical Guidelines on Drug Misuse and Dependence Update 2017. London: Independent Expert Working Group; 2017.
29. NHS. Drug tariff 2018. 2018.
30. PSNC. Changes to oral liquid methadone fee arrangements FAQs – March 2013. 2013.
31. Clinical Guidelines on Drug Misuse and Dependence Update 2017 Independent Expert Working Group. Drug Misuse and Dependence: UK Guidelines on Clinical Management. London: Global and Public Health/Population Health/Healthy Behaviours/25460; 2017.
32. Pinto H, Maskrey V, Swift L, Rumball D, Wagle A, Holland R. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J Subst Abuse Treat. 2010;39(4):340–352. doi:10.1016/j.jsat.2010.07.009
33. Trial record. Clinical trial of CAM2038, long-acting subcutaneous buprenorphine injections for treatment of patients with opioid dependence. Available from: https://clinicaltrials.gov/ct2/show/NCT02651584?term=NCT02651584&rank=1.
34. Tiberg F, Jensen R, Sanjurjo V, Carter J. Substitution therapy with flexible-dose depot buprenorphine injection to treat opioid use disorder in the United Kingdom: a pharmacoeconomic assessment. Value Health. 2017;20(9):A711. doi:10.1016/j.jval.2017.08.1880
35. Jensen R, Carter J, Tiberg F, Sanjurjo V. Flexible-dose depot buprenorphine injection for opioid substitution treatment in heroin-addicted adults: a Swedish pharmacoeconomic perspective. Value Health. 2017;20(9):A711. doi:10.1016/j.jval.2017.08.1879
36. Burkinshaw P, Knight J, Anders P, et al. An Evidence Review of the Outcomes That Can Be Expected of Drug Misuse Treatment in England. 2017. London. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/586111/PHE_Evidence_review_of_drug_treatment_outcomes.pdf
37. National Institute for Health and Care Excellence. Evidence review opioid dependence: buprenorphine prolonged- release injection (Buvidal); 2019. Available from: https://www.nice.org.uk/advice/es19/evidence/evidence-review-pdf-6666819661.
38. Methadone dispensing (FP10 and FP10MDA).
39. PSNC. Pharmaceutical services negotiating committee - fees and allowances; 2018. https://psnc.org.uk/dispensing-supply/endorsement/fees-allowances/.
40. National Institute for Health and Care Excellence (NICE). Costing statement: needle and syringe programmes; 2014. Available from: https://www.nice.org.uk/guidance/ph52/resources/ph52-needle-and-syringe-programmes-costing-statement2.
41. Langham S, Wright A, Kenworthy J, Grieve R, Dunlop WCN. Cost-effectiveness of take-home naloxone for the prevention of overdose fatalities among heroin users in the United Kingdom. Value Health. 2018;21(4):407–415. doi:10.1016/j.jval.2017.07.014
42. Curtis L. Unit costs of health & social care 2017. Pers Social Serv Res Unit. 2017. doi:10.1016/j.cemconcomp.2014.07.014
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

