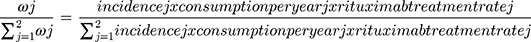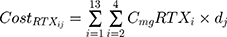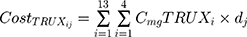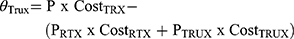Back to Journals » ClinicoEconomics and Outcomes Research » Volume 12
Budget Impact Analysis of Switching to Rituximab’s Biosimilar in Rheumatology and Cancer in 13 Countries Within the Middle East and North Africa
Authors Almaaytah A
Received 17 June 2020
Accepted for publication 11 August 2020
Published 15 September 2020 Volume 2020:12 Pages 527—534
DOI https://doi.org/10.2147/CEOR.S265041
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xing Lin Feng
Ammar Almaaytah1,2
1Faculty of Pharmacy, Middle East University, Amman, Jordan; 2Faculty of Pharmacy, Jordan University of Science and Technology, Irbid, Jordan
Correspondence: Ammar Almaaytah Tel +962777658820
Email [email protected]
Introduction: Biosimilars of monoclonal antibodies are being rapidly developed and approved by public health regulatory authorities worldwide. These biosimilars are expected to bring significant budgetary savings to national governments and consequently increase patients’ accessibility to biological therapy. Rituximab has been used extensively for the treatment of cancer and rheumatoid disorders over the past two decades. New biosimilars of Rituximab have been developed and introduced into clinical practice. We have analyzed the budgetary impact and savings outcome of introducing Rituximab’s biosimilar into 13 countries within the Middle East and North Africa through the implementation of a budget impact analysis model.
Methods: Our model was based on a 1-year full uptake and switch scenario of the Rituximab’s biosimilar CT-P10. The model calculated the total number of patients based on the total national consumption of Rituximab per country. Accordingly, the model produced savings per each indication which were translated into the additional number of patients that would be permitted access to Rituximab’s therapy as a result of these savings.
Results: In our modeling scenario, the total projected savings that will result from the uptake of Rituximab’s biosimilar within the MENA region were estimated to be 46.59 million dollars. The cumulative savings in all 13 countries would allow access of Rituximab’s therapy for a total of 6589 patients which is equivalent to a 14% increase in the number of patients benefiting from Rituximab’s therapy.
Conclusion: The introduction of Rituximab’s biosimilar within the Middle East and North Africa region is associated with significant budgetary savings that will allow public national health authorities to reinvest such economic gains either in expanding access to Rituximab therapy or other costly lifesaving biologicals.
Keywords: budget impact analysis, savings, Rituximab, biosimilar, cost, MENA
Introduction
Rituximab, a chimeric monoclonal antibody, was the first monoclonal antibody to receive approval by the U.S. Food and Drug Administration (FDA) for the treatment of B-Cell lymphomas in 1997.1 Rituximab’s approval for cancer was later followed by its approval for the treatment of rheumatoid arthritis due its mechanism of action which targets CD-20 antigens on B lymphocytes.2,3 The originator drug is marketed under the name MabThera® in Europe and Rituxan® in the United States and its patent expired in 2016 allowing other pharmaceutical companies to develop their own versions of the molecule as biosimilars.4 The first biosimilar to receive both FDA and European approval as a Rituximab biosimilar was CT-P10, later to be renamed Truxima® which was developed and manufactured by Celltrion, Korea.
The Europeans Medicines Agency (EMA) define a biosimilar as a product that proves a high level of similarity with the reference product in an extensive comparability exercise with regards to its quality, safety and efficacy in addition to evidence of bioequivalence.5 Accordingly, CT-P10 received regulatory approval for all therapeutic indications of the originator Rituximab and these include Rheumatoid arthritis (RA), non-Hodgkin’s lymphoma (NHL), chronic lymphocytic leukemia (CLL) and granulomatosis with polyangiitis.6 The approval of biological biosimilars and those of monoclonal antibodies represents a huge and significant economic opportunity to health authorities worldwide as these molecules will generate a significant price reduction and consequently will reduce the overall cost of biological therapy which is considered a significant burden to national health expenditure.7 Additionally, the overall savings that result from biosimilar adoption and a healthy price competition will lead to a substantial increase in patients’ accessibility to these expensive medications and accordingly will enhance the overall health outcomes in those countries.8 Currently, there are over 58 biosimilars that are approved by the European Medicines Agency which consequently would provide huge health outcomes and budget savings within the European Union.9 In the Middle East and North Africa (MENA), the regulatory pathways for biosimilar market authorization are still in their infancy as only 4 countries have adopted official regulations that allow biosimilars to be registered following rigorous guidelines that guarantee patients’ safety and drug efficacy.10 Additionally, little awareness exits within the medical community regarding the use of biosimilars and their clinical efficacy, safety and quality when compared with their originator counterparts.11 In this study, a budget impact analysis of the introduction of CT-P10 into the Middle East and North Africa including 13 countries was conducted to assess the potential savings that will result from adopting this new medication. Budget impact analysis (BIA) or what is also termed as comparative cost efficiency studies are used to estimate the amount of total savings that would result due to the adoption of a new therapeutic intervention. Accordingly, BIA studies provide policymakers with important information regarding public health expenditure which permits more efficient future financial forecasting within national health systems.12 Our BIA model focuses on the use of Rituximab in the treatment of RA, NHL and CLL. These three diseases are of substantial financial impact on public health systems globally. In the MENA region, the World health organization has ranked musculoskeletal diseases among the top ten causes of disability.13 With the chronic progressive nature of RA and its impact on society both in the amount of cost needed to manage the disease and the quality of life of RA patients, the need for increasing patients’ access to biological therapy is becoming significantly essential. For lymphomas and CLL, Rituximab and other biological therapies targeting CD20 antigens has revolutionized the treatment of those types of oncological diseases and has been considered as a major therapeutic option in almost all cancer-related clinical guidelines.14,15 The aim of our study is to assess and evaluate the budgetary impact of introducing CT-P10 in 13 countries within the Middle East and North Africa for the treatment of RA and cancer. BIA studies provide two major data indicators for health policymakers, the first is related to the projected cumulative budget savings per therapeutic intervention and the other with equal importance is the subsequent increase in patients’ accessibility to the therapeutic intervention introduced as a result of the total savings achieved. Since the originator Rituximab and its approved biosimilar are clinically equivalent according to the related clinical studies, we hypothesize that CT-P10 would be associated with significant budget savings and patients’ accessibility in both the Middle East and North Africa.
Methods
Our BIA model was created using a Microsoft Excel platform to estimate the cumulative cost savings of introducing CT-P10 in 13 countries within the Middle East and North Africa for the treatment of RA and cancer. The scenario was developed for a 1-year treatment horizon and a total of three-year time horizon was also calculated. The model focused on all types of Rituximab’s indications for RA and cancer including NHL, CLL and granulomatosis with polyangiitis in accordance with the worldwide guidelines for non-Hodgkin Lymphoma and Chronic Lymphocytic leukemia. The model also focused on projecting the total number of patients for each indication that will benefit from the potential savings in comparison with the originator’s price and thus patient’s accessibility was evaluated. All assumptions included in the model were in accordance with the recommendations established by the International Society for Pharmacoeconomics and Outcomes Research principles of good practice for BIA models.16
Patients
The total number of patients treated with Rituximab was estimated based on the total consumption of Rituximab [MabThera®] per country in milligrams and according to IMS sales data. Accordingly, the patient population represents a real-life application of total Rituximab consumption. However, the IMS data does not include the consumption per therapeutic indication of each disease modality and the number of patients split into each diagnosis was based on data from literature and previous expert assumptions performed in related comparative budget savings studies. The patients’ distribution per therapeutic indication is described in detail in Figure 1, while the assumptions are described in detail in Table S1 (Supplementary data). The first assumption was made that 10% of the total consumption of Rituximab is allocated for the indication granulomatosis with polyangiitis. The remaining 90% of the consumption were allocated as 20% for RA and 80% for the cancer indications including NHL and CLL. For cancer indications, the total consumption per each indication was estimated based on the total annual dose in milligrams per patient (Table 1) and the incidence rate of each given diagnosis in the countries included in the study. The weight of each cancer indication was calculated as per the formula listed in Figure 1. The incidence rate of each cancer indication was obtained for each country from the Global Cancer Observatory (International Agency for Research on Cancer, WHO). In NHL and CLL, total all case incidence rates per 100,000 population were 6.82 (95% confidence interval 4.2–13.3) and 5.16 (3.4–7.4), respectively. For the weight calculations, the assumptions were carried out that the percentage of NHL and CLL patients treated with Rituximab were 87% and 78%, respectively.17 A duration of 1-year treatment scenario was analyzed for all indications including a follow-up 3-year savings outcome. The market assumptions of the uptake of CT-P10 as patient naïve Rituximab were used in this model and applied to a fixed number of patients and the total number of patients remained the same over the fixed time horizons described previously.
 |
Table 1 Dosing Regimens of Rituximab in All Approved Indications |
Uptake of Rituximab Biosimilar
The uptake of CT-P10 by the patient population in all indications in our model was estimated for a complete switch of 100% to Rituximab’s biosimilar over the 1-year time horizon scenario as the data provided for actual costs per country are related to entry agreements financial mechanisms (Tenders) hence no market share would be allowed in such circumstances.
Costs
The pricing of the originator Rituximab in our model depended on real life managed entry agreement financial mechanisms that were endorsed by local public health authorities in each of the 13 individual countries involved in the study. This would reflect more accurate estimation of the national budgetary savings that would incur form biosimilar adoption rather than depending on third-party payer perspective. The price of the biosimilar CT-P10 was assumed to be 70% of the originator‘s price as CT-P10 is still not registered in all of those countries. The total annual cost of patients receiving either the originator Rituximab or its biosimilar were computed for each indication based on the actual cost of the therapeutic dose for 1 year in milligrams. For the cancer indications the average price for 1 mg for both the 100 mg and 500 mg vials in each country was used for the price estimation while for RA, the price of 500 mg vials was applied to compute the price of the single mg. The calculations were performed as follows:
where i represents the country included in the model with a maximal number of 13, j represents the therapeutic indications (RA, NHL, CLL and Granulomatosis polyangiitis) with a maximal number of 4. 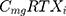 represents the cost of 1 mg MabThera® in each individual country while
represents the cost of 1 mg MabThera® in each individual country while 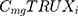 represents the cost of a single mg of CT-P10. Finally, dj represents the total annual dose for each indication j. The annual total dose for cancer patients was calculated based on the Mean body surface area (BSA) of 1.79 m2 that is based on a retrospective study performed in the UK regarding cancer patients and other BIA studies performed on countries in the European Union.18,19 The dose for each indication was determined using clinical guidelines and Rituxan®’s official dosing regimen.3,14 The model did not include the administrative costs associated with therapy as these were assumed to be equal for both the originator Rituximab and its biosimilar.
represents the cost of a single mg of CT-P10. Finally, dj represents the total annual dose for each indication j. The annual total dose for cancer patients was calculated based on the Mean body surface area (BSA) of 1.79 m2 that is based on a retrospective study performed in the UK regarding cancer patients and other BIA studies performed on countries in the European Union.18,19 The dose for each indication was determined using clinical guidelines and Rituxan®’s official dosing regimen.3,14 The model did not include the administrative costs associated with therapy as these were assumed to be equal for both the originator Rituximab and its biosimilar.
Total Budget Impact
The total budget impact of introducing CT-P10 in each individual country (i) within the MENA region for each indication (j) according was computed according to the following formula and as performed previously by Gulácsi et al:18
The total savings that result from introducing CT-P10 in each indication was projected to increase the number of patients that could benefit from these savings and accordingly assess patients’ accessibility to Rituximab treatment.
Results
Our BIA model focused on introducing Rituximab’s biosimilar into 13 countries within the Middle East and North Africa based on the assumption that the price of the newly introduced biosimilar was 70% of the originator and that the entry agreement financial mechanisms would ensure complete dominance of the biosimilar over the market. The total projected savings that will result from the uptake of Rituximab within the region was $46.59 million dollars (of which 15% for RA treatment, 33.3% for the treatment of NHL, 27.9% for CLL treatment and finally 16.3% for granulomatosis with polyangiitis). Seventy-five percent of all savings were accomplished in five countries and these include; KSA (11.6%), Algeria (17.7%), Morocco (16.9%), Lebanon (15.6%) and Iraq (12.4%). (Table 2). The cumulative savings in all 13 countries would allow access of Rituximab’s therapy for a total of 6589 patients (925 for RA, 1635 for NHL, 1397 for CLL and 2628 for granulomatosis with polyangiitis) (Table 2). This is equivalent to a 14% increase in the number pf patients treated with Rituximab.
The highest beneficiary in terms of patients’ access to Rituximab treatment was Iraq with 1390 extra patients permitted therapy followed by Morocco (1610) and (906) in Saudi Arabia.
The total percentage of patients benefitting from the BIA model was also projected for three years and the resultant total amount of savings across the region would surpass $139 million dollars equal to 20,000 thousand additional patients with access to Rituximab treatment within the Middle East and North Africa.
Discussion
The BIA model we utilized in this study has revealed that switching to Rituximab’s biosimilar in 13 countries within the Middle East and North Africa would save $46.59 million dollars over a 1-year time horizon, these savings would accordingly be allocated to give access to a total of 6585 patients across the whole region and in all indications for Rituximab. The three-year projection would permit savings of $139 million dollars and consequently 20,000 patients with access to Rituximab’s significant clinical benefits in all treatment indications. Allowing such savings to incur within the national health services in the region would result in significant economic and social benefit to the governments and the population of these countries, the savings as we have displayed in our model could be allocated for treatment of other patients in need of Rituximab’s therapy or simply to other expensive lifesaving therapies that are currently available in the clinic. Our model assumed that Truximab®’s biosimilar is 30% lower than the originator’s price; however, there are several circumstances during the public tender offerings that allow the price of biosimilars to reach up to 50–60% of the originator’s price. This would mean that the introduction of any biosimilar into to the market would drive a healthy competition within the biological therapeutic category between the different pharmaceutical companies, a process that would reduce costs and increase patients' access to these valuable medications. The current data regarding patient’s access to Rituximab’s therapy and other cancer medications within the Middle East and North Africa show that total public spending on oncology within this region is comparable to both European and the U.S. health systems,20 this would further support the need of performing pharmacoeconomic studies in this region due to the significant potential of budgetary savings attributed to these studies. As with several other BIA models published in literature,21,22 most BIAs have some limitations regarding the model design and implementation due to the scarcity of data in literature regarding the medication process and patient numbers. In our situation, this issue is amplified in the MENA region as the there is a significant shortage of literature in pharmacoeconomic and epidemiological studies. Additionally, there is also a significant scarcity regarding public health data published by national authorities regarding the prevalence of both RA and other oncological diseases which could be considered a limitation to more accurate and expanded future studies. BIA studies are limited in literature globally (Orlewska et al, 2009), but in the Middle East and North Africa they are even less implemented and this could be attributed to the lack of data as mentioned previously. Rituximab’s indications include RA, NHL and CLL, the epidemiological data regarding these diseases in the Middle East and North Africa remains poorly understood and there is significant scarcity in the availability of data regarding the exact incidence and prevalence of each disease. A recent global burden study regarding RA estimated that that RA prevalence in the region to be of 0.16% and consequently among the lowest in the world.23 In oncology, the epidemiological data within the Middle East and North Africa is also considered suboptimal as cancer registries in some of these countries cover only 13% of the total population.24 Accordingly, our model relied on a number of assumptions that were essentially derived from other BIA models in literature and in compliance recommendations established by the International Society for Pharmacoeconomics and Outcomes Research principles of good practice for Budget Impact Analysis models.16,25 These assumptions include, for example, the number of patients which are derived from the total annual consumption of Rituximab per country, the distribution and indication weights were also based on literature and expert views. However, with all these limitations and assumptions, we believe that the findings of our study give a clear guideline to health policymakers within the region regarding the economic advantages of introducing and adopting biosimilars within their public health systems. This in turn would lead to aggregated health gains due to the huge savings and budget relief that could be gained from adopting these policies.
The field of biosimilars in the Middle East and North Africa is still in its infancy in regard to regulatory pathways for biosimilar market authorization.10 Only four countries in the region (Saudi Arabia, Jordan, Lebanon and Egypt) have established scientific guidelines for biosimilar market authorization and we believe that the findings of this study should not lead public health policymakers to focus on the budgetary savings of adopting biosimilars per se, but biosimilar adoption should be correlated with stringent and rigorous guidelines that guarantee the quality and non-inferiority of the biosimilar and preventing the entry of bio replicas or what is known as biomimics which would jeopardize patients health and safety. We recommend that all public health authorities in the Middle East and North Africa fast forward the establishment of regulatory pathways for biosimilar approval and registration or at least adopt ones that have already been validated such as the European Medicines Agency and the World Health Organization biosimilar guidelines. Additionally, the question of therapeutic interchangeability is also of major importance as physicians report low scores of awareness regarding the exact meaning of biological therapeutic interchangeability with biosimilars and several studies indicate that the majority of physicians expect negative health outcomes as a result of biosimilar switching.26,27
In conclusion, we report that the adoption of a Rituximab biosimilar in the MENA region would be associated with significant budgetary savings and in turn would increase patients’ accessibility to Rituximab in sensitive life-threatening indications such as NHL, CLL and granulomatosis with polyangiitis. Additionally, the access to Rituximab’s significant benefits in rheumatology would also be enhanced. This in total would lead to a positive impact on both the patient and the society within the Middle East and North Africa.
Ethical Approval
The requirements for Institutional Review Board (IRB) approval and informed consent were waived as this was a pharmacoeconomic modeling study without patient contact.
Acknowledgment
The author is grateful to both the Middle East University, Amman, Jordan and the deanship of research at Jordan University of Science and Technology for the financial support granted to cover the publication fee of this research article.
Disclosure
The author of this study declares that they do not have any conflict of interest regarding the data and publication of this manuscript. The datasets used and/or analysed during the current study including the Global Cancer observatory and the IMS sales data are freely available and can be provided by the author on reasonable request via email.
References
1. Roche Pharma AG. Summary of product characteristics. Mabthera 100 mg concentrate for solution for infusion; 2016. Available from: https://www.ema.europa.eu/en/documents/product-information/mabthera-epar-product-information_en.pdf.
2. Boumans MJH, Tak PP. Rituximab Treatment in Rheumatoid Arthritis: How Does It Work? Arthritis Res Ther.2009;1:(134) . doi:10.1186/ar2852.
3. Genentech, Inc. Rituxan (rituximab) prescribing information; 2010. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103705s5311lbl.pdf.
4. European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: nonclinical and clinical issues; 2014. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-2.pdf.
5. Abraham I, Sun D, Bagalagel A, et al. Biosimilars in 3D: definition, development and differentiation. Bioengineered. 2013;4(4):203–206. doi:10.4161/bioe.25067
6. Yoo DH, Suh C-H, Shim SC, et al. A multicentre randomised controlled trial to compare the pharmacokinetics, efficacy and safety of CT-P10 and innovator rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76(3):566–570. doi:10.1136/annrheumdis-2016-209540
7. Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. Rand Health Q. 2018;7:4.
8. Simoens S, Jacobs I, Popovian R, Isakov L, Shane LG. Assessing the value of biosimilars: a review of the role of budget impact analysis. Pharmacoeconomics. 2017;35(10):1047–1062. doi:10.1007/s40273-017-0529-x
9. Generics and Biosimilar Initiative Gabi. Biosimilars approved in Europe; 2020. Available from: http://www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe.
10. Almaaytah A, Mashaqbeh H, Haddad R. Current status of biosimilar regulations in the MENA region. Int J Res Pharm Sci. 2020;11(3):3443–3449. doi:10.26452/ijrps.v11i3.2484
11. Farhat F, Othman A, Karak FE, Kattan J. Review and results of a survey about biosimilars prescription and challenges in the Middle East and North Africa region. Springerplus. 2016;5(1):2113. doi:10.1186/s40064-016-3779-8
12. Almaaytah A, Elhajji FD. Comparative cost efficiency of the originator drug of infliximab and its biosimilar for the treatment of rheumatoid arthritis in the MENA region. Int J Pharm Investig. 2019;9(1):12–15. doi:10.5530/ijpi.2019.1.4
13. Watad A, Al-Saleh J, Lidar M, Amital H, Shoenfeld Y. Rheumatology in the Middle East in 2017: clinical challenges and research. Arthritis Res Ther. 2017;19(1):1. doi:10.1186/s13075-017-1359-0
14. Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v78–v84. doi:10.1093/annonc/mdv303
15. Dotan E, Aggarwal C, Smith MR. Impact of rituximab (Rituxan) on the treatment of B-cell non-Hodgkin’s lymphoma. Pharm Ther. 2010;35(3):148.
16. Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis—principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014;17(1):5. doi:10.1016/j.jval.2013.08.2291
17. Ekström-Smedby K. Epidemiology and etiology of non-hodgkin lymphoma–a review. Acta Oncol (Madr). 2006;45(3):258–271. doi:10.1080/02841860500531682
18. Gulácsi L, Brodszky V, Baji P, Rencz F, Márta P. The rituximab biosimilar CT-P10 in rheumatology and cancer: a budget impact analysis in 28 European countries. Adv Ther. 2017;34(5):1128–1144. doi:10.1007/s12325-017-0522-y
19. Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P, Shea BJ. The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. PLoS One. 2010;5(1):1. doi:10.1371/journal.pone.0008933
20. Kasteng F, Wilking N, Jonsson B. Patient access to cancer drugs in nine € countries in the Middle East. Available from: http://www.comparatorreports.se/Middle%20East%20oncology%20drug%20uptake%20Final%20report%20Sept% 2015%202008.pdf.
21. Kim J, Hong J, Kudrin A. Year budget impact analysis of biosimilar infliximab for the treatment of rheumatoid arthritis in UK, Italy, France and Germany. Arthritis Rheum. 2014;11(Suppl):S512.
22. Jha A, Upton A, Dunlop WCN, Akehurst R. The budget impact of biosimilar infliximab (Remsima®) for the treatment of autoimmune diseases in five European countries. Adv Ther. 2015;32(8):742–756. doi:10.1007/s12325-015-0233-1
23. Cross M, Smith E, Hoy D, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316–1322. doi:10.1136/annrheumdis-2013-204627
24. Zanetti R, Tazi MA, Rosso S. New data tells us more about cancer incidence in North Africa. Eur J Cancer. 2010;46(3):462–466. doi:10.1016/j.ejca.2009.11.012
25. Ewa O, Gulácsi L. Budget-impact analyses. Pharmacoeconomics. 2009;27(10):807–827. doi:10.2165/11313770-000000000-00000
26. Teeple A, Ellis LA, Huff L, et al. Physician attitudes about non-medical switching to biosimilars: results from an online physician survey in the United States. Curr Med Res Opin. 2019;35(4):611–617. doi:10.1080/03007995.2019.1571296
27. Cohen H, Beydoun D, Chien D, et al. Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther. 2016;33(12):2160–2172. doi:10.1007/s12325-016-0431-5
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.