Back to Journals » Clinical Ophthalmology » Volume 16
Brolucizumab in Neovascular Age-Related Macular Degeneration – Indian Real-World Experience: The BRAILLE Study – Fifty-Two-Week Outcomes
Authors Chakraborty D , Maiti A, Sheth JU , Mondal S, Boral S, Nandi K, Sinha TK, Das A
Received 6 November 2022
Accepted for publication 15 December 2022
Published 23 December 2022 Volume 2022:16 Pages 4303—4313
DOI https://doi.org/10.2147/OPTH.S395577
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Debdulal Chakraborty,1 Aniruddha Maiti,2 Jay U Sheth,3 Soumen Mondal,1 Subhendu Boral,1 Krishnendu Nandi,4 Tushar Kanti Sinha,1 Arnab Das1
1Department of Vitreoretinal Services, Disha Eye Hospitals, Kolkata, West Bengal, India; 2Department of Vitreoretinal Services, Global Eye Hospitals Eye, Kolkata, West Bengal, India; 3Department of Vitreoretinal Services, Shantilal Shanghvi Eye Institute, Mumbai, Maharashtra, India; 4Department of Vitreoretinal Services, Netralayam Super Speciality Eye Care Centre, Kolkata, West Bengal, India
Correspondence: Debdulal Chakraborty, Department of Vitreoretinal services, Disha Eye Hospitals, Kolkata, West Bengal, India, Tel +91 9433059923, Email [email protected]
Purpose: To report the 52-week real-world efficacy and safety outcomes of brolucizumab therapy for neovascular age-related macular degeneration (nAMD) in Indian eyes.
Patients and Methods: A retrospective, multicentre chart analysis of 82 eyes of 82 patients with nAMD (switch therapy: 65 eyes; treatment-naïve: 17 eyes) with 52-week follow-up data was performed. Pro-re-nata re-treatment was offered based on visual and tomographic criteria. Changes in best-corrected visual acuity (BCVA), intraretinal fluid (IRF), subretinal fluid (SRF), central-subfield thickness (CST), and pigment epithelial detachment (PED) were the key outcome measures, coupled with the safety profile.
Results: The mean age of the study population was 67.65 (± 10.67) years, with 57 male patients (69.5%). The study’s mean number of injections was 4.8 (± 0.77). After brolucizumab therapy, the BCVA improved significantly at weeks 4 (P< 0.001), and maintained up to week 52 (P< 0.001). The CST also reduced significantly at all the visits (Baseline: 413.6 ± 64.6 μm; 52-week: 292.37 ± 13.5 μm; P< 0.001). Significantly fewer eyes demonstrated residual SRF (P< 0.001) and IRF (P< 0.001) at all visits, starting with week 12 and continuing until week 52. The PED resolution was significant from week 24 through week 52 (P=0.004). Each of the 82 eyes received four injections of brolucizumab, with 63.4% (52 eyes) receiving a fifth dose and only 17.1% requiring a sixth. Mild intraocular inflammation (IOI) was seen in three eyes (3.66%) that resolved conservatively. One patient (1.2%) developed mild fever that subsided with oral medications.
Conclusion: The 52-week BRAILLE study demonstrates that brolucizumab is effective and safe in nAMD eyes in a real-world setting. Brolucizumab treatment can reduce the therapeutic burden in patients with nAMD due to its rapid, sustained efficacy and favourable safety profile.
Keywords: brolucizumab, inflammation, age-related macular degeneration
Introduction
Age-related macular degeneration (AMD) is a progressive degenerative eye disorder resulting in permanent visual impairment and blindness.1 It is the commonest cause of macular neovascularization (MNV), requiring periodical intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy. The approved anti-VEGF agents for nAMD include pegaptanib sodium (Eyestech/OSI Pharmaceuticals, NY, USA), ranibizumab (Lucentis®; Genentech, CA/Roche, Basel, Switzerland), aflibercept (Eylea®, Regeneron, Tarrytown, NY), brolucizumab (Beovu®; Novartis, Basel, Switzerland), and faricimab (Vabysmo; Genentech).2,3 Additionally, bevacizumab (Avastin®) has been widely utilized globally as an off-label treatment for nAMD.2,3
For treating nAMD, brolucizumab, a humanized single-chain antibody fragment, has received approval from multiple regulatory authorities, including the United States Food and Drug Administration (US-FDA; 2019), the European Medicines Agency (EMA; 2020), and the Drug Controller General of India (DCGI; 2020).4,5 The Phase III pivotal trials, HAWK and HARRIER, demonstrated the non-inferiority of brolucizumab over aflibercept in visual outcomes at 96 weeks.6 Additionally, in terms of resolving fluid and reducing retinal thickness, brolucizumab fared better than aflibercept.6 However, such excellent visual and anatomical outcomes are rarely replicated in the real-world due to multiple factors such as under-treatment, non-compliance, financial burden, logistical reasons, and an overburdened healthcare system. As a result, the patient experiences gradual visual decline over the long term.7
The BRAILLE study reported the short-term efficacy and safety profile of nAMD patients treated with intravitreal injection (IVI) of brolucizumab under Indian real-world conditions.5 A significant improvement in visual acuity (VA) and a reduction in the central subfield thickness (CST) were noted after a mean follow-up of 7.3±2.2 weeks.5 Of note, no episodes of intraocular inflammation (IOI) were encountered after 126 IVI of brolucizumab.5 Based on these encouraging results, brolucizumab injection was deemed efficacious and safe for nAMD management over the short-term.5 The 52-week data of the BRAILLE study are presented here.
Materials and Methods
The BRAILLE study was a retrospective, multi-center, non-randomized, interventional study conducted at four tertiary eye care centers in India. The study was conducted in accordance with the tenets of the Declaration of Helsinki and was approved by the Institutional review board at each site and Central Ethics Committee at Disha Eye Hospitals (Regn Number ECR/846/Inst/WB/2016/RR-19: EC-CT-2022/138). Written informed consent for treatment and data collection was obtained from each patient.
Design
The details of the study design, inclusion, and exclusion criteria have been published previously.5 Briefly, a medical chart analysis of all nAMD patients treated with IVI brolucizumab between October 2020 and February 2022 was carried out. These included all treatment-naïve and recalcitrant cases of nAMD. As per protocol, the recalcitrant cases were defined as eyes with fluid on the spectral-domain optical coherence tomography (SD-OCT) which was either worsening or persistent (<100µm reduction) despite repeated doses of aflibercept or ranibizumab injections. These patients were advised to switch to brolucizumab therapy. The treatment-naïve patients freely chose the brolucizumab molecule after they were counselled for all the anti-VEGF agents.
All patients received IVI brolucizumab (6 mg/0.05 mL) in the operation theater under strict aseptic conditions. Post-injection topical antibiotic (0.5% moxifloxacin) was advised for 1 week. The patients were reviewed at baseline and subsequently every 4 weekly till 52 weeks. Every appointment involved taking a thorough medical history that included any ocular or systemic adverse events. Additional evaluations included the best-corrected visual acuity (BCVA) by Snellen’s visual-acuity chart; intraocular pressure (IOP) by Goldmann applanation tonometer; and anterior segment and fundus examination; and spectral-domain OCT (SD-OCT). To exclude polypoidal choroidal vasculopathy (PCV), additional fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA) were performed at baseline.
A vast majority of the study population was from the lower socioeconomic strata and so were financially unstable As a result, retreatment based on the pro-re-nata (PRN) regimen was offered. The retreatment criteria included drop of one or more lines in Snellen’s visual acuity; worsening of fluid (intraretinal fluid [IRF] or subretinal fluid [SRF]) or appearance of new fluid as compared to the previous visit; or persistent fluid defined as <100µm reduction from the previous visit. Through an assessment of electronic medical records, all the demographic, clinical, and imaging data were extracted.
Outcome Measures
The primary objective of the BRAILLE study was to assess the functional and anatomical outcomes after brolucizumab therapy from baseline to week 52. The 52-week results presented herein include mean change in BCVA from the baseline to week 52; mean change in central subfield thickness (CST) from the baseline to week 52; and the percentage of eyes with IRF and SRF at baseline and week 52. The interim outcomes from weeks 12, 24, and 36 are also reported. Two independent graders (D.C., S.M.) conducted all imaging analyses. The graders re-analyzed the images jointly and reached agreement if there were any disagreement.
A thorough safety analysis was another key outcome measure of our study. The methodology has been discussed earlier by our group.8 The treating vitreoretinal surgeon analyzed and reported all safety-related incidents based on clinical judgement. The specifics of the adverse events (AEs) and adverse drug reactions (ADRs) were obtained from the electronic patient data.9 According to these guidelines, “Any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have to have a causal relationship with this treatment” is categorized as an AE.9 Likewise, “all noxious and unintended responses to a medicinal product related to any dose should be considered ADR”.9 ADRs and AEs were divided into two categories: serious AEs (SAE) or serious ADRs (sADR), and non-serious AEs (nsAE) or non-serious ADRs (nsADR).5,8 The occurrence of any event resulting in a life-threatening AE, death, persistent or significant disability/incapacity, congenital anomaly/birth defect, or requiring inpatient hospitalization or prolongation of existing hospitalization was labelled as an SAE or sADR.5,8 Those episodes not meeting these criteria were categorized as nsAE or nsADR.5,8 Presence of any uveitic episode, either anterior, intermediate, posterior, or any combination of these was defined as IOI.
Statistical Analysis
The SPSS 23.0 version (SPSS Inc., Chicago, Ill., USA) was used to perform the statistical analysis. Continuous variables were reported as mean, and variation from the mean value (standard deviation [SD]) as mean±SD, or median (Interquartile range [IQR]) if they did not follow a normal distribution. The paired-T-test (for normal distribution) and the Wilcoxon-Signed rank test were used to assess paired continuous data (for non-normal distribution). The percentage was used to describe categorical variables, and the McNemar test was used for analyzing the paired categorical data. Variables with P values <0.05 were considered statistically significant.
Results
Study Cohort
Patients’ baseline demographics have been previously provided,5 and these are also incorporated here as Table 1. In brief, data from 94 eyes (Switch therapy: 74 eyes, 78.7%; treatment-naïve: 20 eyes, 21.3%) of 94 patients were captured from the electronic database. At 52 weeks, 12 eyes were lost to follow-up, and the remaining 82 eyes were included in the final analysis, a significant proportion of which were recalcitrant cases shifted to IVI brolucizumab (Switch therapy: 65 eyes, 79.27%; treatment-naïve: 17 eyes, 20.73%).
 |
Table 1 Demographic Characteristics of the Study Population |
The mean age of the study population was 67.65 (±10.67) years, with a male preponderance (57 patients; 69.5%). Prior to receiving IVI brolucizumab, the switch group had undergone an average of 8.63 (±4.74) anti-VEGF injections (range 3–44).
Best-Corrected Visual Acuity
The median BCVA at baseline was 0.8 (0.47–1.07) logMAR. After IVI brolucizumab therapy, the BCVA improved significantly at week 4 (median BCVA: 0.53 [0.17–1] logMAR; P<0.001), and this gain was maintained through week 52 (median BCVA: 0.47 [0.3–1] logMAR; P<0.001). Subgroup analysis revealed a similar significant improvement in BCVA in the switch therapy groups (Baseline median BCVA: 0.8 [0.53–1.23] logMAR; Final median BCVA: 0.6 [0.38–1] logMAR; P<0.001) while the improvement in treatment-naïve eyes did not reach statistical significance (Baseline median BCVA: 0.3 [0.17–0.53] logMAR; Final median BCVA: 0.17 [0.17–0.3] logMAR; P=0.449). The BCVA results for the study population are listed in Table 2.
 |
Table 2 Changes in the BCVA and CST in the Study Population Through 52-Week |
Central Subfield Thickness
At week 52, the mean CST had significantly decreased from the baseline level of 413.6±64.6 µm to 292.37±13.5 µm (P<0.001). The CST reduction was detected as early as week 12 (284.11±38.43 µm; P<0.001) and continued until week 52. Both the treatment-naive and switch therapy eyes showed significant CST reduction at all visits (Treatment-naïve group: Baseline CST – 395.47±60.47 µm; Final CST – 291.53±15.43 µm; P<0.001; Switch therapy group: Baseline CST – 418.25±65.28 µm; Final CST – 292.6±13.06 µm; P<0.001). The CST outcomes in the study eyes are summarized in Table 2.
Subretinal Fluid and/or Intraretinal Fluid and Pigment Epithelial Detachment
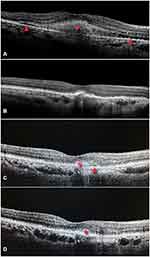
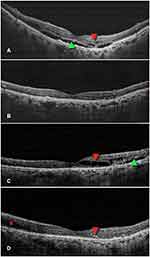
After treatment with IVI brolucizumab, a significantly lower number of eyes showed residual SRF and IRF at all of the visits, beginning at week 12 and continuing through week 52. This was in comparison to the fluid status at the beginning of the study. Among the 82 study eyes, 64 (78.05%) had SRF at baseline; at week 52, this number had decreased significantly to 18 (21.95%; P<0.001). In addition, the proportion of patients with IRF significantly decreased from 81.7% (67 eyes) at baseline to 29.27% (24 eyes) after week 52 (P<0.001). The number of eyes with PED also reduced significantly from 19 (23.17%) at baseline to 9 (10.98%) at 52 weeks (P=0.004). In the subgroup analysis, the proportion of patients with SRF reduced significantly in both groups (Treatment-naïve group: P<0.001; Switch therapy group: P<0.001), while the those with IRF significantly reduced only in the switch therapy arm (Treatment-naïve group: P=0.687; Switch therapy group: P<0.001). Similarly, the PED resolution was significantly only in the switch therapy arm (Treatment-naïve group: P=0.25; Switch therapy group: P=0.031). Changes in the fluid and PED status of the study participants’ eyes throughout the 52-week period are depicted in Table 3. Case examples of treatment-naive and recalcitrant nAMD patients who received IVI brolucizumab are shown in Figures 1 and 2, respectively.
 |
Table 3 Changes in the Proportion of Patients with SRF, IRF, and PED, in the Study Population Through 52-Week |
Number of Injections
The mean number of injections in the study was 4.8 (±0.77). Treatment-naive eyes received a mean of 4.35 (±0.49) injections while the switch therapy groups received a mean of 4.94 (±0.78) injections. Each of the 82 eyes received four injections of brolucizumab. Moreover, 63.4% (52 eyes) of the eyes received a fifth dose, while only 17.1% of the eyes required the sixth injection. The mean duration between doses was: first dose to second dose: 10.98±3 weeks; second dose to third dose: 11.71±2.16 weeks; third dose to fourth dose: 12.27±1.98 weeks; fourth dose to fifth dose: 11.69±2.07 weeks; fifth dose to sixth dose: 12 weeks. Graph 1 (Figure 3) illustrates the mean interval between the injections in the study eyes.
 |
Figure 3 Mean interval between the brolucizumab injections in the study cohort. |
Safety Analysis
Upto 52 weeks, mild ocular pain was the most common nsAE, affecting 21.95% (18 eyes) of patients. This was followed by a burning sensation and subconjunctival hemorrhage in 8 eyes each (9.76%). During the study period, sADR was noted in one patient (1.2%) who experienced mild fever that resolved with oral antipyretics. Among the ocular sADRs, three eyes (3.66%) developed IOI in the form of anterior uveitis and grade 2 vitritis following brolucizumab injection. Additionally, one of the three patients experienced disc edema. The IOI was seen after the fifth dose in two eyes and the sixth dose in one eye. All three eyes recovered completely with conservative management (tapering dose of oral steroids 1 mg/kg body weight over a month and tapering dose of topical steroids, starting at 1 hourly dosage). All three patients with IOI had poor vision from the baseline. The details of their visual acuity are provided in Table 4. Additionally, three eyes each (3.66%) experienced retinal-pigment epithelial (RPE) tear and subretinal hemorrhage (SRH). Two of the three patients who were diagnosed with SRH also developed vitreous hemorrhage (VH), requiring a vitrectomy. The third patient, however, continued to receive IVI brolucizumab medication. Table 5 summarizes the safety data of the study population.
 |
Table 4 Visual Acuity Details of the Patients Having Intraocular Inflammation |
 |
Table 5 List of Adverse Events Reported Up to Week 52 in the Patients Receiving Intravitreal Brolucizumab Injections |
Discussion
This real-world study describes the long-term efficacy and safety data following brolucizumab medication. These findings build on the short-term results that were previously reported in the BRAILLE study.5 Our real-world data demonstrated that brolucizumab therapy resulted in excellent visual outcomes and morphological responses in terms of fluid resolution and retinal thickness reduction. The overall safety profile of brolucizumab was acceptable, with the majority of adverse events being mild. Three of the study eyes developed minor IOI, which were managed conservatively.
The current strategy for treating nAMD concentrates mostly on blocking VEGF in the retinal tissue via intravitreal injection of an anti-VEGF agent. This is done in an effort to maximize the visual outcomes without compromising the safety aspect. Over more than a decade, multiple anti-VEGF molecules have been used to successfully treat nAMD.2,3 The landmark trials and their subsequent extension analyses demonstrate that initial VA improvement can be preserved over time with sustained anti-VEGF therapy and the best functional and anatomic results obtained with a fixed or treat and extend regimen.10,11 In practice, however, early VA benefits generally diminish over time, frequently as a result of undertreatment.7 Additionally, the COVID-19 pandemic has significantly impacted healthcare delivery and forced a restructuring of outpatient clinics to reduce COVID-19 risk.12 The capacity to offer the optimal care in nAMD clinics is adversely affected by this.12 Despite our real-world study being conducted during the COVID-19 pandemic, superior visual outcomes were achieved and maintained over 52 weeks. The REBA study (N=105 eyes), which was a real-world analysis of brolucizumab for nAMD in both treatment-naive and switch therapy patients from Germany, similarly reported significant visual improvement after a mean follow-up of 10.4 months.13 However, in the other real-world studies on brolucizumab, conducted by Walter et al (N = 530 eyes; US),14 Enriquez et al (N = 166 eyes; US),15 the SHIFT study (N = 63 eyes; Germany),16 and the BREW study (N = 42 eyes; US),17 investigators did not observe any evidence of any visual benefits. Visual gains vary from study to study, and this may be due in part to the “ceiling effect” among eyes with good baseline vision. This can explain the lack of significant visual improvement and morphological response (IRF and PED) in the treatment-naïve eyes of the current study. Despite a promising trend towards visual and anatomical improvement (IRF and SRF) in the treatment-naive arm of our study, this “ceiling effect” can account for the lack of statistical significance.
Imaging parameters from SD-OCT, including the retinal thickness, fluid, and PED morphology, are common biomarkers of disease activity and therapeutic response. A systematic literature review and network meta-analysis comparing all anti-VEGF agents, including the most recently approved faricimab, concluded that brolucizumab therapy produces superior retinal thickness reduction with comparable visual outcomes across all molecules at years 1 and 2.18 This was achieved with the fewest number of injections per year (5.7 injections/year).18 Our PRN-based study also revealed a comparable injection frequency (4.74 ± [0.75]). Despite the lower injection frequency in our real-world data, consistent and sustained morphological improvement was observed throughout the 52-week study period. The promising morphological outcomes relating to fluid, retinal thickness, and the PED, together with the excellent visual outcomes with fewer injections in our study, indicate a longer durability with the brolucizumab molecule with prolonged efficacy. This is because the molecular size of brolucizumab is very small, allowing it to deliver a greater molar dose and bind with the VEGF-A molecule in a 2:1 ratio.19 Additionally, even when the molecular concentration of brolucizumab decreases with time, it continues to bind to VEGF-A, albeit at a ratio of 1:1, which allows it to maintain its efficacy.19 These characteristics make it a suitable option for reducing the treatment burden of nAMD patients. This trend was clearly noted in our study where the mean interval between the injection was consistently above 10 weeks.
Anti-VEGF medications have also been linked to non-infectious endophthalmitis. They can be a direct immunological reaction to the principal medication or to drug-related impurities that may occur during the agent’s production, preparation, storage, or delivery. Data from 88,150 anti-VEGF injections for nAMD tracked by the Fight Retinal Blindness! (FRB!) registration were examined by Daien et al.20 They reported a greater rate of non-infectious endophthalmitis with bevacizumab (8/9931, 0.081%) than with ranibizumab (3/54,776; 0.005%) or aflibercept (0/23,425).20 In a comparable study, Williams et al investigated at 100,588 anti-VEGF injections for the development of non-infectious vitritis and found that rates of 0.10% (67 cases) for bevacizumab, 0.02% (6 cases) for ranibizumab, and 0.16% (13 cases) for aflibercept were seen.21 In our study, the safety profile of brolucizumab was found to be adequate. Three eyes developed mild IOI, which was treated conservatively and resolved completely. The rate of IOI in our study (3.19%) was marginally lower than in the HAWK and HARRIER trials (4.4%).6 It was also lower than comparable real-world data such as the SWIFT trial (12.4%)16 and the study by Enriquez et al15 (8.1%). IRIS Registry (10,654 eyes) and Komodo Healthcare Map (11,161 eyes) were analysed for safety, and the incidence of IOI was found to be roughly 2.4% in both datasets.22 This was marginally lower than our study. Additionally, the risk of retinal vasculitis and/or retinal vein occlusion was found to be around 0.6%.20 However, neither vasculitis nor vascular occlusion was seen in our study. In a post-hoc analysis of the HAWK and HARRIER clinical trials, the authors observed that more than half (52%) of IOI incidents occurred 3 months following the first dosage of brolucizumab.23 Also, the median time for IOI to start from the beginning of the study was 100 days (the mean time was 165.6 ± 153.6 days). Furthermore, the IOI was observed after a median of three injections (mean: 3.9 ± 2.21 injections) following the first dose.24 The authors postulated delayed hypersensitivity to immune complexes as a possible reason for the time lag between starting brolucizumab therapy and the appearance of the IOI.24 This can explain the absence of IOI in our earlier short-term data (mean follow-up: 7.3 [± 2.2] weeks; mean injections: 1.36 [± 0.58])5 compared to the 3.19% incidence observed in the current long-term study (mean injections: 4.74 [± 0.74]). The lower number of injections necessary in a PRN regimen with monthly follow-up may have an indirect effect of lowering the rate of IOI as seen earlier in the BRAILLE and BREW studies.5,17 However a PRN regimen with anti-VEGF agents may be inferior and not give the best results when it comes to functional or anatomic improvement.7
The major limitation of our study include the retrospective design. Secondly, we used a PRN regimen for both the treatment-naïve and the recalcitrant cases. This was done because of lack of affordability secondary to lower socioeconomic profile of the study population and a restructured AMD clinic because of COVID-19 pandemic. Despite this, the patients achieved excellent visual and anatomical outcomes over 52 weeks. Also, the study lacked the statistical power to conduct a safety analysis. However, this is in line with a majority of the studies reported in the literature.25
Conclusion
To conclude, the 52-week results of the BRAILLE study shows that brolucizumab is efficacious and safe in the management of nAMD. In real-world, brolucizumab therapy can help reduce the treatment burden in nAMD eyes with a faster and sustained action and an acceptable safety profile. Further long-term real-world studies evaluating and comparing different retreatment regimens, including the treat-and-extend, are warranted to better understand the best possible therapeutic strategy in these nAMD eyes.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stewart MW. Clinical and differential utility of VEGF inhibitors in wet age-related macular degeneration: focus on aflibercept. Clin Ophthalmol. 2012;6:1175–1186. doi:10.2147/OPTH.S33372
2. Thomas CJ, Mirza RG, Gill MK. Age-related macular degeneration. Med Clin North Am. 2021;105(3):473–491. doi:10.1016/j.mcna.2021.01.003
3. Pugazhendhi A, Hubbell M, Jairam P, Ambati B. Neovascular macular degeneration: a review of etiology, risk factors, and recent advances in research and therapy. Int J Mol Sci. 2021;22(3):1170. doi:10.3390/ijms22031170
4. Ferrante N, Ritrovato D, Bitonti R, Furneri G. Cost-effectiveness analysis of brolucizumab versus aflibercept for the treatment of neovascular age-related macular degeneration (nAMD) in Italy. BMC Health Serv Res. 2022;22(1):573. doi:10.1186/s12913-022-07972-w
5. Chakraborty D, Maiti A, Sheth JU, et al. Brolucizumab in neovascular age-related macular degeneration - Indian real-world experience: the BRAILLE study. Clin Ophthalmol. 2021;15:3787–3795. doi:10.2147/OPTH.S328160
6. Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: ninety-six-week outcomes from the Phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128:89–99. doi:10.1016/j.ophtha.2020.06.028
7. Mehta H, Tufail A, Daien V, et al. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog Retin Eye Res. 2018;65:127–146.
8. Chakraborty D, Stewart MW, Sheth JU, et al. Real-world safety outcomes of intravitreal ranibizumab biosimilar (razumab) therapy for chorioretinal diseases. Ophthalmol Ther. 2021;10(2):337–348.
9. European Medicines Agency. ICH topic E 2 A clinical safety data management: definitions and standards for expedited reporting; 1995. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-15.pdf.
10. Maguire MG, Martin DF, Ying G-S, et al.; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123(8):1751–1761. doi:10.1016/j.ophtha.2016.03.045
11. Qin VL, Young J, Silva FQ, Conti FF, Singh RP. Outcomes of patients with exudative age-related macular degeneration treated with antivascular endothelial growth factor therapy for three or more years: a review of current outcomes. Retina. 2018;38(8):1500–1508. doi:10.1097/IAE.0000000000001753
12. Groppe M, Bindra MS. Restructuring wet age-related macular degeneration services during the COVID-19 pandemic to allow Social Distancing Outpatient Clinics (SDOC). Clin Ophthalmol. 2021;15:651–659. doi:10.2147/OPTH.S269596
13. Bilgic A, Kodjikian L, March de Ribot F, et al. Real-world experience with brolucizumab in wet age-related macular degeneration: the REBA study. J Clin Med. 2021;10(13):2758. doi:10.3390/jcm10132758
14. Walter SD, Saba NJ Efficacy and durability of brolucizumab in patients being transitioned from prior anti-VEGF therapy.
15. Enríquez AB, Baumal CR, Crane AM, et al. Early experience with brolucizumab treatment of neovascular age-related macular degeneration. JAMA Ophthalmol. 2021;139(4):441–448. doi:10.1001/jamaophthalmol.2020.7085
16. Bulirsch LM, Saßmannshausen M, Nadal J, Liegl R, Thiele S, Golz FG. Short-term real-world outcomes following intravitreal brolucizumab for neovascular AMD: SHIFT study. Br J Ophthalmol. 2021;106:1288–1294. doi:10.1136/bjophthalmol-2020-318672
17. Sharma A, Kumar N, Parachuri N, et al. Brolucizumab-early real-world experience: BREW study. Eye. 2021;35(4):1045–1047. doi:10.1038/s41433-020-1111-x
18. Finger RP, Dennis N, Freitas R, et al. Comparative efficacy of brolucizumab in the treatment of neovascular age-related macular degeneration: a systematic literature review and network meta-analysis. Adv Ther. 2022;39(8):3425–3448. doi:10.1007/s12325-022-02193-3
19. Chakraborty D, Sheth JU, Boral S, Sinha TK. Off-label intravitreal brolucizumab for recalcitrant diabetic macular edema: a real-world case series. Am J Ophthalmol Case Rep. 2021;24:101197. doi:10.1016/j.ajoc.2021.101197
20. Daien V, Nguyen V, Essex RW, et al. Incidence and outcomes of infectious and noninfectious endophthalmitis after intravitreal injections for age-related macular degeneration. Ophthalmology. 2018;125(1):66–74. doi:10.1016/j.ophtha.2017.07.005
21. Williams PD, Chong D, Fuller T, Callanan D. Noninfectious vitritis after intravitreal injection of anti-VEGF agents: variations in rates and presentation by medication. Retina. 2016;36(5):909–913. doi:10.1097/IAE.0000000000000801
22. Khanani AM, Zarbin MA, Barakat MR, et al. Safety outcomes of brolucizumab in neovascular age-related macular degeneration: results from the IRIS registry and komodo healthcare map. JAMA Ophthalmol. 2022;140(1):20–28. doi:10.1001/jamaophthalmol.2021.4585
23. Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128(7):1050–1059. doi:10.1016/j.ophtha.2020.11.011
24. Singer M, Albini TA, Seres A, et al. Clinical characteristics and outcomes of eyes with intraocular inflammation after brolucizumab: post hoc analysis of HAWK and HARRIER. Ophthalmol Retina. 2022;6(2):97–108. doi:10.1016/j.oret.2021.05.003
25. Esen F, Alhan O, Kuru P, Sahin O. Safety assessment and power analyses in published anti-vascular endothelial growth factor randomized controlled trials. Am J Ophthalmol. 2016;169:68–72. doi:10.1016/j.ajo.2016.06.019
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.