Back to Journals » Cancer Management and Research » Volume 10
Breast cancer laterality and molecular subtype likely share a common risk factor
Authors Cheng SA , Liang LZ , Liang QL , Huang ZY, Peng XX , Hong XC , Luo XB , Yuan GL , Zhang HJ, Jiang L
Received 1 August 2018
Accepted for publication 19 October 2018
Published 29 November 2018 Volume 2018:10 Pages 6549—6554
DOI https://doi.org/10.2147/CMAR.S182254
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rituraj Purohit
Shao-Ang Cheng,1,* Li-Zhong Liang,2,* Qi-Lian Liang,1 Zhen-Yi Huang,3 Xiao-Xia Peng,1 Xiao-Cui Hong,1 Xing-Bo Luo,1 Gao-Le Yuan,1 Hui-Jie Zhang,1 Liang Jiang4
1Oncology Center, Affiliated Hospital of Guangdong Medical University, Zhanjiang 524001, China; 2Medical Insurance Office, Affiliated Hospital of Guangdong Medical University, Zhanjiang 524001, China; 3Department of Finance, Central Hospital of Guangdong Agriculture Reclamation, Zhanjiang 524002, China; 4Interventional Ward, Affiliated Hospital of Guangdong Medical University, Zhanjiang 524001, China
*These authors contributed equally to this work
Background: To investigate the epidemiological features of breast cancer laterality and molecular subtypes in southern China.
Materials and methods: A total of 2,049 cases who were diagnosed with unilateral breast cancer in the past 5 years were classified based on laterality and molecular subtypes. Molecular subtypes were defined in accordance with the 2013 St. Gallen recommendations.
Results: Breast cancer was more likely to be diagnosed in the left breast than in the right at a rate of around 5%. In the case of invasive carcinomas, the right breast was more commonly affected than the left in young (<40 years old) patients (left-to-right [L:R] ratio 0.80, 95% CI 0.65, 0.98), whereas the opposite trend was found in old (≥40 years old) patients (L:R ratio 1.06, 95% CI 1.02, 1.73). Except for invasive mucinous and invasive medullary breast cancers, the other histological types occurred more frequently on the left side than on the right. In situ cancer with a defined subtype was likely to be diagnosed as luminal B(HER-2+). Except for invasive medullary and invasive nonspecific cancers, other invasive carcinomas with a defined subtype were most likely to be diagnosed as luminal B(HER-2–). The age of ≥40 years was a risk factor for luminal B(HER-2+), and a significant correlation was present between the right breast and luminal B(HER-2+).
Conclusion: We explored the risk factors of breast cancer laterality and various molecular subtypes and found that age may be a predictor of breast cancer laterality. We found that age and laterality are the probable risk factors of the luminal B(HER-2+) type of breast cancer. These results provide a basis for the epidemiological characterization of breast cancer.
Keywords: epidemiology, breast carcinoma, tumor laterality, molecular subtype, histological type, risk factor, China
Background
Consistently, breast cancer in women is more likely to occur in the left breast than in the right.1 This finding has gained the attention of many researchers and numerous hypotheses have been proposed to account for the left-side dominance of breast cancer, but none have been uniformly accepted or confirmed. Among the earliest suggestions are breast-feeding patterns and lactation dysfunction of the left breast,2,3 which have not been supported by subsequent research. The left breast is somewhat larger than the right and therefore poses more tissues at risk, which may be the most intuitively plausible explanation.4 However, subsequent studies have supported the research finding that cancer risk is not associated with breast size.5,6 In recent years, handedness and brain hemispheric laterality specification have become popular assumptions for breast cancer laterality.7,8 Most women are believed to be right-handed, and thus, a palpable lump in the left chest is more likely to be detected.9,10 Furthermore, breast cancer predisposition involves the same genetic factors as the brain hemispheric laterality specification.8
Similarly, the relationship between the risk factors of breast cancer and the risk factors of the molecular subtypes of breast cancer has attracted increasing attention. Most epidemiological studies have found some differences in the distribution of risk factors, although the specific findings are inconsistent in different studies.11–14 Luminal B is rarely subdivided in similar researches in the past.15 However, in our study, we classified breast cancer into five molecular subtypes according to the definition of ER, PR, HER-2, and Ki-67 status, including luminal B(HER-2+) and luminal B(HER-2–), and investigated the epidemiological characteristics of breast cancer.16,17
In the present study, we analyzed the epidemiological characteristics of laterality and molecular subtypes of breast cancer in Asian breast cancer patients. We collected and analyzed clinical data of breast cancer patients from the Affiliated Hospital of Guangdong Medical University who were diagnosed in the last 5 years. To a great extent, this study represents the status of breast cancer patients in South China. The characteristics or risk factors of the laterality and molecular typing of breast cancer were estimated to elucidate the occurrence pattern of breast cancer and to provide help for development and testing of hypotheses.
Materials and methods
According to WHO, breast cancers are defined as cases with morphological codes C50 and D05 of the ICD-O, 10th edition. In this retrospective clinical study, we used the hospital database to locate all eligible patients who were treated at the Affiliated Hospital of Guangdong Medical University in Guangdong Province of South China. Those cases with a definite diagnosis of breast cancer during a 5-year period from 2013 to 2017 met our inclusion criteria. Some patients visited our hospital more than once, and thus, less than three duplicates per 1,000 records were present. We adopted all edits using standardized protocols and kept records of cases that were first diagnosed as breast cancer and were estimated to show at least 95% case ascertainment through verification by the statistical department of hospital. Those who suffered from bilateral breast cancer and second primary cancer were excluded. Bilateral breast tumor refers to tumors with the same type of histology and diagnosis within 2 months. Tumors showing invasive and in situ components together are relatively rare and were ruled out.
Excluding the ineligible cases, 2,049 unilateral primary breast cancers were included in the analysis. Of these, 652 breast cancers could not be classified into subtypes due to lack of tissue availability or staining results for one or more of the four markers. Finally, 1,397 cases for which immunohistochemical data were available were included for further analysis.
Data on ER, PR, HER-2, and Ki-67 expression status were obtained from medical record review and were assessed by immunohistochemistry. Both ER and PR were considered positive if the nuclear staining of tumor cells was more than 5%. For PR, the nuclear staining in more than 20% of the tumor cells was considered to indicate high expression. For HER-2, strong complete membrane staining in more than 10% of tumor cells was defined as positive expression (3+). Scores of 0 and 1 were considered to indicate negative expression, and all tumor cells with a 2+ score were further tested by fluorescence in situ hybridization. The determination value of Ki-67 expression may be different in different pathological experimental centers; we used 20% as the threshold value to judge the level of Ki-67 expression. Finally, the cases of breast cancers were divided into the following molecular subtypes: luminal A breast cancer (ER positive and/or PR positive and high expression/HER-2 negative/Ki-67 low expression), luminal B(HER-2–) breast cancer (ER positive and/or PR positive and low expression/HER-2 negative/Ki-67 high expression), luminal B(HER-2 +) breast cancer (ER positive and/or PR positive/HER-2 positive), HER-2-overexpressing breast cancer (ER negative/PR negative/HER-2 positive), and basal-like breast cancer (ER negative/PR negative/HER-2 negative).
This research was approved by the Medical Sciences Ethics Committee of the Affiliated Hospital of Guangdong Medical University. All study participants received detailed written information in advance and signed a written informed consent prior to the study. They were also informed that the results of the study will be published.
Statistical analysis
Mean, frequency, proportion, and SD were used to describe the data and further calculate the number and proportion of laterality and molecular subtypes. The number and ratio of left- to right-sided tumors among different types of breast cancer in different age groups were calculated. We focused on diverse pathological subtypes of invasive breast carcinoma. Chi-squared test was used to evaluate the significance of differences in laterality among the groups. When calculating the CI of the ratio, the number of breast cancer on the left side was assumed to be binomially distributed and was approximated using the normal distribution.
We analyzed three major groups of breast cancer (luminal, HER-2-overexpressing, and basal-like breast cancer). The luminal group was subdivided into three subtypes: luminal A, luminal B(HER-2–), and luminal B(HER-2+). The number and proportion of molecular subtypes with different risk factors were analyzed. Logistic regression was used to evaluate the significance of differences in molecular subtypes within groups. Multivariate logistic regression analysis was conducted to calculate the independent contribution of age and laterality to the occurrence of molecular subtypes and estimate ORs and 95% CIs for the correlation between risk factors and each subtype. All statistical analyses were performed using SPSS version 21.0 and Microsoft Excel version 2016. P-values of <0.05 were considered statistically significant.
Results
The 381 unilateral in situ tumors analyzed in women showed that these tumors occurred more frequently in the left breast than in the right at a rate of 10.2% (left-to-right [L:R] ratio 1.23, 95% CI 0.97, 1.51). Similarly, the 1,668 unilateral invasive tumors analyzed showed that these tumors occurred more frequently in the left breast than in the right at a rate of 0.8% (L:R ratio 1.02, 95% CI 1.00, 1.04). In the group of young (<40 years) patients, invasive breast cancer was more likely to be located in the right breast than in the left at a rate of 11.0% (L:R ratio 0.80, 95% CI 0.65, 0.98). On the contrary, in the group of older (≥40 years) patients, invasive breast cancer occurred more likely in the left breast than in the right at a rate of 3.0% (L:R ratio 1.06, 95% CI 1.02, 1.73) (Table 1).
  | Table 1 Occurrence of left- and right-sided unilateral breast cancer by age at diagnosis and behavior Abbreviation: L:R, left-to-right. |
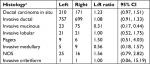
Except for mucinous and medullary types, other invasive histological types were more likely to occur in the left breast than in the right. Invasive ductal carcinoma accounted for 87% of invasive carcinoma, and the probability of this carcinoma being diagnosed on the left side was 4.0% higher than on the right (L:R ratio 1.08, 95% CI 0.80, 1.17). All carcinomas in situ were ductal carcinomas in situ, and their rate of diagnosis on the left side was 10.2% higher than that on the right (L:R ratio 1.23, 95% CI 0.97, 1.51) (Table 2).
In subsequent analysis, 1,397 breast cancer cases were classified into five molecular phenotypes. A total of 12.8% (n=263) of the patients were found with luminal A, 19.5% (n=399) with luminal B(HER-2–), 16.3% (n=334) with luminal B(HER-2+), 10.0% (n=205) with HER-2-overexpressing, and 9.6% (n=196) with basal-like breast cancer, whereas 31.8% (n=652) were found with cancers with no specific subtype. The mean age at diagnosis ranged from 47.6 years for women with luminal B(HER-2+) tumors to 51.8 years for women with HER-2-overexpressing tumors. We also calculated the number and proportion of histological types for each molecular subtype (Table 3).
  | Table 3 Tumor characteristics according to breast cancer molecular subtypes Note: aInformation obtained from pathological records. Abbreviation: NOS, not otherwise specified. |
We observed differences in the correlation between risk factors and molecular subtypes of breast cancer. To investigate a possible association between age at diagnosis and molecular subtype, we divided the breast cancer patients into two groups (<40 years and ≥40 years). The age of ≥40 years was found to be a risk factor for luminal B(HER-2+) (OR 0.46, 95% CI 0.29, 0.71; P<0.01). In terms of tumor location, right-side predominance was found to be a risk factor for luminal B(HER-2+) (OR 1.44, 95% CI 1.11, 1.88; P<0.05) (Table 4).
Discussion
In this study, 2,049 cases with unilateral breast cancer hospitalized in the Affiliated Hospital of Guangdong Medical University in the recent 5 years were classified based on laterality and molecular subtypes. In general, the probability of diagnosis of breast cancer on the left side was ∼5% higher than that on the right, which is consistent with previous studies.1,4 However, the same result was not found in terms of age. The probability of breast cancer diagnosis on the right side was 10.0% higher than that on the left in the group of young (<40 years) patients but was 5.0% higher on the left side than the right in the group of older (≥40 years) patients. This result is similar to some previous reports, and thus, age may be considered a significant predictor of breast cancer laterality.4 Except for invasive mucinous and invasive medullary types, all other histological types showed left-side dominance. The number of carcinomas in situ was significantly less than that of invasive carcinomas, and the histological classification was relatively few. Thus, we were unable to further explore the correlation between different histological types and laterality of carcinoma in situ. Little research has been conducted on the correlation between laterality and histological type. A previous large-scale US study showed that all the types except the phyllodes are left-side dominant, but the right-side predominance of phyllodes is not obvious.1 Thus, the correlation between laterality and histological type is unclear or irrelevant and must be studied further.
We divided 1,397 cases with available immunohistochemical data into five molecular subtypes, including luminal A, luminal B(HER-2–), luminal B(HER-2+), HER-2-overexpressing, and basal-like types. We excluded 652 tumors from the analyses due to lack of tissue availability or staining results for one or more of the four markers. Since the data of patients were extracted from the same database and non-subtype group did not affect risk factors, we believe that their exclusion had little effect on the results. To the best of our knowledge, no similar research on Asian breast cancer patients has been reported.12–15 In the aspect of age, patients with the luminal type were younger compared with those with HER-2-overexpressing and basal-like types. The luminal B(HER-2–) was the most obvious. This finding is consistent with the results of Kwan et al.11 Many studies have reported that young age is associated with basal-like breast cancer and that old age is related to HER-2-overexpressing breast cancer.18–21 In the present study, although we failed to find statistical significance, young age was most likely associated with both HER-2-overexpressing and basal-like breast cancers. Statistically, old age and left-side dominance were found to be the risk factors for the luminal B(HER-2+) subtype compared with others. This finding is inconsistent with previous studies, which may be due to racial differences. A large-scale breast cancer study on Asians must be conducted to explore the correlation between age and breast cancer.
In the aspect of histological type, carcinoma in situ was more likely to be diagnosed as luminal B(HER-2+) than other molecular subtypes. Except for invasive medullary and invasive nonspecific types, other invasive carcinomas were most likely to be diagnosed as luminal B(HER-2–). The correlation between histological types and molecular subtypes was less explored in similar literature.12–15 The diverse pathological types of breast carcinoma differ in immune response, protein translation efficiency, and metabolism which may be one of the mechanisms underlying the different molecular subtypes. However, additional follow-up studies are needed to confirm this view.22
Conclusion
We conducted an epidemiological study on the laterality and molecular subtypes of breast cancer in Asian patients. This study indicates that age may be a predictor of the laterality of breast cancer. Except for invasive mucinous and medullary types, other invasive histological types were more likely to occur in the left breast than in the right. We are looking forward to further research on the correlation between different histological types of carcinoma in situ and laterality. Our analysis showed that breast cancer is more likely to be diagnosed as luminal B(HER-2–) and that patients with luminal B(HER-2 +) are young. Compared with invasive carcinoma, cancer in situ showed a better chance to be diagnosed as luminal B(HER-2+), but the correlation between histological types and molecular subtypes needs further investigation. In addition, age and laterality were found to be the probable risk factors of the luminal B(HER-2+) type of breast cancer.
Data sharing statement
The data sets generated during and/or analyzed in the current study are not publically available but are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank the Guangdong Medical University, China and Affiliated Hospital of Guangdong Medical University, China, for providing database and other research support. They would also like to thank for setting up and managing the database dedicated to the collection of such a large amount of data. This work was supported by major application special topics of the science and technology department of Guangdong Province, China (No. 2015B010131016) and Science and Technology Planning Project of Guangdong Province, China (No. 2014A020212291).
Disclosure
The authors report no conflicts of interest in this work.
References
Perkins CI, Hotes J, Kohler BA, Howe HL. Association between breast cancer laterality and tumor location, United States, 1994-1998. Cancer Causes Control. 2004;15(7):637–645. | ||
Segi M, Fukushima I, Fujisaku S. An epidemiological study on cancer in Japan. J-STAGE. 1957;48:1–63. | ||
Ing R, Ho JHC, Petrakis N. Unilateral breast-feeding and breast cancer. Lancet. 1977;310(8029):124–127. | ||
Ekbom A, Adami HO, Trichopoulos D, Lambe M, Hsieh CC, Pontén J. Epidemiologic correlates of breast cancer laterality (Sweden). Cancer Causes Control. 1994;5(6):510–516. | ||
Senie RT, Saftlas AF, Brinton LA, Hoover RN. Is breast size a predictor of breast cancer risk or the laterality of the tumor? Cancer Causes Control. 1993;4(3):203–208. | ||
Thurfjell E, Hsieh CC, Lipworth L, Ekbom A, Adami HO, Trichopoulos D. Breast size and mammographic pattern in relation to breast cancer risk. Eur J Cancer Prev. 1996;5(1):37–41. | ||
Altundag K, Isik M, Sever AR. Handedness and breast cancer characteristics. J Buon. 2016;21(3):576–579. | ||
Klar AJ. Breast cancer predisposition and brain hemispheric laterality specification likely share a common genetic cause. Breast Dis. 2011;33(1):49–52. | ||
Senie RT, Rosen PP, Lesser ML, Snyder RE, Schottenfeld D, Duthie K. Epidemiology of breast carcinoma II: factors related to the predominance of left-sided disease. Cancer. 1980;46(7):1705–1713. | ||
Hartveit F. The side and size of breast tumours. Clin Oncol. 1983;9(2):135–142. | ||
Kwan ML, Kushi LH, Weltzien E, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11(3):R31. | ||
Yang XR, Sherman ME, Rimm DL, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16(3):439–443. | ||
Phipps AI, Malone KE, Porter PL, Daling JR, Li CI. Body size and risk of luminal, HER2-overexpressing, and triple-negative breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2078–2086. | ||
Dizdar O, Aksoy S, Altundag K. Reproductive and hormonal risk factors for postmenopausal luminal, HER-2-overexpressing, and triple-negative breast cancer. Cancer. 2009;115(8):1802–1803. | ||
Tamimi RM, Colditz GA, Hazra A, et al. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast Cancer Res Treat. 2012;131(1):159–167. | ||
Vuong D, Simpson PT, Green B, Cummings MC, Lakhani SR. Molecular classification of breast cancer. Virchows Arch. 2014;465(1):1–14. | ||
Falck AK, Bendahl PO, Chebil G, Olsson H, Fernö M, Rydén L. Biomarker expression and St Gallen molecular subtype classification in primary tumours, synchronous lymph node metastases and asynchronous relapses in primary breast cancer patients with 10 years’ follow-up. Breast Cancer Res Treat. 2013;140(1):93–104. | ||
Britton JA, Gammon MD, Schoenberg JB, et al. Risk of breast cancer classified by joint estrogen receptor and progesterone receptor status among women 20-44 years of age. Am J Epidemiol. 2002;156(6):507–516. | ||
Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. | ||
Yang XR, Sherman ME, Rimm DL, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16(3):439–443. | ||
Phipps AI, Malone KE, Porter PL, Daling JR, Li CI. Reproductive and hormonal risk factors for postmenopausal luminal, HER-2-overexpressing, and triple-negative breast cancer. Cancer. 2008;113(7):1521–1526. | ||
Du T, Zhu L, Levine KM, et al. Invasive lobular and ductal breast carcinoma differ in immune response, protein translation efficiency and metabolism. Sci Rep. 2018;8(1):7205. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.