Back to Journals » International Medical Case Reports Journal » Volume 17
BRCA2, PALB2, RECQL4 Germline Pathogenic Variants, and Somatic TP53 Mutation in Triple Metachronous Malignancies: A Case Report and Literature Review
Authors Liu Y, Yang H, Fu X, Zhong L, Xu P, Fang F, Liu Y, Li Q, Yan Y, Wei S, Wang J, Zhang C
Received 30 October 2023
Accepted for publication 30 December 2023
Published 10 January 2024 Volume 2024:17 Pages 23—29
DOI https://doi.org/10.2147/IMCRJ.S440132
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Yang Liu,1,2,* Hui Yang,1,2,* Xueshu Fu,1,2 Luting Zhong,1,2 Ping Xu,1,2 Fang Fang,1,2 Ying Liu,1,2 Qing Li,1,2 Ya’nan Yan,1,2 Shanchuang Wei,1,2 Junqing Wang,1,2 Chunhua Zhang1– 3
1Department of Gynaecology, Affiliated Hospital of YangZhou University Huai ‘an Maternal and Child Health Care Center, Huai’an, Jiangsu, People’s Republic of China; 2Department of Gynaecology, The Huai’an Maternity and Child Clinical College of Xuzhou Medical University, Huai’an, Jiangsu, People’s Republic of China; 3Department of Integrated Traditional Chinese & Western Clinical Medicine, Macau University of Science and Technology, Macau, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chunhua Zhang, Department of Gynaecology, The Huai’an Maternity and Child Clinical College of Xuzhou Medical University, Huai’an, Jiangsu, People’s Republic of China, Tel +86-13952326117, Email [email protected]
Background: Multiple primary cancer (MPC) refers to the presence of more than one cancer in an individual. Triple primary malignancies are uncommon.
Case: We report the case of a 50-year-old postmenopausal woman in our gynecology department, diagnosed with endometrial cancer, ovarian cancer, and unilateral breast cancer. She carried germline mutations in BRCA2, PALB2, and RECQL4, along with a somatic pathogenic variant in TP53. Endometrial cancer patients harboring germline pathogenic variants in BRCA2 exhibit a heightened risk of ovarian and breast cancer. BRCA2 is known to play a role in the development of ovarian and breast cancer, while PALB2 is identified as a gene associated with breast cancer susceptibility. RECQL4 has been linked to breast cancer, cervical cancer, and other tumors.
Conclusion: Genetic testing may be imperative for identifying MPC in endometrial cancer patients. For individuals with BRCA2 and other gene pathogenic variants, routine examination and monitoring of the endometrium, ovaries, breasts, and other sites prone to polygenic cancer are recommended.
Keywords: multiple primary cancer, endometrial cancer, ovarian cancer, breast cancer, genetic test
Introduction
Multiple primary cancer (MPC) refers to the occurrence of more than one cancer in a single patient. MPC can be categorized as synchronous cancer or metachronous cancer, based on the timing of their occurrence relative to the initial malignant tumor. Synchronous cancer occurs when two tumors arise simultaneously or sequentially within a six-month period, whereas metachronous cancer involves tumors discovered consecutively with an interval exceeding six months.1
Double primary malignancies are more prevalent, with triple primary malignancies being relatively uncommon.2
Notably, 63.6% of initial cancers in females are reported to manifest in endocrine-related organs such as the breast, uterus, and thyroid.3 Beyond estrogen-related factors, genetic variations also contribute to the development of these cancers. BRCA2 is implicated in the onset of ovarian and breast cancer,4 PALB2 is recognized as a gene linked to breast cancer susceptibility,5 and RECQL4 has been associated with breast cancer, cervical cancer,6 and other malignancies.7
Herein, we reported the case of a 50-year-old postmenopausal woman with BRCA2, PALB2, RECQL4 germline pathogenic variants, and somatic TP53 pathogenic variant who was diagnosed with endometrial cancer, ovarian cancer and unilateral breast cancer.
Case Report
A 50-year-old female had been diagnosed with three different types of cancer, namely endometrial cancer, ovarian cancer and right-breast cancer, between 2018 and 2022. The patient underwent transabdominal resection of ovarian cyst in 2008, and the postoperative pathology was benign. The patient had a history of hypertension, type 2 diabetes mellitus, BMI of 25.84 kg/m2, two pregnancies, no long-term use of estrogen supplements, no family history of colon cancer, endometrial cancer, breast cancer or ovarian cancer, and denied any history of smoking.
In 2018, she has been menopausal for five years, underwent diagnosis and curettage due to persistent postmenopausal vaginal bleeding. Histological examination confirmed an adenomatous polyp with local glandular epithelial dysplasia. Magnetic Resonance Imaging (MRI) revealed irregular signal shadows in the uterine cavity, measuring approximately 2.6cm1.8cm3.0cm, and a slightly enhanced 1.6cm*1.4cm mass on the posterior uterine wall. There was no apparent invasion, no pelvic lymph node enlargement, and both ovaries appeared normal (Figure 1). Based on the examination findings and the patient’s decision, a total laparoscopic hysterectomy and bilateral salpingectomy were performed in August (Figure 2). Although the option to concurrently remove bilateral ovaries was available due to the patient’s health condition, she declined. During the operation, erosive changes in the endometrium were observed, and a rapid intraoperative pathology examination indicated atypical hyperplasia of the endometrium. Subsequent permanent postoperative histopathology revealed well-differentiated endometrioid adenocarcinoma (T1aN0M0) (Figure 3), eliminating the need for adjuvant treatment. In the postoperative follow-up period, serum CA153 levels first increased on December 24, 2019, followed by elevations in CA125 on January 4, 2020, and HE4 on November 9, 2020. Tumor markers exhibited a slow-growth trend, but there were no accompanying symptoms, signs, or imaging evidence of tumor recurrence.
 |
Figure 1 2018.07.26 MRI. |
 |
Figure 2 2018.08.05 laparoscopic image. |
 |
Figure 3 2018.08.05 HE×40. |
Later, the patient exhibited signs of decreased appetite and progressive weight loss. Pelvic color Doppler ultrasound and pelvic MRI, conducted for the first time on March 5, 2021, revealed the presence of a pelvic mass. Subsequent MRI on May 17, 2021, displayed an irregular mixed signal mass measuring about 4.6cm3.2cm4.2cm on the vaginal stump and the anterior aspect of the rectum. A PET-CT on May 28, 2021, described a soft tissue mass located in the vaginal stump and the anterior rectum, raising suspicions of tumor recurrence. The triad examination confirmed a mass approximately 5cm in diameter in the anterior rectum. Electing for reoperation on June 12, 2021, the surgical procedure uncovered extensive tumor metastasis in the pelvic and abdominal cavities, involving the peritoneum, greater omentum, liver, spleen, hepatorenal recess, rectum, colon, mesentery, and right diaphragm. Cytoreductive surgery was performed, resulting in the absence of gross residual lesions. Postoperative pathology indicated moderately differentiated ovarian serous papillary carcinoma, with cancer tissues detected in other excised pelvic and abdominal lesions (Figure 4). Therefore, the patient commenced six cycles of TP (paclitaxel and carboplatin) chemotherapy starting from June 28.
 |
Figure 4 2021.06.16 HE×40. |
Considering the patient’s rapid onset of endometrial and ovarian cancers, a genetic test was recommended, revealing germline pathogenic variants in BRCA2, PALB2, RECQL4, and a TP53 somatic pathogenic variant, with HRD (homologous recombination deficiency) (-) and MMR (mismatch repair) (-). Due to the BRCA2 pathogenic variant, the patient underwent bilateral breast examination, revealing no abnormalities. Despite recommendations, the patient and her family declined preventive mastectomy.
Unexpectedly, on March 7, 2022, a grade 4A mass was detected in the patient’s right breast by Doppler ultrasound. Subsequently, on March 9, 2022, a radical mastectomy was performed on the right side, accompanied by prophylactic mastectomy on the left side. Pathological results indicated ductal carcinoma in the right breast and normal breast tissue in the left breast (Figures 5 and 6).
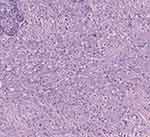 |
Figure 5 2022.03.09 right breast HE×40. |
 |
Figure 6 2022.03.09 left breast HE×40. |
Currently, the patient is undergoing treatment and follow-up with targeted drugs such as Olaparib.
Discussion
Endometrial cancer (EC) is one of the most common gynecological tumors, and its incidence rate is increasing every year. EC often manifests as irregular vaginal bleeding. A few patients have no obvious symptoms.8 EC almost always shows growth into the uterine cavity with necrosis. A small portion of the atrophic uterus may also diffusely infiltrate the muscle.9 Genetic testing is helpful for individualized and accurate treatment of patients with EC.
In this case report, we described a patient with well-differentiated EC, a low-risk type, with no family history of tumor or genetic disease. Both ovaries were retained during total laparoscopic hysterectomy and bilateral salpingectomy based on the requirements of the patient and her family. Moderately differentiated serous papillary carcinoma of the ovary was diagnosed two years after the operation. The patient was advised to undergo genetic testing, which showed BRCA2, PALB2, RecQL4 germline pathogenic variants, and TP53 somatic pathogenic variant. No abnormality was found in breast examination, and the patient and her family refused preventive mastectomy. Nine months later, the right breast mass was found by color Doppler ultrasound, and the right breast radical mastectomy + left breast preventive resection were performed. The pathological results indicated right breast intraductal cancer and left breast normal tissue.
Three primary malignant tumors in a single patient are relatively rare. In the present case, timely genetic testing and appropriate intervention at the first diagnosis of EC in 2018 may have prevented the subsequent end-stage ovarian cancer and breast cancer.
From this case, we can summarize the following based on literature review. First, genetic testing is critical for patients with multiple primary cancers. For patients with endometrial cancer, ovarian cancer and breast cancer, which are hormone-related tumors, genetic testing should be performed regardless of pathological type and differentiation degree, and timely preventive surgical resection should be performed, if necessary. Second, to improve the awareness of the recognition of multiple primary cancers, close follow-up of tumors with the same mechanism is required to avoid missed diagnosis and misdiagnosis.
Genetic testing plays an important role in the prevention, diagnosis, treatment and follow-up of cancer patients.10 BRCA2 is a tumor suppressor gene that is critical for homologous DNA repair. It is located on chromosome 13, 13q12 Position 3, with a total length of 84,193 bp. BRCA2 is a familial breast cancer/ovarian cancer susceptibility gene that was discovered after BRCA1.11 A study reported that the cumulative lifetime risk of ovarian cancer in women with BRCA2 gene pathogenic variant is 16.5%.12 Germline pathogenic variants, including BRCA1/2, RAD51C, RAD51D, BRIP1, PALB2, ATM and Lynch syndrome-related genes (MLH1, MSH2, MSH6, PMS2, EPCAM), can increase the risk of epithelial ovarian cancer.13–15 Approximately 1–5% of breast cancers are attributed to inherited pathogenic variants in BRCA1 or BRCA2.16 It has been reported that BRCA2 has pathogenic variant defects due to which double-stranded DNA cannot be repaired, resulting in an increase in p53 level, apoptosis and cell cycle arrest.17 P53 is an important anti-cancer gene. Wild-type P53 causes apoptosis of cancer cells to prevent cancer development. It also helps to repair cellular defects. Pathogenic variants of p53 can increase carcinogenesis.18 PALB2 is a BRCA2-interacting protein. Functional deletion of the familial PALB2 gene is a predisposing factor for breast cancer. PALB2 is a significant breast cancer susceptibility gene, and germline PALB2 pathogenic variants are associated with ovarian, pancreatic and male breast cancers.19 As a member of RecQ helicase family, RecQL4 plays an important role in maintaining genomic stability. Its absence can lead to ontogeny, premature aging and tumor susceptibility.20
The case review aligns with literature findings, affirming the link between genetic testing and clinical diseases. Combining literature insights and this case, along with the latest information, we suggest that women with pathogenic mutations in BRCA2 and other genes should focus on monitoring the primary sites of multi-gene cancers, such as the endometrium, ovaries, and breast.
Conclusions
Genetic testing may be necessary for cancer patients. For women with BRCA2 and other gene pathogenic variants, once a tumor is found, regular examination and follow-up of endometrium, ovary, breast and other polygenic cancer-prone sites is recommended. Moreover, timely preventive surgical resection should be performed, if necessary. With the continuous development of gene detection technology, it is expected that new targets for the occurrence and development of polygenic carcinoma will be found in the future to guide clinical practice.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study as it was a single case report.
Data Sharing Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Informed Consent Statement
We have obtained the written informed consent from the patient for publication of this case report and any accompanying images. No institutional approval is required to publish case details.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all there areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no external funding.
Disclosure
The authors declare no conflict of interest.
References
1. Marius-Ioan B, Doina P. Triple metachronous malignancies with thyroid involvement: a brief overview of five case reports over 20 years of institutional experience. Diagnostics. 2020;10(3):168. doi:10.3390/diagnostics10030168
2. Tripathy S, Mirdha AR, Shamim SA, et al. A case of metachronous triple carcinoma with synchronous double primary carcinoma on 18F-fluorodeoxyglucose positron emission tomography-computed tomography. Indian J Nuclear Med. 2020;35(2):174–175. doi:10.4103/ijnm.IJNM_19_19
3. Jung GS, Lee O, Kim SS, et al. Multiple primary cancer. J Korean Radiol Soc. 1989;25(5):770. doi:10.3348/jkrs.1989.25.5.770
4. Germani A, Petrucci S, De Marchis L, et al. Beyond BRCA1 and BRCA2: deleterious variants in DNA repair pathway genes in Italian families with breast/ovarian and pancreatic cancers. J Clin Med. 2020;9(9):3003. doi:10.3390/jcm9093003
5. Kwon WK, Jang H, Lee JE, et al. Discovery of BRCA1/BRCA2 founder variants by haplotype analysis. Cancer Gen. 2022;266–267:19–27. doi:10.1016/j.cancergen.2022.05.042
6. Arora A, Agarwal D, Abdel-Fatah TM, et al. RECQL4 helicase has oncogenic potential in sporadic breast cancers. J Pathol. 2016;238(4):495–501. doi:10.1002/path.4681
7. Das M, Prasad SB, Yadav SS, et al. Over expression of minichromosome maintenance genes is clinically correlated to cervical carcinogenesis. PLoS One. 2013;8(7):e69607. doi:10.1371/journal.pone.0069607
8. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 426: the role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. Obstetrics Gynecol. 2009;113(2 Pt 1):462–464. doi:10.1097/AOG.0b013e31819930cc
9. Erickson LA. Endometrial adenocarcinoma, endometrioid type. Mayo Clin Proc. 2018;93(7):963–964. doi:10.1016/j.mayocp.2018.03.017
10. O’Shea R, Rankin NM, Kentwell M, et al. Stakeholders’ views of integrating universal tumour screening and genetic testing for colorectal and endometrial cancer into routine oncology. Eur J Hum Genet. 2021;29(11):1634–1644. doi:10.1038/s41431-021-00871-4
11. Audeh MW, Carmichael D, Prof J, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376(9737):245–251. doi:10.1016/S0140-6736(10)60893-8
12. Kuchenbaecker K, Antoniou A, Barnes DR. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2403–2416. doi:10.1001/jama.2017.7112
13. Lynch HT, Snyder CL, Shaw TG, et al. Milestones of Lynch syndrome: 1895–2015. Nat Rev Cancer. 2015;15(3):181–194. doi:10.1038/nrc3878
14. Moschetta M, George A, Kaye SB, et al. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann Oncol. 2016;27(8):1449–1455. doi:10.1093/annonc/mdw142
15. Eoh KJ, Kim HM, Lee JY, et al. Mutation landscape of germline and somatic BRCA1/2 in patients with high-grade serous ovarian cancer. BMC Cancer. 2020;20(1):204. doi:10.1186/s12885-020-6693-y
16. Davies H, Glodzik D, Morganella S, et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nature Med. 2017;23(4):517–525. doi:10.1038/nm.4292
17. Chang Z, Zhang W, Song M, et al. Expression characteristics of FHIT, p53, BRCA2 and MLH1 in families with a history of oesophageal cancer in a region with a high incidence of oesophageal cancer. Oncol Lett. 2014;9(1):430–436. doi:10.3892/ol.2014.2682
18. Soragni A, Janzen DM, Johnson LM, et al. A designed inhibitor of p53 aggregation rescues p53 tumor suppression in ovarian carcinomas. Cancer Cell. 2016;29(1):90–103. doi:10.1016/j.ccell.2015.12.002
19. Yang X, Leslie G, Doroszuk A, et al. Cancer risks associated with germline PALB2 pathogenic variants: an international study of 524 families. J clin oncol. 2019;38(7):674–685. doi:10.1200/JCO.19.01907
20. Martin-Giacalone BA, Rideau T, Scheurer ME, et al. Cancer risk among RECQL4 heterozygotes. Cancer Gen. 2022;262–263:107–110. doi:10.1016/j.cancergen.2022.02.001
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
