Back to Journals » Clinical Ophthalmology » Volume 16
Branch Retinal Artery Occlusions, Paracentral Acute Middle Maculopathy and Acute Macular Neuroretinopathy After COVID-19 Vaccinations
Authors Ishibashi K, Yatsuka H, Haruta M , Kimoto K, Yoshida S , Kubota T
Received 7 January 2022
Accepted for publication 8 March 2022
Published 31 March 2022 Volume 2022:16 Pages 987—992
DOI https://doi.org/10.2147/OPTH.S357359
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Koki Ishibashi,1,2 Hiroyuki Yatsuka,3 Masatoshi Haruta,1 Kenichi Kimoto,3 Shigeo Yoshida,1 Toshiaki Kubota3
1Department of Ophthalmology, Kurume University School of Medicine, Kurume, Japan; 2Department of Ophthalmology, Yame General Hospital, Yame, Japan; 3Department of Ophthalmology, Faculty of Medicine, Oita University, Yufu, Japan
Correspondence: Masatoshi Haruta, Department of Ophthalmology, Kurume University School of Medicine, 67 Asahi-machi, Kurume, Fukuoka, 830-0011, Japan, Tel +81 942 31 7574, Fax +81 942 37 0324, Email [email protected]
Purpose: Potential retinal adverse events after COVID-19 vaccinations reported previously include paracentral acute middle maculopathy (PAMM), acute macular neuroretinopathy (AMN), and central serous chorioretinopathy. We report four cases of branch retinal artery occlusion (BRAO), one case of PAMM, and one case of AMN that occurred after administration of the Pfizer-BioNTech COVID-19 vaccine.
Patients and Methods: We retrospectively reviewed the medical records of six patients who presented to Yame General Hospital or Oita University Hospital from July through October 2021.
Results: Four patients (2 males) presented with visual field defects associated with BRAO, one male patient with PAMM, and one female patient with AMN after receiving the Pfizer-BioNTech COVID-19 vaccine. The mean age was 59.3 years; the mean best-corrected visual acuity was 20/21. The mean time from the last vaccination to the onset of visual field defect was 22.8 days. Five patients had received two doses of the vaccine and one patient one dose. Patients’ medical history included diabetes mellitus in case 2, hypertension in cases 2, 3 and 6, and Alport syndrome and end-stage renal disease in case 6 for which the patient was undergoing regular hemodialysis.
Conclusion: Although rare, retinal adverse events may occur after COVID-19 vaccinations. Further studies with a larger sample size should determine whether these retinal abnormalities are causally associated with COVID-19 vaccinations or just coincidental. Potential risks of BRAO/PAMM/AMN after COVID-19 vaccinations must be carefully weighed against the substantial benefit of COVID-19 vaccinations.
Keywords: acute macular neuroretinopathy, branch retinal artery occlusion, paracentral acute middle maculopathy, Pfizer-BioNTech COVID-19 vaccine, retinal adverse events
Introduction
Potential retinal adverse events after COVID-19 vaccinations reported previously include paracentral acute middle maculopathy (PAMM), acute macular neuroretinopathy (AMN), and central serous chorioretinopathy.1,2 PAMM is believed to result from retinal ischemia through the intermediate capillary plexus and/or deep capillary plexus; whereas AMN through the deep capillary plexus and/or choriocapillaris.3 The typical optical coherence tomography (OCT) findings in PAMM show hyperreflectivity at the inner nuclear layer; whereas in AMN at the outer plexiform and outer nuclear layers. We report four cases of branch retinal artery occlusion (BRAO), one case of PAMM, and one case of AMN that occurred after administration of the Pfizer-BioNTech COVID-19 vaccine (Cambridge, MA, USA/Mainz, Germany).
Patients and Methods
We retrospectively reviewed the medical records of six patients who presented to Yame General Hospital or Oita University Hospital from July through October 2021. This study adhered to the tenets of the Declaration of Helsinki. The Ethical Committee of Yame General Hospital approved this study and waived the need for individual patient consent due to the retrospective nature of this study (No. 21-005). The data were appropriately anonymized to protect confidentiality during the analyses. Written informed consent was provided by the patients to have the case details and accompanying images published for cases 1, 3, 5 and 6. The best-corrected visual acuities (BCVAs) were converted to the logarithm of the minimum angle of resolution (logMAR) units for statistical analyses. Data are expressed as the mean ± standard deviation.
Results
The clinical characteristics of 6 patients are shown in Table 1. Four patients (2 males) presented with visual field defects associated with BRAO (cases 1–4), one male patient with PAMM (case 5), and one female patient with AMN (case 6) after receiving the Pfizer-BioNTech COVID-19 vaccine. The mean age was 59.3 ± 21.4 years; the mean BCVA was 0.03 ± 0.14 logMAR (approximate Snellen equivalent 20/21). Five patients had received two doses of the vaccine and one patient one dose. The mean time from the last vaccination to the onset of visual field defect was 22.8 ± 23.3 days. Patients’ medical history included diabetes mellitus in case 2, hypertension in cases 2, 3 and 6, and Alport syndrome and end-stage renal disease in case 6 for which the patient was undergoing regular hemodialysis.
 |
Table 1 Clinical Characteristics of Retinal Adverse Events After COVID-19 Vaccinations |
Selected Cases
Case 1. A 38-year-old woman presented with a 1-day history of lower visual field defects in her right eye. She had received the first dose of the Pfizer-BioNTech COVID-19 vaccine 16 days previously. She took no medications (including oral contraceptives). At presentation, the BCVA was 20/13 in the right eye. Fundus examination of the right eye showed superotemporal retinal whitening, which was consistent with BRAO (Figure 1).
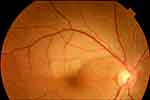 |
Figure 1 Branch retinal artery occlusion (BRAO) after COVID-19 vaccinations. A fundus photograph of the right eye of case 1 shows superotemporal BRAO. |
Case 3. An 86-year-old man presented with a 21-day history of lower visual field defects in his left eye. He had received the second dose of the Pfizer-BioNTech COVID-19 vaccine 25 days previously. At presentation, the BCVA was 20/25 in the left eye. Fundus examination of the left eye showed superotemporal retinal whitening, which was consistent with BRAO (Figure 2).
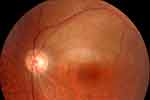 |
Figure 2 Branch retinal artery occlusion (BRAO) after COVID-19 vaccinations. A fundus photograph of the left eye of case 3 shows superotemporal BRAO. |
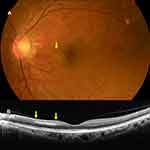
Case 5. A 62-year-old man presented with a 15-day history of visual field defects in his left eye. He had received the second dose of the Pfizer-BioNTech COVID-19 vaccine 22 days previously. At presentation, the BCVA was 20/17 in the left eye. A fundus examination of the left eye showed inferonasal parafoveal retinal whitening and a horizontal OCT image of that eye demonstrated parafoveal hyperreflective band at the inner nuclear layer, which were consistent with PAMM (Figure 3).
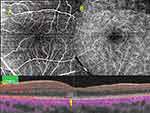
Case 6. A 33-year-old woman presented with a 3-day history of visual field defects in her left eye. She had received the second dose of the Pfizer-BioNTech COVID-19 vaccine 11 days previously. At presentation, the BCVA was 20/29 in the left eye. OCT angiography images of the left eye showed possible flow deficits in the deep capillary plexus and a horizontal OCT image of that eye demonstrated hyperreflective lesions affecting the outer plexiform and outer nuclear layers with disruption of the ellipsoid zone, which were consistent with AMN (Figure 4).
Discussion
The COVID-19 pandemic accelerated development of highly effective vaccines that were produced with unprecedented speed with the use of diverse new technologies. There are currently two mRNA COVID-19 vaccines (Pfizer-BioNTech and Moderna [Cambridge, MA, USA]) and one vector COVID-19 vaccine (AstraZeneca [Frederick, MD, USA]) approved in Japan. As of January 3, 2022, more than 101 million people in Japan, ie, more than 79% of the population, had received at least one dose of the COVID-19 vaccines. In the current case series, four patients presented with visual field defects associated with BRAO, one patient with PAMM, and one patient with AMN after receiving the Pfizer-BioNTech COVID-19 vaccine. Although the Pfizer-BioNTech COVID-19 vaccine has been shown not to be associated with an elevated risk of arterial embolism and thrombosis,4 PAMM and AMN have been reported after Sinopharm COVID-19 vaccinations (Shanghai, China).1 The exact mechanisms by which COVID-19 vaccinations cause BRAO/PAMM/AMN remain unclear; however, common proposed mechanisms include both molecular mimicry and antigen-specific cell and antibody-medicated hypersensitivity reactions.1 Furthermore, central retinal artery occlusion, PAMM and AMN have been reported after confirmed COVID-19 infections.5–7 COVID-19 infections can directly affect the endothelial cells, causing inflammatory and procoagulatory state, which may lead to vascular thromboembolic complications.8–10 Substantial overlap between the retinal adverse events of COVID-19 infections and COVID-19 vaccinations suggests that the immune response to COVID-19 may be involved in the pathogenesis of the retinal adverse events after COVID-19 vaccinations.2
Several cases of rare unusual thrombotic events and thrombocytopenia were reported recently following the AstraZeneca COVID-19 vaccine.11 After the onset of BRAO, the patient in case 1 underwent systematic evaluations including blood tests, electrocardiography, carotid/cardiac ultrasonography, and head magnetic resonance imaging, with no evidence of thrombocytopenia or underlying risk factors for thromboembolism.
The limitations of the current study were its retrospective nature, no follow-up data and that only six cases were reported. We could not estimate an incidence rate of BRAO/PAMM/AMN after COVID-19 vaccinations and compare incidence rates of BRAO/PAMM/AMN between vaccinated and unvaccinated patients. Although BRAO/PAMM/AMN in the current case series occurred after administration of the Pfizer-BioNTech COVID-19 vaccine, a definite causal relationship between BRAO/PAMM/AMN and COVID-19 vaccinations could not be established.
Conclusion
Considering that six cases of relatively rare retinal abnormalities occurred shortly after the administration of COVID-19 vaccine during the study period from July through October 2021 and that no patients with BRAO/PAMM/AMN had presented during the control period from July through October 2018 (before the COVID-19 pandemic), BRAO/PAMM/AMN may occur in patients who received the COVID-19 vaccine. Further studies with a larger sample size should determine whether these retinal abnormalities are causally associated with COVID-19 vaccinations or just coincidental. Potential risks of BRAO/PAMM/AMN after COVID-19 vaccinations must be carefully weighed against the substantial benefit of COVID-19 vaccinations.
Acknowledgment
The authors thank Lynda Charters for English editing.
Disclosure
The authors report no conflicts of interest associated with this work.
References
1. Pichi F, Aljneibi S, Neri P, et al. Association of ocular adverse events with inactivated COVID-19 vaccination in patients in Abu Dhabi. JAMA Ophthalmol. 2021;139:1131–1135. doi:10.1001/jamaophthalmol.2021.3477
2. Ng XL, Betzler BK, Testi I, et al. Ocular adverse events after COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29:1216-1224. doi:10.1080/09273948.2021.1976221
3. Iovino C, Au A, Ramtohul P, et al. Coincident PAMM and AMN and insights into a common pathophysiology: coincident PAMM and AMN. Am J Ophthalmol. 2022;236:136-146. doi:10.1016/j.ajo.2021.07.004
4. Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi:10.1056/NEJMoa2110475
5. Virgo J, Mohamed M. Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Eye. 2020;34:2352–2353. doi:10.1038/s41433-020-1069-8
6. Acharya S, Diamond M, Anwar S, et al. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases. 2020;21:e00867. doi:10.1016/j.idcr.2020.e00867
7. Ozsaygılı C, Bayram N, Ozdemir H. Cilioretinal artery occlusion with paracentral acute middle maculopathy associated with COVID-19. Indian J Ophthalmol. 2021;69:1956–1959. doi:10.4103/ijo.IJO_563_21
8. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi:10.1182/blood.2020006000
9. Levi M, Thachil J, Iba T, et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi:10.1016/S2352-3026(20)30145-9
10. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi:10.1016/S0140-6736(20)30937-5
11. Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384:2254–2256. doi:10.1056/NEJMe2106315
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.