Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Brain-Reactive Antibodies are Potential Biomarkers for Evaluating Therapeutic Efficacy in NPSLE Patients
Authors Wang X , Feng D, Ke Y, Gu L, Lv C, Zhang M, Wang Q, Wang Y
Received 24 January 2022
Accepted for publication 23 June 2022
Published 4 July 2022 Volume 2022:18 Pages 1329—1340
DOI https://doi.org/10.2147/NDT.S359698
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Xiujiao Wang,1,* Dongju Feng,2,* Yao Ke,1 Lei Gu,1 Chengyin Lv,1 Miaojia Zhang,1 Qiang Wang,1 Yanyan Wang1
1Department of Rheumatology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, 210029, People’s Republic of China; 2Department of Immunology, Nanjing Medical University, Nanjing, Jiangsu, 211166, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiang Wang; Yanyan Wang, Department of Rheumatology, The First Affiliated Hospital of Nanjing Medical University, No. 300 Guangzhou Road, Nanjing, Jiangsu, 210029, People’s Republic of China, Tel +86-13913889877 ; +86-13813892892, Fax +86-25-68306893, Email [email protected]; [email protected]
Purpose: Neuropsychiatric systemic lupus erythematosus (NPSLE) is the main cause of disability and death in systemic lupus erythematosus (SLE). It can cause cognitive impairment and organic brain syndrome. Brain-reactive antibodies, such as anti-DNA/anti-N-methyl-D-aspartate receptor (NMDAR) antibodies (DNRAbs), anti-microtubule-associated protein 2 (anti-MAP2) antibodies, and anti-glial fibrillary acidic protein (anti-GFAP) antibodies are thought to participate in the progression of NPSLE and thus considered potential diagnostic biomarkers, but whether they can be used for evaluating therapeutic efficacy in NPSLE is unknown.
Patients and methods: Overall, 17 NPSLE patients and 10 non-SLE controls were included in this study. All the patients were treated with glucocorticoid (GC) pulse therapy. Serum and cerebrospinal fluid (CSF) concentrations of DNRAbs and anti-MAP2 and anti-GFAP antibodies were measured using enzyme-linked immunosorbent assay. The differences between the CSF concentrations of these antibodies in NPSLE patients before and after GC pulse therapy were analyzed.
Results: CSF concentrations of DNRAbs and anti-MAP2 and anti-GFAP antibodies were significantly higher in NPSLE patients compared to the non-SLE controls. Among the patients, CSF concentration of DNRAbs was significantly higher in the patients with acute confusional state (ACS) than in those with non-ACS diffuse NPSLE or focal NPSLE. Additionally, CSF concentration of DNRAbs was significantly correlated with QIgG (r=0.4884, P=0.0467) and IgG index (r=0.5319, P=0.0280) in NPSLE patients. Moreover, CSF concentrations of DNRAbs, anti-MAP2, and anti-GFAP antibodies and QIgG were significantly decreased after GC pulse therapy in NPSLE patients.
Conclusion: These results indicate that CSF DNRAbs and anti-MAP2 and anti-GFAP antibodies are potential biomarkers for evaluating therapeutic efficacy in NPSLE.
Keywords: autoantibodies, neuropsychiatric systemic lupus erythematosus, efficacy evaluation, CSF biomarkers
Introduction
Systemic lupus erythematosus (SLE) is a connective-tissue disease characterized by the production of autoantibodies and multiple organ damage, including renal, cardiovascular, hematological, and neural damages.1 The neural damages can cause neuropsychiatric symptoms termed neuropsychiatric SLE (NPSLE), which includes cognitive impairment and encephalopathy. Manifestations of NPSLE have been divided into focal neurological syndromes, diffuse psychiatric syndromes (including cognitive, mood, and anxiety disorders, psychosis, and acute confusional state [ACS]), and peripheral nervous system manifestations.2 Among these syndromes, ACS is the most severe one, which usually has a poor prognosis.3 Neuropsychiatric symptoms present in 28–60% of SLE patients4,5 and can lead to disability or even death.6
Autoantibodies can mediate neuronal damage and promote the pathogenesis of NPSLE.7–9 Damage to neural cells, including neurons and glial cells, has been observed in NPSLE patients.10 Increasing evidence has shown that autoantibodies against components of neural cells (which are also referred to as brain-reactive antibodies) are associated with the neuropsychiatric events in SLE, and therefore are considered to be potential biomarkers of NPSLE.11 N-methyl-D-aspartate receptors (NMDAR) are ion channels located on the postsynaptic membrane of neurons. Antibodies against the GluN2A/GluN2B subunits of NMDAR also bind to DNA and are thus termed anti-DNA/anti-NMDAR antibodies (DNRAbs).12 Previous studies have shown that the total concentration of DNRAbs in the cerebrospinal fluid (CSF) of NPSLE patients is significantly increased7 and is associated with the dysfunction of the nervous system as well as the neuronal damage in SLE.13,14 Microtubule-associated protein 2 (MAP2) is a cytoskeleton protein that is highly expressed in neurons. Anti-MAP2 antibodies have been found to be significantly upregulated in the CSF of NPSLE patients.7,15 Glial fibrillary acidic protein (GFAP) is the predominant component of the intermediate filaments in astrocytes and has been reported to be upregulated in the CSF of SLE patients.10 Accordingly, brain-reactive antibodies presumably participate in the pathogenesis of NPSLE and promote the development of neuropsychiatric symptoms in SLE patients. However, whether these antibodies can be used for evaluating therapy efficacy in NPSLE is not known.
Therefore, this study focused on the CSF and serum concentrations of DNRAbs and anti-MAP2 and anti-GFAP antibodies in NPSLE patients and analyzed the differences between these concentrations in NPSLE patients before and after glucocorticoid (GC) pulse therapy to assess the potential of these antibodies as biomarkers for evaluating therapeutic efficacy in NPSLE.
Methods
Patients and Samples
For this study, 17 NPSLE patients (n=16 females; n=1 male) were recruited. In addition, 10 patients with non-inflammatory neurological diseases, namely intracerebral hemorrhage (n=4), neurodegenerative diseases (n=3), steroid psychosis (n=2), and headache (n=1), were studied as non-SLE controls. All NPSLE patients fulfilled the American College of Rheumatology (ACR) 2012 revised criteria for the classification of SLE16 and the 1999 ACR definitions for NPSLE syndromes.2 Disease activity was assessed based on the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI). Permanent and irreversible damage due to SLE was assessed using the systemic lupus international collaborating clinics (SLICC)/ACR damage index (SDI). According to the ACR definition of NPSLE,2 the neuropsychiatric syndromes in this study included focal (n=4) and diffuse (n=13) NPSLE. The patients with diffuse NPSLE included those with ACS (n=7) and non-ACS, including anxiety disorder, cognitive dysfunction, mood disorder, and psychosis (n=6). All the patients with NPSLE were hospitalized in the Department of Rheumatology of the First Affiliated Hospital of Nanjing Medical University between 2015 to 2021. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committees of the First Affiliated Hospital of Nanjing Medical University (Ethical number: 2021-SR-464). Informed consent was obtained from all the study participants. All the NPSLE patients were treated with intravenous injection of 500 mg/day methylprednisolone for 3 days (GC pulse therapy). CSF samples were obtained from the participants via a lumbar puncture. The serum and CSF samples of the NPSLE patients were collected on the day of admission or diagnosis and two weeks after the GC pulse therapy.
CSF Analyses
CSF analyses were performed as a part of the routine diagnostic workshop. Serum and CSF concentrations of albumin IgG were assessed using immunonephelometry. Quotients of albumin (QAlb) and IgG (QIgG) were defined as the ratios of the CSF concentrations of these fractions to the corresponding serum concentrations. The IgG index was defined as the ratio of QIgG to QAlb. The reference ranges for QAlb, QIgG and IgG index were set according to Reiber17 and Bonnan.18
Measurement of Serum and CSF Concentrations of Autoantibodies
Serum and CSF concentrations of DNRAbs and anti-MAP2 and anti-GFAP antibodies were measured using enzyme-linked immunosorbent assay (ELISA) as described previously, with minor modifications.19 Briefly, we used synthetic DWEYSVWLSN peptide and purified MAP-2 and GFAP proteins as coating antigens for detecting DNRAbs and anti-MAP2 and anti-GFAP antibodies, respectively. Each coating antigen was diluted in phosphate-buffered saline (PBS) to a final concentration of 0.1 μg/mL, and 100 μL was used to coat each well of an Immulon microtiter 96-well plate at 4 °C for 24 h. Then, all the samples (including serum and CSF) were diluted in 1:5, and the non-diluted and diluted samples were incubated in antigen-coated wells at 4 °C overnight. After washing the wells with PBS three times, horseradish peroxidase (HRP)–conjugated goat anti-human IgG (Caltag, San Francisco, CA) and the substrate 2,2-azinobis-3-ethylbenzothiazoline sulfonic acid was used for detection and visualization. The optical density at 450 nm was used for data analysis.
Statistical Analysis
Continuous variables are described by median and range, and categorical variables by numbers and percentages. Normal distribution was tested using the Kolmogorov–Smirnov test. The significance of differences was assessed using the Mann–Whitney U-test or Fisher’s exact test as indicated. The SLEDAI scores and CSF concentrations of brain-reactive antibodies before GC pulse therapy were compared with those after the therapy by using the paired t-test. The QIgG, QAlb and IgG index before GC pulse therapy were compared with those after the therapy by using the Wilcoxon-matched-pairs signed ranks test. Correlations between QIgG, QAlb, IgG index, SLEDAI scores and concentrations of brain-reactive antibodies were assessed by Pearson’s correlation test. P<0.05 was considered to represent statistically significant differences. All the statistical analyses were performed using GraphPad Prism version 7.0 for Windows (GraphPad Software; Microsoft, Redmond, WA).
Results
Patient Characteristics
Patient characteristics, including demographic, clinical, and CSF parameters are reported in Table 1. No difference in age (Mann–Whitney U-test P=0.5213) or sex (Fisher’s exact test P=1.000) between the NPSLE patients and non-SLE controls was observed. Among the NPSLE patients, the median of SLEDAI was 24 (range: 10–36), and the median of SDI was 2 (range: 1–5). In addition, the CSF parameters of the NPSLE patients showed that all of the NPSLE patients had abnormal QAlb. On the other hand, 64.7% of them had abnormal QIgG, and 5.9% of them had abnormal IgG indexes. We also assessed for the presence of anti-nuclear antibodies in the sera and CSF of NPSLE patients (see Table 1).
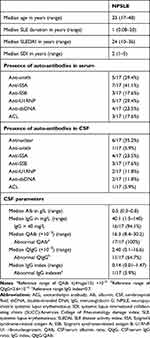 |
Table 1 Patient Characteristics: Demographic, Clinical, and CSF Data |
CSF Concentrations of Brain-Reactive Antibodies Were Elevated in NPSLE Patients
Our initial experiments compared the CSF and serum concentrations of brain-reactive antibodies in NPSLE patients with those in the non-SLE controls. The NPSLE patients had significantly increased CSF concentrations of brain-reactive antibodies (DNRAbs and anti-MAP2 and anti-GFAP antibodies) compared with the concentrations in the non-SLE controls. The total CSF concentration of DNRAbs in the NPSLE patients was nearly twice as much as that in the non-SLE controls (median 385.9 pg/mL, range 280.8–523.7 pg/mL vs median 189 pg/mL, range 100.2–271 pg/mL; P<0.0001). The total CSF concentration of anti-MAP2 antibodies in the NPSLE patients was significantly higher than that in the non-SLE controls (median 68.5 pg/mL, range 39.0–85.6 pg/mL vs median 31.52 pg/mL, range 25.94–37.47 pg/mL; P<0.0001). Likewise, the total CSF concentration of anti-GFAP antibodies in the NPSLE patients was also significantly higher than that in the non-SLE controls (median 108.3 pg/mL, range 51.6–155.1 pg/mL vs median 39.85 pg/mL, range 31.5–48.2 pg/mL; P<0.0001) (Figure 1A–C).
Next, we examined the concentrations of these autoantibodies in the sera of NPSLE patients and non-SLE controls. There were no significant differences in the serum concentrations of DNRAbs and anti-MAP2 and anti-GFAP antibodies between NPSLE patients and non-SLE controls (Figure 1D–F).
CSF Concentrations of Brain-Reactive Antibodies are Correlated with the Severity of NPSLE
We compared the CSF concentrations of brain-reactive antibodies in patients with various NPSLE subgroups. There was no significant difference in CSF concentrations of DNRAbs and anti-MAP2 and anti-GFAP antibodies between the patients with focal NPSLE and those with diffusion NPSLE (Figure 2A–C). Then, we further analyzed the CSF concentrations of the antibodies in the focal, non-ACS, and ACS NPSLE patients. The results revealed that total CSF concentration of DNRAbs was significantly increased in the ACS patients compared with the concentration in the focal (P=0.0381) or non-ACS diffuse NPSLE (P=0.0221) patients (Figure 2D). By contrast, no significant difference was found in CSF concentrations of anti-MAP2 and anti-GFAP antibodies among the different sub-groups of NPSLE patients (Figure 2E and F).
CSF DNRAbs are Associated with Intrathecal Immunoglobulin Synthesis
Next, we examined the correlation between CSF concentrations of brain-reactive antibodies and intrathecal immunoglobulin synthesis in NPSLE patients. QIgG was moderately correlated with total CSF concentration of DNRAbs (r=0.4884; P=0.0467) in the NPSLE patients (Figure 3A) but displayed no relationship with CSF concentration of anti-MAP2 (r=−0.0401; P=0.8787) or anti-GFAP antibody (r=−0.1415; P=0.5879) (Figure 3B and C). QIgG reflects both intrathecal IgG synthesis as well as IgG penetration into CSF from circulation.17 In order to remove the influence from QAlb, we analyzed the IgG index and found that the IgG index (r=0.5319; P=0.0280) was moderately correlated with total CSF concentration of DNRAbs in NPSLE patients (Figure 3D) but displayed no relationship with CSF concentration of anti-MAP2 (r=0.0428; P=0.8703) or anti-GFAP antibody (r=−0.1212; P=0.6431) (Figure 3E and F).
CSF Brain-Reactive Antibodies are Not Associated with the Disruption of the Blood-Brain Barrier (BBB)
We asked whether CSF concentrations of brain-reactive antibodies were associated with BBB function in the NPSLE patients. Results showed that QAlb was not correlated with CSF concentration of DNRAbs (r=−0.1796; P=0.4905), anti-MAP2 antibody (r=−0.3371; P=0.1858), or anti-GFAP antibody (r=−0.1839; P=0.4798) in NPSLE patients (Figure 3G–I).
The CSF Concentrations of Brain-Reactive Antibodies in NPSLE Patients Decreased Upon GC Pulse Therapy
GC pulse therapy is accepted as the most effective therapy for SLE patients with serious organ damage, including NPSLE patients.20 Then, we examined the CSF concentrations of brain-reactive antibodies in the NPSLE patients after GC pulse therapy. Results revealed that the CSF concentrations of DNRAbs and anti-MAP2 and anti-GFAP antibodies in the NPSLE patients all decreased upon GC pulse therapy (Figure 4A–C). The total CSF concentration of DNRAbs in the NPSLE patients was reduced from median 385.9 (range, 280.8–523.7) pg/mL to 310.4 (60.95–448.2) pg/mL (P=0.0006) (Figure 4A); Anti-MAP2 antibodies in the CSF were reduced from 68.5 (39.0–85.6) pg/mL to 55.6 (5.5–93.1) pg/mL (P=0.0337) (Figure 4B); Anti-GFAP antibodies in the CSF range from 108.3 (51.6–155.1) pg/mL to 88.3 (21.5–128.4) pg/mL (P=0.0167) (Figure 4C). These results suggest that the CSF concentrations of DNRAbs and anti-MAP2 and anti-GFAP antibodies in the NPSLE patients were decreased after the GC pulse therapy.
The Disease Activity in the NPSLE Patients was Significantly Decreased After GC Pulse Therapy But Not Associated with the CSF Concentrations of Brain-Reactive Antibodies
We found that GC pulse therapy significantly relieved the neuropsychiatric symptoms in all the NPSLE patients and decreased the SLEDAI score of the NPSLE patients from median 24 (range, 10–36) to 4 (1–21) (P < 0.0001) (Figure 5A). Then, we asked whether disease activity was associated with CSF concentrations of brain-reactive antibodies. However, we did not observe any correlation between SLEDAI score and CSF concentrations of DNRAbs (r=−0.2255; P=0.3842) and anti-MAP2 (r=0.0874; P=0.7389) and anti-GFAP (r=−0.2169; P=0.4031) antibodies in NPSLE patients (Figure 5B–D).
The QIgG was Decreased After GC Pulse Therapy in NPSLE Patients, Whereas the IgG Index and QAlb Were Unchanged
We next compared intrathecal immunoglobulin synthesis and BBB function before GC pulse therapy with those after the therapy. The QIgG in the NPSLE patients were decreased from median 2.4 (range 0.1–16.6) × 10−3 to 2.0 (0.1–3.1) × 10−3 (P = 0.0389) after GC pulse therapy (Figure 6A). However, the IgG index and QAlb concentration did not significantly change upon the GC pulse therapy (Figure 6B and C).
Discussion
The most important finding of this study is that the CSF concentrations of brain-reactive antibodies (DNRAbs and anti-MAP2 and anti-GFAP antibodies) in the NPSLE patients were significantly decreased after effective GC pulse therapy. This finding indicates that these brain-reactive antibodies can serve as biomarkers for evaluating therapeutic efficacy in NPSLE.
The pathogenesis of NPSLE is still mostly unknown. Autoantibody-mediated responses have been considered to play important roles in pathogenesis.21 Increasing evidence has demonstrated the diagnostic value of brain-reactive antibodies in NPSLE.7,14,15,19,22 Consistent with previous findings, the present study found that CSF concentrations of DNRAbs and anti-MAP2 and anti-GFAP antibodies were significantly elevated in NPSLE patients. Further analyses demonstrated that CSF concentrations of DNRAbs correlated with ACS in NPSLE patients. However, the serum levels of these antibodies were similar between NPSLE patients and non-SLE controls. Williams et al reported that serum anti-MAP2 antibodies were significantly associated with NPSLE,15 which was not consistent with our findings. Additionally, factors that affect serum levels of brain-reactive antibodies remain unknown. Previous studies indicated that they were probably associated with subgroups of NPSLE8 and racial/ethnic groups.23 The study by Williams et al included 100 SLE patients and 34 patients with NPSLE. Among the 100 SLE patients, 54 were white, 37 were black, eight were Hispanic, and one was Asian. They did not report the proportion of NPSLE subgroups. Our study only included 17 NPSLE patients, all of whom were Asians. Four of them were focal NPSLE patients, 13 were diffuse NPSLE patients (n=7 ACS; n=6 non-ACS). Therefore, we speculated that the discrepancy was caused by different study populations and sample sizes.
Intrathecal immunoglobulin synthesis and BBB disruption have been considered important pathological changes in NPSLE. There is still debate over whether brain-reactive antibodies are produced intrathecally or passively transferred from the systemic circulation into the central nervous system (CNS) through the impaired BBB. This study showed that 64.7% of NPSLE patients had increased QIgG and 5.9% had abnormal IgG index. Furthermore, CSF concentration of DNRAbs was significantly correlated with both QIgG and IgG index in NPSLE patients. Previous studies have shown that DNRAbs can cause neuronal damage in SLE.13,14 Our results indicate that CSF DNRAbs are associated with intrathecal immunoglobulin synthesis and probably contribute to the development of NPSLE. Then, we found that all NPSLE patients had abnormal QAlb, but none of the brain-reactive antibodies is significantly correlated with QAlb in NPSLE patients. A previous study has reported that autoantibodies are produced intrathecally and that the presence of autoantibodies correlate with oligoclonal antigen-specific B cells, which infiltrate from the circulation through the disrupted BBB.24 Therefore, combined with the previous findings, we speculate that the disruption of the BBB in NPSLE patients possibly allow immune cells (including B cells) to infiltrate into the CNS from the circulation and then lead to the intrathecal production of brain-reactive antibodies (such as DNRAbs), thereby contributing to neuronal damage in NPSLE pathogenesis.
Neuropsychiatric symptoms are one of the best indications for GC pulse therapy in SLE patients.20,25 In line with previous findings, our study found that CSF concentrations of DNRAbs and anti-MAP2 and anti-GFAP antibodies were significantly increased in NPSLE patients. Additionally, this study revealed that these concentrations significantly decreased upon GC pulse therapy in NPSLE patients. These results indicate that DNRAbs and anti-MAP2 and anti-GFAP antibodies can serve as biomarkers for evaluating therapeutic efficacy in NPSLE.
In addition to decreased CSF concentrations of brain-reactive antibodies in NPSLE patients after GC pulse therapy, we found that QIgG was also significantly reduced. QIgG may be affected by intrathecal immunoglobulin synthesis, as well as BBB permeability.17 GC has been shown to stabilize the BBB by modulating tight junction proteins and repressing the inflammatory response.26 However, results indicated that GC pulse therapy did not significantly affect QAlb, which indicates that GC pulse therapy likely did not influence BBB permeability in NPSLE patients. Though there were no significant changes in the IgG index after GC pulse therapy, a tendency revealed that the IgG index was lower after GC pulse therapy. The small sample size may be the reason that our results did not show any significant differences. These results indicate that GC pulse therapy reduces intrathecal synthesis of immunoglobulin. Previous studies have suggested that GC reduces production of antibodies in SLE by modulating B-lymphocyte function, such as restricting the differentiation into plasma cells,27,28 reducing the expression of immunoglobulin, and promoting the secretion of immunomodulatory cytokines.29 The PRDM1 gene encodes the terminal-differentiation factor BLIMP-1, which reduces B-cell proliferation. A recent study has reported that GC significantly upregulates the expression of PRDM1 and also impairs B-cell receptor signaling and Toll-like receptor 7 signaling.29 Therefore, GC pulse therapy probably reduces the concentrations of brain-reactive antibodies by regulating B lymphocytes. In addition, recent studies have suggested that brain-reactive antibodies, such as DNRAbs, may promote the development of NPSLE by affecting the function of microglia. DNRAbs are associated with the impairment of cognitive functions in NPSLE and play important roles in the related microglia-mediated neuronal damage.13,14 Thus, we speculate that GC pulse therapy inhibits the activation of microglia by decreasing the concentration of DNRAbs and thereby effectively relieves the neuropsychiatric symptoms of NPSLE.
Although total CSF concentration of anti-MAP2 antibodies was significantly decreased in most NPSLE patients after undergoing GC pulse therapy, one of the patients displayed a CSF concentration nearly twice as much as before (93.1 pg/mL vs 57.7 pg/mL). We followed up with this patient and found that her neuropsychiatric symptoms (mood disorder and psychosis) were improved after the GC pulse therapy and were completely relieved three months after the therapy. However, new neuropsychiatric symptoms (headache and anxiety) appeared approximately 8 months after the therapy. Therefore, a high CSF concentration of anti-MAP2 antibodies may indicate that the neuropsychiatric symptoms of NPSLE patients are still not effectively controlled. CSF concentration of anti-MAP2 antibodies may serve as a potential biomarker for predicting relapse of neuropsychiatric symptoms in NPSLE patients, but further studies are needed.
This research has some limitations. In clinical practice, it is nearly impossible to obtain CSF from SLE patients without neuropsychiatric symptoms because of the lack of indication for a lumbar puncture. Therefore, this study did not have any non-NPSLE controls (ie, SLE patients who did not suffer from neuropsychiatric events). In addition, the sample size was relatively small and there was only one male patient. It is extremely difficult to obtain CSF samples, and we could obtain both serum and CSF samples from only 17 NPSLE patients. In the future, we plan to cooperate with other teams to increase our sample size of NPSLE patients.
The present study suggests that the CSF concentrations of DNRAbs and anti-MAP2 and anti-GFAP antibodies in NPSLE patients are increased but significantly decreased after GC pulse therapy. Furthermore, DNRAbs in the CSF may be related to ACS-NPSLE. Additionally, all the NPSLE patients in this study displayed abnormal QAlb, indicating BBB disruption. Moreover, CSF DNRAbs were found to correlate with QIgG and IgG index in NPSLE patients, indicating that they are associated with intrathecal immunoglobulin synthesis and probably play very important roles in the pathogenesis of NPSLE.
Conclusion
In summary, this study confirmed the diagnostic value of brain-reactive antibodies in NPSLE. More importantly, our study proved that DNRAbs, anti-MAP2 antibodies, and anti-GFAP antibodies are potential biomarkers for evaluating therapeutic efficacy in NPSLE.
Ethical Statement
This study complied with the Declaration of Helsinki and was approved by the Ethics Committees of the First Affiliated Hospital of Nanjing Medical University (Ethical number: 2021-SR-464).
Acknowledgments
This work was supported by grants from the Program of Innovative and Entrepreneurial Talent of Jiangsu province ([2020]30099) and Young Scholars Fostering Fund of the First Affiliated Hospital of Nanjing Medical University (PY2021033).
Disclosure
The authors declare that they have no conflict of interest.
References
1. Stojan G, Petri M. Epidemiology of systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2018;30:144–150. doi:10.1097/BOR.0000000000000480
2. Ad AC. Committee HOC, Neuropsychiatric ON, Nomenclature L. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608. doi:10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F
3. Bertsias GK, Ioannidis JP, Aringer M, et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis. 2010;69:2074–2082. doi:10.1136/ard.2010.130476
4. Unterman A, Nolte JE, Boaz M, et al. Neuropsychiatric syndromes in systemic lupus erythematosus: a meta-analysis. Semin Arthritis Rheum. 2011;41:1–11. doi:10.1016/j.semarthrit.2010.08.001
5. Hanly JG, Urowitz MB, Gordon C, et al. Neuropsychiatric events in systemic lupus erythematosus: a longitudinal analysis of outcomes in an international inception cohort using a multistate model approach. Ann Rheum Dis. 2020;79(3):356–362. doi:10.1136/annrheumdis-2019-216150
6. Wu XY, Yang M, Xie YS, et al. Causes of death in hospitalized patients with systemic lupus erythematosus: a 10-year multicenter nationwide Chinese cohort. Clin Rheumatol. 2019;38:107–115. doi:10.1007/s10067-018-4259-z
7. Hirohata S, Arinuma Y, Yanagida T, et al. Blood-brain barrier damages and intrathecal synthesis of anti-N-methyl-D-aspartate receptor NR2 antibodies in diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematosus. Arthritis Res Ther. 2014;16(2):R77. doi:10.1186/ar4518
8. Hirohata S, Sakuma Y, Matsueda Y, et al. Role of serum autoantibodies in blood brain barrier damages in neuropsychiatric systemic lupus erythematosus. Clin Exp Rheumatol. 2018;36:1003–1007.
9. Diamond B, Bloom O, Al Abed Y, et al. Moving towards a cure: blocking pathogenic antibodies in systemic lupus erythematosus. J Intern Med. 2011;269(1):36–44. doi:10.1111/j.1365-2796.2010.02318.x
10. Trysberg E, Nylen K, Rosengren LE, et al. Neuronal and astrocytic damage in systemic lupus erythematosus patients with central nervous system involvement. Arthritis Rheum. 2003;48(10):2881–2887. doi:10.1002/art.11279
11. Diamond B, Honig G, Mader S, et al. Brain-reactive antibodies and disease. Annu Rev Immunol. 2013;31(1):345–385. doi:10.1146/annurev-immunol-020711-075041
12. DeGiorgio LA, Konstantinov KN, Lee SC, et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7(11):1189–1193. doi:10.1038/nm1101-1189
13. Nestor J, Arinuma Y, Huerta TS, et al. Lupus antibodies induce behavioral changes mediated by microglia and blocked by ACE inhibitors. J Exp Med. 2018;215:2554–2566. doi:10.1084/jem.20180776
14. Chan K, Nestor J, Huerta TS, et al. Lupus autoantibodies act as positive allosteric modulators at GluN2A-containing NMDA receptors and impair spatial memory. Nat Commun. 2020;11:1403. doi:10.1038/s41467-020-15224-w
15. Williams RC, Sugiura K, Tan EM. Antibodies to microtubule-associated protein 2 in patients with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2004;50:1239–1247. doi:10.1002/art.20156
16. Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–2686. doi:10.1002/art.34473
17. Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci. 2001;184:101–122. doi:10.1016/s0022-510x(00)00501-3
18. Bonnan M. Intrathecal IgG synthesis: a resistant and valuable target for future multiple sclerosis treatments. Mult Scler Int. 2015;2015:296184. doi:10.1155/2015/296184
19. Yamada Y, Nozawa K, Nakano S, et al. Antibodies to microtubule-associated protein-2 in the cerebrospinal fluid are a useful diagnostic biomarker for neuropsychiatric systemic lupus erythematosus. Mod Rheumatol. 2016;26:562–568. doi:10.3109/14397595.2015.1123345
20. Xiong W, Lahita RG. Pragmatic approaches to therapy for systemic lupus erythematosus. Nat Rev Rheumatol. 2014;10:97–107. doi:10.1038/nrrheum.2013.157
21. Schwartz N, Stock AD, Putterman C. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol. 2019;15:137–152. doi:10.1038/s41584-018-0156-8
22. Sato S, Temmoku J, Fujita Y, et al. Autoantibodies associated with neuropsychiatric systemic lupus erythematosus: the quest for symptom-specific biomarkers. Fukushima J Med Sci. 2020;66:1–9. doi:10.5387/fms.2020-02
23. Hanly JG, Urowitz MB, Su L, et al. Autoantibodies as biomarkers for the prediction of neuropsychiatric events in systemic lupus erythematosus. Ann Rheum Dis. 2011;70:1726–1732. doi:10.1136/ard.2010.148502
24. Stern JN, Yaari G, Vander Heiden JA, et al. B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci Transl Med. 2014;6:248ra107. doi:10.1126/scitranslmed.3008879
25. Parker BJ, Bruce IN. High dose methylprednisolone therapy for the treatment of severe systemic lupus erythematosus. Lupus. 2007;16:387–393. doi:10.1177/0961203307079502
26. Witt KA, Sandoval KE. Steroids and the blood-brain barrier: therapeutic implications. Adv Pharmacol. 2014;71:361–390. doi:10.1016/bs.apha.2014.06.018
27. Yan SX, Deng XM, Wang QT, et al. Prednisone treatment inhibits the differentiation of B lymphocytes into plasma cells in MRL/MpSlac-lpr mice. Acta Pharmacol Sin. 2015;36:1367–1376. doi:10.1038/aps.2015.76
28. Haneda M, Owaki M, Kuzuya T, et al. Comparative analysis of drug action on B-cell proliferation and differentiation for mycophenolic acid, everolimus, and prednisolone. Transplantation. 2014;97:405–412. doi:10.1097/01.TP.0000441826.70687.f6
29. Franco LM, Gadkari M, Howe KN, et al. Immune regulation by glucocorticoids can be linked to cell type-dependent transcriptional responses. J Exp Med. 2019;216:384–406. doi:10.1084/jem.20180595
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






