Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Brain-Imaging Mechanisms on Female Abdominal Obesity Treated by “Shu-Mu” Acupoint Catgut Embedding and Compatibility Relation: Study Protocol for a 12-Week Randomized Controlled Trial
Authors Li Q , Lu Y, Zhang X , Chen Z , Feng J, Zeng X, Zhao S, Huang G, Li L, Xing C, Liang F , Guo T
Received 9 January 2023
Accepted for publication 7 March 2023
Published 11 March 2023 Volume 2023:16 Pages 733—747
DOI https://doi.org/10.2147/DMSO.S400197
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Qifu Li,1,* Yi Lu,2,* Xinghe Zhang,1 Ziwen Chen,3 Jialei Feng,4 Xuanxiang Zeng,1 Siwen Zhao,1 Gaoyangzi Huang,1 Li Li,5 Chonghui Xing,6 Fanrong Liang,3 Taipin Guo1
1School of Second Clinical Medicine/The Second Affiliated Hospital, Yunnan University of Chinese Medicine, Kunming, People’s Republic of China; 2The Department of Medical Imaging, The First Affiliated Hospital of Kunming Medical University, Kunming, People’s Republic of China; 3College of Acupuncture and Tuina, Chengdu University of Traditional Chinese Medicine, Kunming, People’s Republic of China; 4Institute for History of Medicine and Medical Literature, China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China; 5The Third Affiliated Hospital, Yunnan University of Chinese Medicine, Kunming, People’s Republic of China; 6The Sports Trauma Specialist Hospital of Yunnan Province, Kunming, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Taipin Guo, School of Second Clinical Medicine/The Second Affiliated Hospital, Yunnan University of Chinese Medicine, Kunming, People’s Republic of China, Email [email protected] Fanrong Liang, School of Acupuncture and Tuina, Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China, Email [email protected]
Background: Acupoint catgut embedding (ACE) has been proven to be effective and safe in the treatment of obesity, but few studies have been conducted involving its central mechanisms. Our previous study has demonstrated the effectiveness of Shu-Mu ACE in the treatment of abdominal obesity (AO). However, the neurological mechanism of Shu-Mu ACE for weight loss has not yet been elucidated. The mechanism of the combination of the Shu and Mu acupoints may be related to the central integrative effects of the brain. This paper aims to explore the potential neural mechanisms of Shu-Mu ACE in female patients with AO.
Methods and Analysis: A total of 100 eligible female AO patients and 20 healthy female subjects will be recruited for this study. 100 AO patients will be randomly allocated to five groups: Shu-Mu ACE (Group A), Shu ACE (Group B), Mu ACE (Group C), sham ACE (Group D), and waiting-list (Group E). Treatment will be administrated once every two weeks for 12 weeks. The body mass index (BMI), waist circumference (WC), Visual Analog Scales (VAS) of appetite, Self-Rating Anxiety Scale (SAS), and Self-Rating Depression Scale (SDS) will be utilized to evaluate the clinical efficacy. Outcomes will be assessed at baseline and at each time point of treatment. Multimodal MRI will be performed at baseline and after 12-week treatment and the results will be used to investigate the neural mechanisms of ACE for obesity. Neurological changes and clinical data will be analysed for correlation.
Discussions: This study hypothesized that Shu-Mu ACE therapy has a synergistic effect and may treat AO by modulating the neuropathological alterations in the brain. Our findings will demonstrate the neurological mechanism of AO treated by “Shu-Mu” Acupoint Catgut Embedding and compatibility relation.
Trial Registration: This trial is registered at the Chinese Clinical Trial Registration Center (No. ChiCTR2100048920).
Keywords: acupoint catgut embedding, female, abdominal obesity, Shu-Mu acupoints, compatibility relation, study protocol, neurological mechanism
Introduction
Obesity is an abnormal or excessive fat accumulation that can be detrimental to health.1 Abdominal obesity (AO) is a high risk type of obesity characterized by a large accumulation of fat in the abdomen region, which has also been considered a high-risk type of obesity.2,3 According to the World Health Organization (WHO), the global prevalence of obesity is increasing every year and the overweight and obese populations are expected to reach 2.16 billion and 1.12 billion by 2030, further increasing the global clinical and public economic burden.4 In addition, obesity is linked to more deaths worldwide than being underweight, and AO is considered to be a risk factor for a variety of non-communicable diseases such as diabetes, musculoskeletal disorders, cardiovascular diseases, chronic respiratory disease and some cancers.1,5,6 According to statistics, more than two-thirds of deaths in China in 2008 can be attributed to chronic diseases, and one of the main risk factors is obesity.7
Currently, the treatment methods used in various clinical institutions are largely the same, mainly including diet therapy, physical activities, medicine, surgery and complication treatment. However, both diet and exercise require long-term habits that can be difficult for patients to stick to.8 Medication can cause many side effects, including nausea, insomnia, headaches, abdominal pain, and dysgeusia.9 Surgical treatment can effectively reduce weight, but the extremely high safety requirements, high price, strict indications and high incidence of postoperative complications limit the spread.10 Acupoint catgut embedding (ACE) is a type of acupuncture in Traditional Chinese Medicine (TCM). Clinical studies have shown that ACE has a good effect on obesity.11–13 We have conducted a systematic review and Meta-analysis on 43 clinical studies involving 3520 cases of ACE in the treatment of obesity and confirmed that ACE in the treatment of obesity has advantages compared with hand acupuncture, drugs, electroacupuncture, and sham acupuncture.14 There is also a systematic review and Meta-analysis showing that ACE has a higher effect on abdominal obesity, and there are few adverse events.15
The mechanism of the stimulation method of weight loss through acupuncture points has not been fully elucidated by current research. Studies have shown that diet control is an effective treatment for obesity.16 Existing neuroimaging studies have found that the appetite control network plays an important role in the pathological changes of the obesity center through the analysis of structure, function and neural circuits.17,18 Our study has demonstrated that Shu-Mu ACE can effectively reduce waist circumference and body mass index (BMI), as well as showing the effect on appetite in female patients with AO.19 However, the neurological mechanism of Shu-Mu ACE for weight loss has not yet been elucidated. As a classical collocation of acupoints in TCM, Shu-Mu Acupoints have excellent synergistic effects,20 which are complementary in clinical applications,21 but the mechanism of the synergistic effect is still not well clarified. We hypothesize that Shu-Mu ACE therapy has a synergistic effect and may be useful in treating AO by modulating neuropathic changes in the brain.
Therefore, this study aims to carry out clinical trials using multimodal brain imaging technology to (1) elaborate on the changes in white or gray matter mass of brain structures and related functional brain regions in AO patients by comparing them with healthy individuals and establish a brain imaging model for AO patients; (2) To correlate the clinical efficacy of Shumu ACE for AO with the pathological central changes in the brain regions of AO, and to clarify the brain network regulation mechanism of Shu-mu ACE on AO; (3) To analyze the respective modulation of AO brain structure and function by Shu ACE and Mu ACE, and then compare them with Shumu ACE, respectively, elucidate the central mechanism of the synergistic effect of the two Shu and Mu acupoints in combination for the treatment of AO.
Methods and Analyses
Study Design
This randomized sham-controlled, participant-blinded trial will be conducted from September 2021 to December 2023 in the Sports Trauma Specialist Hospital of Yunnan Province and the Second Affiliated Hospital of Yunnan University of Chinese Medicine. This study will enroll 100 female patients with AO and 20 healthy subjects. Treatment will be administrated once two weeks for 12 weeks. The relevant scale assessments and multimodal MRI data acquisition will be taken at the corresponding time points specified. A study flowchart is presented in Figure 1. We designed this protocol following the guidelines of the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT)22 (see File 1). The schedule of participant enrolment, intervention, and assessment is illustrated in Table 1. This trial has been approved by the ethics committee of the Sports Specialist Hospital of Yunnan Province (ethical approval number: 2021–01), and it has been registered at the Chinese Clinical Trial Registration Center, the registration number is ChiCTR2100048920. The participants will receive treatment once two weeks for a total of 12 weeks in the four intervention groups except for the control group.
 |
Table 1 Study Schedule for Data Measurements |
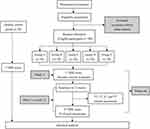 |
Figure 1 Flowchart of the study design. |
Participants
Inclusion criteria for female patients with AO are as follows:
- Satisfies the diagnostic criteria for abdominal obesity, ①BMI≥28kg/m2; ②female waistline≥80cm;
- Right handedness;
- Between the ages of 18 and 40.
- Educated for≥6 years and able to understand the entire research process;
- Have signed an informed consent form;
- Have not participated in another trial within three months.
Exclusion Criteria for female patients with AO are as follows:
- BMI≥40kg/m2;
- Obesity that is secondary to endocrine disorders (hypothalamic disorders, gonadal disorders, Cushing’s syndrome, pituitary disorders, thyroid disorders, etc.) and medication (antipsychotics or glucocorticoid);
- Those who are pregnant, breastfeeding, or who have had a request to have children in the last 6 months or who cannot provide adequate contraception;
- Diabetes, cardiovascular diseases, mental disorders, immunodeficiency, liver and kidney impairment, blood diseases;
- Nuclear magnetic contraindications (such as metal implant, vascular stents, cardiac pacemakers, false tooth, claustrophobia, etc.);
- Brain with definite organic lesions or significant asymmetry between the right and left skull structures;
- Obesity surgery history;
- Those with chronic dysmenorrhea;
- Bleeds easily, allergic to alcohol or animal protein.
Inclusion criteria for healthy subjects are as follows:
- BMI: 18.5–23.9 kg/m2;
- Right handedness, females;
- Aged between 18 and 40 years;
- No physical abnormalities during the trial period (eg flu, fever, etc.);
- No drugs or alcohol for at least 2 days before participation in the trial, and no stimulant drug use for at least 1 month;
- Have signed an informed consent form.
Exclusion Criteria for healthy subjects are as follows:
- Serious primary diseases such as significant cognitive dysfunction, cardiovascular system, brain, hematopoietic system, liver, kidney damage and mental disease;
- Women during pregnancy and lactation;
- Patients with long-term dysmenorrhea;
- Brain with definite organic lesions or significant asymmetry between the right and left skull structures;
- Nuclear magnetic contraindications (such as metal implant, vascular stents, cardiac pacemakers, false tooth, claustrophobia, etc.);
- Has received acupuncture or ACE treatment within 6 months;
- Transsexual, homosexuality, non traditional sexual orientation, special fetishes, alcohol or drug abuse, etc.
Recruitment
All patients with AO and healthy subjects will be recruited from the two hospitals and the community. Recruitment methods mainly include posters, public notice boards and WeChat public numbers in hospitals and communities. Researchers will evaluate and screen participants according to the eligible criteria. All participants will be fully informed of the study before signing the informed consent form. They can withdraw from the study at any time. The reason for withdrawal will be recorded.
Randomization and Allocation
Eligible AO participants will be randomly assigned to groups in equal proportions. The random number will be computer generated and sealed in an opaque envelope by a separate researcher Participants will be randomly selected to receive an envelope to be allocated a serial number, which will be recorded in the CRF. Only the acupuncturist will be informed of the group allocation prior to treatment.
Blinding
Participants, outcome assessors, and statisticians will all be blinded in this trial. Due to the specific nature of the ACE operation, acupuncturists are unblinded throughout the procedure. The allocation of participants will only be revealed in exceptional cases such as severe allergies, serious infections, uncontrollable pain, etc.
Interventions
The ACE operation refers to the relevant regulations in the National Standard of the People’s Republic of China (GB/T21709.10–2008) “Acupuncture Technical Operation Specification Part 10: Acupoint Catgut Embedding”.23 Patients in the Shu-Mu ACE (Group A), Shu ACE (Group B), Mu ACE (Group C) and the sham ACE (Group D) will receive 6 treatments over 12 weeks (1 every 2 weeks). Patients in the waiting-list (Group E) will not have any intervention. During the study period, they will only undergo the same tests and scale assessments as the other groups and then receive a further 6 free ACE sessions after 12 weeks. Healthy subjects will not receive any treatment during the trial. Brain imaging data will be acquired only once.
Acupoints
According to the TCM theory of strengthening the spleen and resolving dampness, acupoints are selected from Shu and Mu acupoints of the spleen, stomach, and large intestine, including BL 20 (Pishu), BL 21 (Weishu), BL 25 (Dachangshu), RN 12 (Zhongwan), LR 13 (Zhangmen), and ST 25 (Tianshu), with a total of 11 points (see Figure 2). Both Group A and Group D selected these acupoints, group B selected 6 Shu acupuncture points, group C selected 5 Mu acupoints, and 6 Shu acupoints were selected for Group B and 5 Mu acupoints for Group C. The positioning of the acupoints will be referred to the People’s Republic of China in 2006 (GB/T 12346–2006),24 the specific location of acupoints is shown in Table 2 and Figure 2.
 |
Table 2 The Location of Acupoints |
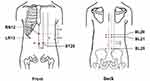 |
Figure 2 Locations of acupoints. |
Appliance Selection
The embedding needles are made of Huahong brand disposable embedding needles (Jiangsu Huahong Medical Instruments Co., Ltd.), the specifications are 7# (diameter 0.7mm, length 55 mm), 8# (diameter 0.8mm, length 55mm), 9 # (Diameter 0.9mm, length 55mm), 11# (diameter 1.1mm, length 55mm). The medical protein suture uses Jinhuan brand medical protein suture (Shanghai Pudong Jinhuan Medical Products Co., Ltd.), the specifications are 2–0 and 3–0.
Operation
Depending on the grouping, patients will be placed in a supine or prone position and the skin around the acupuncture points will be routinely disinfected. Use tweezers to take a section of sterilized medical protein suture about 1–3 cm in length, place it on the front end of the disposable needle tube, and then connect the needle core. Use the thumb and index finger of your left hand to tighten or lift the skin, hold the needle in the right hand, and pierce it to the desired depth. Then push the needle core in and withdraw the needle tube outward to fill the medical protein suture in the subcutaneous tissue or muscle of the acupoint. Finally, the needle hole is covered with sterile gauze. The four groups are operated in the same way, with the Group D differing in that no medical protein suture is placed in the embedding needles.
Multimodal Data Acquisition
Multimodal data will be acquired within 1 week prior to treatment and within 1 week after 12 weeks of treatment. All participants will be scanned under GE 750W 3T MRI and collected using 16-channel head coils. During the scans, participants lie on their backs in the scanner with earplugs in their ears to dampen the noise, remain awake, relax, and avoid systematic thinking throughout the procedure.
The high-resolution T1 BRAVO sequence, the parameters are as follows: TR = 7.7 ms, TE = 3.6 ms, matrix = 228 × 228, FOV = 250 mm × 250 mm, 230 axial slices, acquisition time = 6 min 53s. Diffusion tensor image sequence, the parameters are as follows: TR = 7173 ms, TE = 78 ms, matrix = 115 × 115, FOV = 230 mm × 230 mm, 50 axial slices, slice thickness = 3 mm, b value = 1000, diffusion directions = 32, acquisition time = 9 min 7 s. In addition, the axial T2-weighted MR image will also be collected. The parameters used are as follows: TR = 2500 ms, TE = 80 ms, matrix = 332 × 225, FOV = 250 mm × 220 mm, slice thickness = 6 mm, 18 axial slices, acquisition time = 55s.
During the scan, if the participants complain of discomfort it will be terminated. MRI scans will be performed by the same technician using the same machine. The quality of the images and compliance with procedures are reviewed by a professional staff member after each scan. They are all highly qualified radiologists who are proficient in MRI.
Outcome Measures
Primary Outcome Measures
BMI Data
Changes in BMI will be assessed at baseline, week 2, 4, 6, 8 and 12. The calculation formula of BMI is BMI = weight (kg) ÷ height 2 (m2). The method of height measurement is as follows: the subject stands upright with his heels close to the measuring scale, and the shoulders and buttocks are also close to the measuring scale. The weight measurement method was as follows: the subject has an empty stomach, takes off his shoes, and only wear light clothes. Using the calibrated lever-type weight scale, the subject relaxed and stood upright in the middle of the chassis of the scale. The researcher reads the cursor indication number on the lever scale, and the measured number was accurate to 10 grams, and then weighed the shoes and clothes. The final weight is equal to the total weight minus the weight of the shoes and clothes, in kilograms (kg).
Secondary Outcomes
Secondary outcomes include waist circumference (WC), Visual Analog Scales (VAS) of appetite, Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), total cholesterol (TC), Triglycerides (TG), high-density lipoprotein (HDL) and Low-density lipoprotein (LDL). Changes in WC and VAS of appetite will be assessed at baseline, week 2, 4, 6, 8, 10 and 12. Changes in SAS and SDS will be assessed at baseline and after 12 weeks.
The measure of WC: take the midpoint between the lowest rib and the iliac crest and measure twice with a standard tape measure and then take the average.
Appetite measurement: The VAS of appetite of Canadian E Doucet scholars25 will be used, including the desire to eat, hunger, satiety and willingness to eat VAS, with a total score of 10, 0: no feeling, very little intake; 1–3: mild, little intake; 4–6: moderate, moderate intake; 7–10 strong feeling, huge intake. The appetite VAS scale is shown in Figure 3.
 |
Figure 3 The scale of VAS of appetite. |
The SAS and SDS scale scores will be used to assess ratings of anxiety and depressive states over the past 7 days to determine whether changes in brain areas are influenced by emotional factors. Both scales consist of 20 entries and are divided into four levels. A score of <50 indicates no symptoms, 50–59 indicates minimal to mild symptoms, 60–69 indicates moderate to significant symptoms, and ≥70 indicates severe symptoms.
Other outcomes, including heart rate (HR), blood pressure (BP), TC, TG, HDL, and LDL levels, will be tested at baseline and after 12 weeks.
MRI Data
In this study, the original data was first converted to Nifti format by using the Dcm2nii software in the Micron software package. Then, the PANDA toolbox26 (http://www.nitrc.org/projects/panda/), an integrated toolbox, is used to preprocess the diffusion tensor imaging (DTI) data and construct the brain structural networks. Besides, network properties analysis was performed using the GRETNA toolbox27 (www.nitrc.org/projects/gretna) and visualized by using the BrainNet Viewer toolbox28 (www.nitrc.org/projects/bnv).
Structural Network Construction
We used the MATLAB-based open source software PANDA for the network construction.26 In this study, the structural network procedures we constructed were as follows: (1) The basal ganglia region of the autopsy marker 116 template was removed to make template A.29 (2) The atlas of the basal ganglia,30,31 including striatum, external globus pallidus, globus pallidus interna, red nucleus, substantia nigra, and subthalamic nucleus were assembled, with the overlaps removed, to make template B; (3) Template A and template B were added, and each brain region was numbered. Finally, we obtained a brain template containing 124 brain regions. A single T1-weighted image was co-registered with the b0 image in DTI native space using the affine transformation. The transformed T1 images were normalized to the T1 template in MNI space by a non-linear. Templates of 124 brain regions were warped into DTI raw space. Diffusion MRI mapping was then performed to calculate all mappings in the DTI dataset. In this process, the atlas was terminated by seeding each voxel with fractional anisotropy (FA) > 0.2 if the turn angle was >45 degrees or if a voxel with an anisotropy coefficient <0.2 was reached. As a result, all fiber pathways between the ROIs were constructed using a deterministic bundle plot approach. For the weight of the edge between each pair of ROIs i and j, we will define this in terms of the number of connecting fiber streamlines (FN) in the two regions. Therefore, a fan FN-weighted 124×124 structural connectivity matrix can be constructed for each participant, and the same applies to the FA-weighted structural connectivity matrix.
DTI Data Preprocessing
In brief, preprocessing procedures for DTI data included skull-stripping, eddy-current and head-motion correction, FA calculation and whole-brain deterministic DTI fiber tractography.32 FA was calculated in this preprocess. Besides, whole-brain fiber tractography will be reconstructed by seeding at every voxel in the brain and using fiber assignment with continuous tracking (FACT) algorithm.33 From the deep WM region, the algorithm calculates fiber trajectories terminating in voxels with a turning angle greater than 45° or arriving at an FA less than 0.15.
Graph Theoretical Analysis of Structural Brain Networks
In this study, we mainly calculate global network characteristics and local network characteristics to better characterize the brain structural network topology changes, by using graph theoretical analysis.34 The global network characteristics include clustering coefficient (Cp), characteristic path length (Lp) and “small-world” network attributes (σ), global efficiency (Eglob), and local efficiency (Eloc); and local network parameters mainly include node efficiency (Enodal).
Safety Evaluation
ACE-associated side effects such as allergic reactions to the protein suture, local redness, itching, fever, or even protein suture spillage or fat liquefaction at the acupoints after treatment. When adverse events occur, the time of occurrence, symptoms, severity, treatment measures, and time to resolution will be recorded in detail and their relevance to the treatment will be assessed. The doctor will decide whether to suspend the study based on the condition. Serious adverse events will be reported to the Medical Ethics Committee of Yunnan Sports Trauma Specialist Hospital by the person in charge within 24 hours.
Data Management and Quality Control
The outcome assessor will fill in the initial data in the CRF and enter them into an Excel sheet. The ACE, neurologist and statistician will conduct several reviews of the experimental protocol. To ensure consistency of observations, all observations are performed according to the relevant standards. Laboratory test results must be printed on a computer. To ensure the smooth running of the study, the study director should provide uniform training to all study staff before the study starts, and provide detailed operational training on the project implementation plan and observation indicators to be familiar with the study process and specific implementation details to ensure the reliability of the study findings. The project leader should supervise and check the entire research process, confirming that all research data recording and reporting and case report forms are true, accurate and complete and that they are consistent with the original data. Any deviation from the protocol will be reported to the Ethics Committee who will ultimately decide whether we must change the protocol or terminate the trial.
Finally, to promote participant compliance, all treatments and tests will be provided free of charge. For those in the sham ACE group participants will also be able to continue with 6 free ACE treatments after the trial if they wish.
Sample Size
There is no consensus on the sample size for neuroimaging studies at present. A sample size evaluation of an MRI suggested that the minimum sample size (n=12) for MRI studies can achieve 80% power for typical activation at the single-voxel level.35 A sample size of 12–24 individuals per group is a reasonable sample size to obtain brain functional analysis.36 Therefore, we took 18 samples for each group, considering the dropout rate of 10%, each group included 20 cases, for a total of 100 female AO patients and 20 healthy subjects.
Statistical Analysis
Clinical Data Analysis
Statistical analyses will be performed using the statistical software SPSS 26.0 Statistics (SPSS Inc., Chicago, IL, USA). Percentages or proportions will be used to describe qualitative data, using the chi-square (χ2) test. Data will be expressed as mean ± standard deviation for quantitative data. For continuous variables, a one-way analysis of variance (ANOVA) will be used. For longitudinal and repeated measures data, analysis of variance (ANOVA) with repeated measures will be used. All clinical data will be analysed in association with imaging data after conversion to domain Z-scores. Missing data for dropped participants will be imputed using multiple imputations. All analyses will be performed using a two-sided test, with a p-value < 0.05 being considered statistically significant.
MRI Data Analysis
The structural MRI (sMRI) data will be analysed using the voxel-based morphometry (VBM) toolbox within SPM12. These steps include interlaminar time difference correction, spatial difference correction, head motion correction, normalisation and Gaussian smoothing. During head motion correction, images of subjects with translations of less than 1 mm and rotational movements of less than 2° will be involved in the subsequent analysis. MRI data will be analysed using PANDA, GRETNA and BrainNet Viewer toolboxes and SurfStat. These steps involve removing the high-frequency component through band-pass filters, removing the average signal from the whole brain as well as the signal from the ventricles and white matter, and then registering the data into the surface space of FreeSurfer. Multiple comparison correction will be used for each brain regions measured. Differences between brain regions in each group will be analysed using t-tests and repeated measures ANOVA. Correlations between MRI data and clinical variables will be analysed using Pearson correlation. Comparisons between groups will be ANOVA and Wilcoxon ranking sums (if normality is not met).
Discussion
The Pathological Central Mechanism of Obesity
In the study of brain structure imaging, it is found that obese patients have changes in the structure of most functionally related brain regions, accompanied by certain changes in white or gray matter mass. An analysis of 690 obese men’s brain magnetic resonance imaging voxel-based morphology (VBM) found that the brain has extensive gray mass changes. Among them, the gray mass in the medial temporal lobe, anterior cerebellar lobe, occipital lobe, frontal lobe, precuneus, and midbrain are negatively correlated with BMI, while low frontal gyrus, posterior cerebellar lobe, temporal lobe, thalamus and caudate nucleus are positively correlated with BMI.37 Studies have also found that the BMI of elderly female obese patients is correlated with the decrease of gray mass in brain areas such as the left orbital lobe, right lower frontal lobe, right anterior central gyrus, hippocampus, spindle, and right cerebellum, as well as the increase of white mass in the frontal lobe, temporal lobe and apical lobe.38 A study combining VBM to analyze the brain DTI of obese patients showed that some of its anisotropy values, average diffusion rate, white and gray mass were lower than those of normal people, and showed a negative correlation with body fat rate. Among them, AO is more negatively correlated with gray matter density. The functional areas of these changes are mainly rewarded control and appetite areas.39
According to the analysis of neural circuit models and a large number of obesity central researches, the central brain network response mechanism of obesity is the following four important loops: learning-memory, reward-prominence, motivation-drive, and inhibition-control. In this network, the learning-memory processing center processes related signals, reward-prominence loop, and motivation-drive loop are activated to issue instructions to reduce the activity of the inhibitory-control loop. These four loops together constitute the core pathological center area of the obesity network.17,18
Appetite Control Network Plays an Important Role in the Pathological Changes of the Obesity
Neuroimaging studies have found that the appetite control network plays an important role in the pathological changes of the obesity center through the analysis of structure, function and neural circuits.17,18 In functional brain neuroimaging studies of the brain, food picture induction, feeding, and animal experiments have been confirmed that the core brain areas related to obesity are mainly related to reward, emotion and memory, self-balance regulation, motor and sensory processing, cognitive control and attention.40 The use of high-energy food pictures and non-food pictures for visual stimulation found that obese patients more than normal people activate the dorsal striatum, insula, lateral prefrontal cortex, orbital prefrontal cortex, posterior cingulate and other processing related to reward expectation, taste processing, and emotional motivation.41 The use of food odors and non-food to stimulate, the insula, taste area, cingulate and other border systems, and reward areas are also activated.42 After meals, the areas of increased cerebral blood flow in obese patients and normal-weight people are the prefrontal cortex, the decreased areas are the limbic system/paralimbic system, temporal lobe, occipital cortex, and cerebellum, and the attenuation areas of cerebral blood flow in obese patients are hypothalamus and thalamus.43 Animal experiments also prove that these core brain areas are closely related to the functional response of the obese brain. For example, deep brain stimulation of the lateral hypothalamus in mice can inhibit food intake. At the same time, deep brain stimulation will increase the metabolism of the papillary body, hippocampus, and amygdala, while the metabolism of the thalamus, caudate nucleus, temporal lobe, and cerebellum will decrease.44–46
Multimodal MRI is an Effective Approach to Investigate the Neural Mechanism of ACE for Weight Loss
A combination of two or more MRI scans is referred to as multimodal MRI. The three most commonly used neuroimaging modalities in acupuncture-related studies are functional MRI, sMRI, and DTI.47 MRI, which uses atomic nuclei resonating in a magnetic field to produce image signals that can be processed to study central structural and functional changes, is a non-invasive, non-invasive detection technique that can be used to study central mechanisms in patients with AO. It is a good means to explore the acupuncture point effect from the central function and structure.48 Previous studies on the central mechanism of acupuncture intervention in obesity have shown that acupuncture can activate or even change the function and structure of obesity-related brain regions. For example, the immediate effect of electroacupuncture to stimulate the Zusanli (ST36) and Neiting (ST44) in obese patients can induce the activation or negative activation of the frontal lobe, temporal lobe, thalamus, hippocampus and other brain regions related to obesity circuits.49 The hypothalamus related areas also changed after acupuncture treatment for obesity.50 In addition, related studies have shown that acupuncture treatment of obesity can change the volume of gray matter and white matter in the relevant brain regions of obese patients. After acupuncture treatment, the gray matter volume of the right superior frontal gyrus and posterior cerebellar vertebrae decreased, and the gray matter volume of the right central anterior gyrus increased. At the same time, the volume of white matter in the fusiform gyrus, pontine, and anterior central gyrus of the subiculum hippocampi decreased, and the white matter volume of the right precuneus was increased.51
Existing researches on the central imaging mechanism of ACE therapy for weight loss have factors such as small sample size, lack of clinical biological information, and single research and analysis methods, which cannot be discussed from the central network. The central network effect mechanism of ACE therapy for weight loss has not been clarified yet. Our previous study has demonstrated the effectiveness of ACE in treating obesity, and this study will continue to explore the promising mechanisms of Shu-Mu ACE treatment for patients with AO by using multimodal MRI techniques.
For our inclusion of only females in the study, in addition to the higher prevalence in females, the differences in the structure and function of the brain regions in men and women is also a factor we considered.52 The sham ACE group was set up to reduce as much as possible the interference of other complex factors in the brain function imaging analysis. The waiting-list group was set up to consider the diversity of dietary types in the Chinese and to see if differences in diet affected brain imaging without intervention. Studies have shown that the mechanism of action of the collocation of acupoints correlates with the central integrative action of the brain.53–55 We added separate Shu and Mu groups to the grouping to observe the difference in efficacy between the two groups of acupoints used together and separately, and also to provide a basis for validating the classical acupoint theory and the synergistic effect mechanism of acupoints in TCM. In addition, the brain function imaging of patients with gastrointestinal diseases accompanied by anxiety and depression is significantly different from normal subjects,56 hence we included the SAS and SDS scales as secondary observations to differentiate and exclude this confounding factor.
The combination of Shu and Mu acupoints is one of the classical methods of acupuncture, which is mainly used to treat metabolic diseases of the internal organs in clinical practice. According to TCM’s identification theory, obesity is caused mainly by the malfunction of the spleen, stomach and large intestine, so we choose the Shu and Mu acupoints of the three internal organs as the prescription for treatment. Our bibliometric analysis of 175 ACE treatments for obesity also found that the existing high-frequency acupoints for ACE for obesity are mainly the Shu-Mu acupoints of the spleen, stomach and large intestine.57
This study is the first to explore the neurological mechanism of Shu-Mu ACE in the treatment of AO functionally and structurally by using multimodal MRI. Secondly, this study subdivided the Shu-Mu group of acupoints into two separate groups, the Shu group and the Mu group, which could provide evidence to demonstrate the mechanism of synergistic effect of acupoints. Finally, the design of this trial’s sham buried and wait-for-treatment groups will minimise differences in the central nervous effects of the intervention; contact between participants in the different groups will be avoided in order to maintain the success of the blinded approach. Outcome assessors and statisticians will also be concealed from group allocation. These methods were constructed to reduce possible bias in the results.
Limitations of this study are as follows. First, because of the specific nature of ACE treatment, it will be difficult to blind the acupuncturist. Acupuncturists are not blind to subgroup situations and their attitudes for or against interventions may inevitably transfer to participants. Second, this study included only one centre and its reproducibility needs to be assessed with more centres. Third, due to the limited funding of this study, other types of obesity are not studied, the sample size of the groups was relatively small and no follow-up period was set.
In summary, our trial aims to investigate the mechanisms of brain network modulation by Shu-Mu ACE on AO, and the synergistic mechanisms of the central network in Shu and Mu ACE for AO. These findings will provide feasible data and basic information to reveal the mechanism of ACE and compatibility relation in the treatment of AO.
Ethics and Dissemination
This study will be conducted in accordance with the Declaration of Helsinki. This protocol involving clinical trials was reviewed and approved by the Ethics Committee of the Sports Trauma Specialist Hospital of Yunnan Province. All participants will provide written signed informed consent to participate in this study. This trial is registered at the Chinese Clinical Trial Registration Center (No. ChiCTR2100048920). Results will be published in peer-reviewed journals and disseminated at academic conferences.
Acknowledgments
All authors are grateful for the support of all participants or participants who will join the trial, as well as the support of other institutions.
Author Contributions
All authors contributed significantly to the conception, design, and acquisition of data for the article; TPG and FRL are the principal investigators and were primarily responsible for the conception and design of this study; QFL and JLF wrote the original manuscript and were responsible for major revisions to the article; ZWC, XXZ, SWZ, GYZH, LL, and CHX are primarily responsible for the execution of the study and acquisition of data; YL and XHZ are primarily responsible for the analysis of the data. All authors provided final approval of the forthcoming version and agreed to be responsible for all aspects of the work.
Funding
This study is financially supported by Yunnan Province Applied Fundamental Research Project-Joint General Project of Traditional Chinese Medicine (No. 2019FF002-021; 202101AZ070001-096), Top-notch Young Talent Project of Yunnan Ten Thousand Talents (YNWR-QNBJ-2019-257) and “Famous Doctor” special talent program of the Yunnan Provincial Xing Dian Talent Support Program (Yunnan Party Talent Office [2022] No. 18). The funding agencies do not play any role in the design, collection, analysis, or writing manuscripts of the study.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. World Health Organization. Obesity and overweight. Fact sheet 2021; 2021. Available from: www.who.int/mediacentre/factsheets/fs311/en/.
2. Misra A, Chowbey P, Makkar BM, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–170.
3. Dhawan D, Sharma S. Abdominal obesity, adipokines and non-communicable diseases. J Steroid Biochem Mol Biol. 2020;203:105737. doi:10.1016/j.jsbmb.2020.105737
4. Kelly T, Yang W, Chen CS, et al. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32(9):1431–1437. doi:10.1038/ijo.2008.102
5. Sadeghi O, Saneei P, Nasiri M, et al. Abdominal obesity and risk of hip fracture: a systematic review and meta-analysis of prospective studies. Adv Nutr. 2017;8(5):728–738. doi:10.3945/an.117.015545
6. Kailash KP, Jebamalar J. A correlation study between types of obesity and hypertension. Int J Med Sci Public Health. 2018;7(11):1.
7. Jaacks LM, Gordon-Larsen P, Mayer-Davis EJ, et al. Age, period and cohort effects on adult body mass index and overweight from 1991 to 2009 in China: the China health and nutrition survey. Int J Epidemiol. 2013;42(3):828–837. doi:10.1093/ije/dyt052
8. Mastellos N, Felix LM, Car J, et al. Transtheoretical model stages of change for dietary and physical exercise modification in weight loss management for overweight and obese adults. Cochrane Database Syst Rev. 2014;2014(2):4. doi:10.1002/14651858
9. Krentz AJ, Fujioka K, Hompesch M. Evolution of pharmacological obesity treatments: focus on adverse side-effect profiles. Diabetes Obes Metab. 2016;18(6):558–570. doi:10.1111/dom.12657
10. Arterburn DE, Telem DA, Kushner RF, et al. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324(9):879–887. doi:10.1001/jama.2020.12567
11. Zhou Y-M, Yan B, Yuan W-Q, et al. Efficacy of verum and sham acupoint catgut embedding for treatment of obesity: study protocol for a randomized controlled trial. Trials. 2019;20(1):644. doi:10.1186/s13063-019-3730-8
12. Chen L-S, Li -Y-Y, Chen H, et al. Polyglycolic acid sutures embedded in abdominal acupoints for treatment of simple obesity in adults: a randomized control trial. Chin Med. 2019;14(1):32. doi:10.1186/s13020-019-0258-5
13. Dai L, Wang M, Zhang K-P, et al. Modified acupuncture therapy, long-term acupoint stimulation versus sham control for weight control: a multicenter, randomized controlled trial. Front Endocrinol. 2022;13:952373. doi:10.3389/fendo.2022.952373
14. Guo T, Ren Y, Kou J, et al. Acupoint catgut embedding for obesity: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2015;2015:401914. doi:10.1155/2015/401914
15. Sheng J, Jin X, Zhu J, et al. The effectiveness of acupoint catgut embedding therapy for abdominal obesity: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2019;2019:9714313. doi:10.1155/2019/9714313
16. Meidi L, Deqin L, Chuanshan J, et al. Optimization of ultrasonic extraction of saponins from asparagus cochinchinensis by response surface methodology with box-behnken design. Anhui Univ Chinese Med. 2016;35(5):93–96.
17. Zhang Y, Liu J, Yao J, et al. Obesity: pathophysiology and intervention. Nutrients. 2014;6(11):5153–5183. doi:10.3390/nu6115153
18. Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15(1):37–46. doi:10.1016/j.tics.2010.11.001
19. Zhang X, Li Q, Yi R, et al. Effect of acupoint catgut embedding for abdominally obese female with strong appetite: mixed analysis of a randomized clinical trial. Diabetes Metab Syndr Obes. 2022;15:3387–3395. doi:10.2147/DMSO.S388485
20. Wang H, Shen GM, Liu WJ, et al. The neural mechanism by which the dorsal vagal complex mediates the regulation of the gastric motility by Weishu (RN12) and Zhongwan (BL21) stimulation. Evid Based Complement Alternat Med. 2013;2013:291764. doi:10.1155/2013/291764
21. Zheng H, Liu ZS, Zhang W, et al. Acupuncture for patients with chronic functional constipation: a randomized controlled trial. Neurogastroenterol Motil. 2018;30(7):e13307. doi:10.1111/nmo.13307
22. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–207. doi:10.7326/0003-4819-158-3-201302050-00583
23. Embedding PtoATOSPAC. National standard of the People’s Republic of China (GB/T 21709.10—2008) acupuncture technical operation specification part 10: acupoint catgut embedding. Chin Acupuncture Moxibustion. 2009;29(5):405–406.
24. General Administration of Quality Supervision, InspectionQuarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China. Nomenclature and Location of Acupuncture Points, GB/T 12346-2006. Beijing: Standards Press of China; 2006:7–41.
25. Doucet E, Imbeault P, St-Pierre S, et al. Appetite after weight loss by energy restriction and a low-fat diet-exercise follow-up. Int J Obes Relat Metab Disord. 2000;24(7):906–914. doi:10.3389/fnhum.2013.00042
26. Cui Z, Zhong S, Xu P, et al. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci. 2013;7:42. doi:10.3389/fnhum.2013.00042
27. Wang J, Wang X, Xia M, et al. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci. 2015;9:386. doi:10.3389/fnhum.2015.00386
28. Xia M, Wang J, He Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8(7):e68910. doi:10.1371/journal.pone.0068910
29. Rolls ET, Huang CC, Lin CP, et al. Automated anatomical labelling atlas 3. Neuroimage. 2020;206:116189.
30. Keuken MC, Bazin PL, Schäfer A, et al. Ultra-high 7T MRI of structural age-related changes of the subthalamic nucleus. J Neurosci. 2013;33(11):4896–4900. doi:10.1523/JNEUROSCI.3241-12.2013
31. Keuken MC, Müller-Axt C, Langner R, et al. Corrigendum: brain networks of perceptual decision-making: an fMRI ALE meta-analysis. Front Hum Neurosci. 2017;11:139. doi:10.3389/fnhum.2017.00139
32. Sun Y, Chen Y, Collinson SL, et al. Reduced hemispheric asymmetry of brain anatomical networks is linked to schizophrenia: a connectome study. Cereb Cortex. 2017;27(1):602–615. doi:10.1093/cercor/bhv255
33. Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269. doi:10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3
34. Korgaonkar MS, Fornito A, Williams LM, et al. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol Psychiatry. 2014;76(7):567–574. doi:10.1016/j.biopsych.2014.02.018
35. Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods. 2002;118(2):115–128. doi:10.1016/s0165-0270(02)00121-8
36. Yu S, Dong X, Sun R, et al. Effect of acupuncture and its influence on cerebral activity in patients with persistent asthma: study protocol for a randomized controlled clinical trial. Trials. 2020;21(1):406. doi:10.1186/s13063-020-04319-w
37. Taki Y, Kinomura S, Sato K, et al. Relationship between body mass index and gray matter volume in 1428 healthy individuals. Obesity. 2008;16(1):119–124. doi:10.1038/oby.2007.4
38. Walther K, Birdsill AC, Glisky EL, et al. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2010;31(7):1052–1064. doi:10.1002/hbm.20916
39. Karlsson HK, Tuulari JJ, Hirvonen J, et al. Obesity is associated with white matter atrophy: a combined diffusion tensor imaging and voxel-based morphometric study. Obesity. 2013;21(12):2530–2537. doi:10.1002/oby.2038
40. Carnell S, Gibson C, Benson L, et al. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. 2012;13(1):43–56. doi:10.1111/j.1467-789X.2011.00927.x
41. Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37(2):410–421. doi:10.1016/j.neuroimage.2007.05.008
42. Bragulat V, Dzemidzic M, Bruno C, et al. Food-related odor probes of brain reward circuits during hunger: a pilot FMRI study. Obesity. 2010;18(8):1566–1571. doi:10.1038/oby.2010.57
43. Gautier JF, Chen K, Salbe AD, et al. Differential brain responses to satiation in obese and lean men. Diabetes. 2000;49(5):838–846.
44. Melega WP, Lacan G, Gorgulho AA, et al. Hypothalamic deep brain stimulation reduces weight gain in an obesity-animal model. PLoS One. 2012;7(1):e30672. doi:10.1371/journal.pone.0030672
45. Soto-Montenegro ML, Pascau J, Desco M. Response to deep brain stimulation in the lateral hypothalamic area in a rat model of obesity: in vivo assessment of brain glucose metabolism. Mol Imaging Biol. 2014;16(6):830–837. doi:10.1007/s11307-014-0753-0
46. Whiting DM, Tomycz ND, Bailes J, et al. Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. J Neurosurg. 2013;119(1):56–63. doi:10.3171/2013.2.JNS12903
47. Qiu K, Jing M, Sun R, et al. The status of the quality control in acupuncture-neuroimaging studies. Evid Based Complement Alternat Med. 2016;2016:3685785. doi:10.1155/2016/3685785
48. Huang W, Pach D, Napadow V, et al. Characterizing acupuncture stimuli using brain imaging with FMRI--a systematic review and meta-analysis of the literature. PLoS One. 2012;7(4):e32960. doi:10.1371/journal.pone.0032960
49. Xiaoyan H. Electro-Acupuncture Treatment of Simple Obesity with the Stomach-Intestine Excessive Heat Type Clinical Observation and on the Hypothalamic Feeding Central fMRI Imaging Analysis. Nanjing, China: Nanjing University of Chinese Medicine; 2009.
50. Shuyu X. Gastric Electrical Stimulation Through Acupuncture on Simple Obesity Stomach Heat Type of Clinical Observation and on the Hypothalamic Feeding Center fMRI Image Analysis. Nanjing, China: Nanjing University of Chinese Medicine; 2010.
51. Xiaojie L, Hong Z, Yun P, et al. Neural mechanism study of acupuncture therapy for obese adolescence with VBM-DARTEL approach. J Med Imaging. 2013;23(03):350–354.
52. Ruigrok AN, Salimi-Khorshidi G, Lai MC, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39(100):34–50. doi:10.1016/j.neubiorev.2013.12.004
53. Wu MT, Hsieh JC, Xiong J, et al. Central nervous pathway for acupuncture stimulation: localization of processing with functional MR imaging of the brain--preliminary experience. Radiology. 1999;212(1):133–141. doi:10.1148/radiology.212.1.r99jl04133
54. Shen GM, Wang H, Qin Y. Effect of electroacupuncture at point weishu and front-mu points on rat gastric motility and c-fos expression in bulbar DVC area. Shanghai J Acu-mox. 2012;31(05):357–359.
55. Chen Y. Integrative Mechanism in Limbic System of Combination of Stomach Back-Shu Point and Front-Mu Point for Treating of Functional Dyspepsia. Chengdu, China: Chengdu University of Chinese Medicine; 2012.
56. Qi R, Liu C, Ke J, et al. Intrinsic brain abnormalities in irritable bowel syndrome and effect of anxiety and depression. Brain Imaging Behav. 2016;10(4):1127–1134. doi:10.1007/s11682-015-9478-1
57. Kou J, Guo TP, Wen PP, et al. Bibliometric analysis of the clinical literature of acupoint thread-embedding for simple obesity. Shanghai J Acu-mox. 2016;35(9):1122–1125. doi:10.13460/j.issn.1005-0957.2016.09.1122
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
