Back to Journals » Vascular Health and Risk Management » Volume 14
Brachial–ankle pulse wave velocity, cardio-ankle vascular index, and prognosis
Authors Ato D
Received 7 July 2018
Accepted for publication 5 September 2018
Published 24 October 2018 Volume 2018:14 Pages 321—348
DOI https://doi.org/10.2147/VHRM.S179366
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Dai Ato
Gakujutsu Shien Co., Ltd, Tokyo, Japan
Background: Brachial–ankle pulse wave velocity (baPWV) and cardio-ankle vascular index (CAVI) are indices of arterial stiffness, and several studies have used these indices. However, there is no comprehensive review of these parameters in the prognostic significance.
Methods: The aim of this study was to review the articles exploring the prognostic significance of these parameters. Articles demonstrating independent significance after multivariate analysis on the Cox proportional hazards model were defined as “successful.” The success rate was compared using Fisher’s exact test. In addition, multivariate logistic regression analysis was performed to explore the independent determinants of the success of prognostic prediction.
Results: The success rate of the baPWV articles (65.7% [46/70]) tended to be higher than that of the CAVI articles (40.0% [6/15]; P=0.083). Multivariate analysis demonstrated that log (number of patients) (OR 11.20, 95% CI 2.45–51.70, P=0.002) and dialysis population (OR 0.28, 95% CI 0.08–0.94, P=0.039) were positive and negative independent determinants of the success of prognostic prediction, respectively. In addition, after redefining two studies as the absence of arteriosclerosis obliterans (ASO) exclusion, baPWV (OR 3.36, 95% CI 0.86–13.20, P=0.083) and the existence of exclusion criteria of ASO (OR 3.08, 95% CI 0.96–9.93, P=0.060) exhibited statistical tendency in the multivariate analysis.
Conclusion: This study demonstrated that the number of study participants and dialysis population were the independent determinants of the success of prognostic prediction. This study also showed the importance of exclusion criteria of ASO when using these indices. In addition, a prospective large-scale study to confirm the superiority in the prognostic prediction of these indices is warranted.
Keywords: peripheral arterial disease, ankle–brachial index, diabetes, hemodialysis, cardiovascular events
Introduction
The burden of managing atherosclerotic diseases is increasing globally as economic development continues. Pulse wave velocity (PWV) and ankle–brachial index (ABI) have long been used to quantify arteriosclerosis and atherosclerosis,1,2 and the clinical significance of each has been established.3–5 Carotid–femoral PWV (cfPWV) is a representative marker of arterial stiffness, and several meta-analyses have demonstrated its independent prognostic predictability.6–8 Vascular Profiler (VP; BP-203RPE, VP-1000, and VP-2000 series, Japanese product name “form”) and VaSera (VS; VS-1000, VS-1500, VS-2000, and VS-3000 series) were first sold in Japan at the end of 19999,10 and in the first half of 2002,11,12 respectively, which were new devices that can simultaneously measure brachial–ankle PWV (baPWV) and ABI. The specifications of VS changed to measure cardio-ankle vascular index (CAVI) and ABI in the first half of 2004, and it was continued to be sold.13,14 Several English-language articles about both devices have been published. The first article discussing the prognostic predictability of the baPWV was published in 2005,15 whereas the first article reporting that of the CAVI was published in 2009.16 The number of articles detailing the prognostic predictability of baPWV rapidly increased after 2012, nearing 40 at the end of 2014.17 Moreover, three meta-analyses and one rapid communication article using the data derived from one meta-analysis were also published.18–21 As information accumulated, the baPWV threshold of 18 m/s was set as “high risk,”3,22,23 whereas that of 14 m/s was set as “middle risk”22 in the related guidelines. Five articles reported the prognostic significance of baPWV and CAVI in the same population.24–28 However, no studies have comprehensively discussed the differences in prognostic predictability among those indices.29 Therefore, this study aimed to identify articles that researched the prognostic predictability of both indices and compare the success rate wholly and in each category. Moreover, this review also aimed to explore the independent predictors of the success of prognostic prediction.
Methods
Identifying and defining articles
The concept and measurement method of baPWV and CAVI are available elsewhere.10,14,22 The articles identified in this study were written in English (at least in the abstract) and indexed to PubMed or released publicly on the Internet. Each was obtained by the end of April 2018. Figure 1 shows the selection process of the objective studies. The PubMed search was performed using the related keywords such as “pulse wave velocity,” “brachial–ankle pulse wave velocity,” “cardio-ankle vascular index,” “arterial stiffness,” and “ankle–brachial index.” Longitudinal studies that discussed the prognostic predictability of both indices were identified. End points included all-cause mortality (ACM), cerebrovascular–cardiovascular mortality (CCVM), cerebrovascular–cardiovascular events (CCVE), ischemic heart disease (IHD), major adverse cardiac events, and heart failure. When the other end points such as peripheral arterial disease (PAD) were included, they were explained additionally (Tables 1–3). Studies of functional prognosis such as a decline in cognitive function, kidney function, or activities of daily living were not included. Three meta-analyses and one rapid communication article detailing prognostic predictability of baPWV were excluded.18–21 Longitudinal studies that did not include baPWV or CAVI as a prognostic variable were also excluded.30–37
  | Figure 1 A flowchart of identifying prognostic studies of baPWV and CAVI articles. Abbreviations: baPWV, brachial–ankle pulse wave velocity; CAVI, cardio-ankle vascular index. |
Moreover, among the interventional studies in which those indices were measured, those that did not research their prognostic significance were also excluded because of the discrepancy in the research purpose.38,39 One study reporting perioperative adverse events was also excluded.40 Furthermore, one study that demonstrated the significance of baPWV using a Kaplan–Meier analysis (log-rank test, P<0.0001) was excluded because of considerable difficulty in adopting the Cox proportional hazards model, as the Kaplan–Meier curves apparently showed nonproportional changes in the event rates.41 Finally, a total of 71 baPWV articles and 15 CAVI articles were identified. The identified studies were categorized according to patient characteristics and the presence or absence of the clarified exclusion criteria of lower extremity (LE)-arteriosclerosis obliterans (ASO)/PAD. Any criteria such as “other vascular diseases” were not defined as the exclusion criteria of LE-ASO/PAD in this study. The studies demonstrating independent prognostic predictability of those indices on a multivariate Cox proportional hazards model or multivariate logistic regression model were defined as “successful.” The studies showing significance on only Kaplan–Meier analysis (log-rank test) and/or not demonstrating statistical significance on a multivariate Cox proportional hazards model were defined as “failed.” Comparisons between baPWV and CAVI were performed in all included articles, in the presence or absence of the clarified exclusion criteria of LE-ASO/PAD, in the population of dialysis (hemodialysis and peritoneal dialysis) or other than dialysis, and in the articles discussing both indices in a same cohort. However, one article studying both baPWV and CAVI in the same patient cohort did not describe the prognostic significance of baPWV as a primary end point. As such, that study was excluded from the statistical analysis as a baPWV article because the independent prognostic significance of baPWV for the primary end point was not clarified.28 Moreover, because of the relatively ample number of baPWV articles, the studies with or without the clarified exclusion criteria of LE-ASO/PAD only among baPWV articles were compared to confirm the effect on success rate. Furthermore, multivariate logistic regression analysis was performed to explore the independent factors of the success of prognostic prediction.
Statistical analyses
Statistical analyses were performed using EZR (EZR on R commander version 1.33, September 1, 2016).42 All comparisons between groups were performed using Fisher’s exact test. However, a statistical analysis was not performed among the five articles that simultaneously studied baPWV and CAVI because of the too small sample and of the heterogeneity in the condition.24–28 Moreover, logistic regression analysis was performed to explore the independent determinants of the success of prognostic prediction (success =1, failure =0). The following covariates were analyzed in the univariate analysis: baPWV or CAVI (baPWV =1, CAVI =0), presence of the clarified exclusion criteria of LE-ASO/PAD (yes =1, no =0), dialysis population (yes =1, no =0), follow-up period (years), age (years), male gender (%) in the study cohort, and log-transformed number of patients (log NoP). The number of patients was log transformed because of skewed distribution. In this analysis, the mean values of the patients’ age and follow-up years were primarily used, and if not available, median values were used. The value of the mean/median patient age was missing in two studies.43,44 The proportion of gender was also missing in one study.44 Nevertheless, the analysis was performed without these missing data. The covariates whose P-value was ≤0.2 in the univariate analysis were entered into the multivariate model. Furthermore, reanalysis was performed by redefining two studies as the absence of LE-ASO/PAD exclusion,27,45 because these studies were considered to insufficiently exclude LE-ASO/PAD patients (symptomatic PAD only). P-values of ≤0.05 were considered statistically significant, whereas P-values of 0.05<P≤0.10 were considered to have a statistical tendency.
Results
All articles
A total of 70 articles on baPWV15,24–27,43–107 and 15 articles on CAVI16,24–28,108–116 were identified. Table 1 presents a summary of these articles. The success rate of independent prognostic predictability of the baPWV articles (65.7% [46/70]) tended to be higher than that of the CAVI articles (40.0% [6/15]; P=0.083; Figure 2).
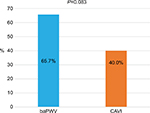  | Figure 2 The success rate of baPWV and CAVI articles. Abbreviations: baPWV, brachial–ankle pulse wave velocity; CAVI, cardio-ankle vascular index. |
Articles clarifying the exclusion criteria of LE-ASO/PAD
Table 2 presents the detailed information about the articles in this category. In this category, the success rates of baPWV and CAVI articles were 75.9% (22/29) and 57.1% (4/7), respectively (P=0.37). After excluding two studies that insufficiently excluded LE-ASO/PAD patients,27,45 the success rates of baPWV and CAVI articles were 81.5% (22/27) and 66.7% (4/6), respectively (P=0.58).
Articles lacking or not clarifying the exclusion criteria of LE-ASO/PAD
In this category, the success rates of baPWV articles and CAVI articles were 58.5% (24/41) and 25% (2/8), respectively (P=0.12). After adding two studies that insufficiently excluded LE-ASO/PAD patients27,45, the success rates of baPWV articles and CAVI articles were 55.8% (24/43) and 22.2% (2/9), respectively (P=0.14).
Population other than dialysis (hemodialysis and peritoneal dialysis)
In this category, the success rate of baPWV articles was 71.43% (41/57) which was similar to that of CAVI articles (54.5% [6/11]; P=0.30).
Studies of dialysis population only
In this category, the success rate of baPWV articles was 38.5% (5/13) which was similar to that of CAVI articles (0% [0/4]; P=0.26).
Studies comparing baPWV and CAVI in the same cohort
Table 3 presents the detailed information of the five articles in this category. Among the baPWV articles, the result was one success, two studies with statistical significance in the log-rank test, one failure, and one without a clarified result of the primary end point. Among the CAVI articles, the result was two successes and three failures. In the study that presented the success of baPWV, CAVI showed a statistical tendency in the Kaplan–Meier analysis (log-rank test, P=0.06). However, the trend disappeared after the adjustment for age, gender, and diabetes on the multivariate Cox proportional hazards model (P=0.49).25 The two studies that showed a statistical significance of baPWV in the Kaplan–Meier analysis (log-rank test) did not reveal significance for CAVI.24,27 In the study that demonstrated the success of CAVI and failure of baPWV, CAVI was analyzed with respect to the presence or absence of improvement for ≥6 months (persistently impaired CAVI). However, the baPWV’s raw value at the second occasion of the measurement was analyzed.26 In a study in which the baPWV result was defined as “not clarified,” the prognostic significance of baPWV in the multivariate Cox proportional hazards model analysis on the primary end point was not described. However, the prognostic significance of CAVI (not baPWV) on the secondary end point of nonfatal stroke was presented.28
Comparison of baPWV articles according to the presence or absence of the clarified exclusion criteria of LE-ASO/PAD
The success rate of the articles in the presence of these criteria (75.9% [22/29]) was similar to that in their absence (58.5% [24/41]; P=0.20; Figure 3A). However, after redefining two studies as the absence of LE-ASO/PAD exclusion,27,45 the success rate of the articles in the presence of these criteria (81.5% [22/27]) was significantly higher than that of the articles in the absence (55.8% [24/43]; P=0.039; Figure 3B).
Multivariate logistic regression analysis to identify the independent determinants for the success of prognostic prediction
Table 4 presents this result. In the univariate analysis, log NoP (P=0.0006) and dialysis population (P=0.005) were significantly associated with the success of prognostic prediction, whereas baPWV and exclusion of LE-ASO/PAD showed a statistical tendency (P=0.071, P=0.076, respectively). In the multivariate analysis, log NoP (OR 11.20, 95% CI 2.45–51.70, P=0.002) and dialysis population (OR 0.28, 95% CI 0.08–0.94, P=0.039) were identified as the independent determinants of the success of prognostic prediction.
Table 5 summarizes the result after redefining two studies as the absence of LE-ASO/PAD exclusion.27,45 In the univariate analysis, exclusion of LE-ASO/PAD (P=0.01), dialysis population (P=0.005), and log NoP (P=0.0006) were significantly associated with the success of prognostic prediction, whereas baPWV showed statistical tendency (P=0.071). In the multivariate analysis, log NoP and dialysis population were the statistically significant factors (for log NoP: OR 9.04, 95% CI 1.90–43.00, P=0.006; for dialysis population: OR 0.27, 95% CI 0.07–0.96, P=0.043). However, baPWV and the exclusion of LE-ASO/PAD showed statistical tendency in the success of prognostic prediction (for baPWV: OR 3.36, 95% CI 0.86–13.20, P=0.083; for the exclusion of LE-ASO/PAD: OR 3.08, 95% CI 0.96–9.93, P=0.060).
Discussion
Overview
To the best of our knowledge, this study is the first to comprehensively review the prognostic predictability of baPWV and CAVI. The current number of English articles using the indices of VP and VS is approximately 1,800 and 550, respectively.117 The ratio of articles studying the prognostic significance of these parameters did not differ significantly (baPWV 70/1,800; CAVI 15/550, P=0.24). There were five articles that simultaneously studied prognostic significance of baPWV and CAVI in a same patient population. The success rate of baPWV articles tended to be higher than that of CAVI articles (65.7% vs 40.0%, P=0.083). Dialysis population and log NoP were the independent determinants for the success in the multivariate logistic regression analysis (Table 4). Moreover, after redefining two studies as the absence of exclusion of LE-ASO/PAD,27,45 the success rate in the presence of these criteria was significantly higher than that in the absence of these criteria among the baPWV articles only (81.5% vs 55.8%, P=0.039). Furthermore, multivariate logistic analysis showed that baPWV and exclusion of LE-ASO/PAD had a statistical trend on the success of prognostic prediction (P=0.083, P=0.060, respectively). The multivariate analysis also showed that the effects of dialysis population and log NoP were attenuated, although these parameters were still significant (P=0.043, P=0.006, respectively; Table 5). Therefore, log NoP had the strongest power on the success of prognostic prediction among the articles investigated in this study. Actually, the most studies involving >1,000 participants were successful in the prognostic prediction.47,48,52,53,55,58,68,88,93,97,103,107,109 These studies mostly used baPWV, and one study used CAVI.109 However, the largest study of CAVI involving >2,000 participants failed.110 Moreover, this review confirmed that the dialysis population (mostly hemodialysis) was a negative determinant of the success of prognostic prediction of the baPWV and CAVI. This is not surprising because the incidence of having false-negative LE-ASO/PAD is high even if the exclusion criteria are defined as ABI of ≤0.9 in the hemodialysis population. Age, proportion of male gender, and follow-up period in the study population had no effect on the success of baPWV and CAVI in the prognostic prediction. In general, age and gender affect the progression of arterial stiffness and thus prognosis.118 However, the result of this study is plausible because this study explored the key factors for the success of prognostic prediction of baPWV and CAVI, not investigating the factors affecting arterial stiffness. This study also confirmed that more than half of the articles did not clarify the exclusion criteria of LE-ASO/PAD or did not exclude patients with LE-ASO/PAD when using baPWV and CAVI in the prognostic studies.
baPWV
Main findings
The success rate of baPWV articles (65.7%) tended to be higher than that of CAVI articles (40.0%) (P=0.083). This difference may be partly caused by the number of the study population. The success rate of baPWV articles (75.9%) was similar to that of CAVI articles (57.1%) in the studies clarifying the exclusion criteria of LE-ASO/PAD. Among the 29 baPWV articles that had the patient exclusion criteria of LE-ASO/PAD, seven articles failed to prove prognostic significance of baPWV. Among the seven articles, two excluded those patients only with symptomatic PAD.27,45
The former study consisted of patients with multiple risk factors for cardiovascular disease (CVD), and the mean ABI in this cohort was 1.01±0.17 (SD). Therefore, patients with an ABI of ≤0.9 existed at high probability. In this study, baPWV, but not CAVI, showed statistical significance in the Kaplan–Meier analysis. Nevertheless, its significance was lost after multivariate adjustment by the Cox model including ABI as a covariate, and the cutoff ABI of 1.04 was selected as an independent predictor.27 Moreover, the mean baPWV and CAVI values of both sides were used in the analysis. This condition meant that the decreased baPWV or CAVI on the side with asymptomatic PAD would lower the mean parameter. However, in reality, a patient was considered to have a high-risk prognosis.5,22,117,119 Therefore, the risk of a patient with asymptomatic PAD who was considered at equivalently high risk as a patient with symptomatic PAD was considerably underestimated by the falsely lowered baPWV or CAVI.27
In the study by Lau et al,45 the study cohort included patients with diabetes vintage of 15.2±7.5 years. Thus, the high probability of a falsely overestimated ABI due to arterial calcification in the lower limbs was considered.5 The mean ABI of both sides, 1.1±0.1, was used in the analysis, and the high probability of asymptomatic LE-ASO/PAD was considered. Furthermore, the mean baPWV of both sides was also used; as such, a similar phenomenon observed in the study by Kusunose et al would be most likely.27 As a result, no prognostic significance of baPWV was proven in this study.45 Thus, at least when utilizing baPWV and CAVI as prognostic predictors that include the lower-limb arteries in the measuring path, these findings imply that the exclusion of symptomatic PAD is insufficient. Therefore, redefining of these two articles and the reanalysis were performed, and this change presented the statistically higher success rate in the presence of exclusion criteria of LE-ASO/PAD than that in the absence of the criteria among the baPWV articles. At the same time, baPWV and exclusion of LE-ASO/PAD showed a statistical tendency in the multivariate logistic model (Table 5).
However, within the articles that clarified the exclusion criteria using ABI (≤0.9) or an expression of LE-ASO/PAD exclusion, the other five studies did not prove an independent prognostic significance of baPWV. The cohorts of these studies were as follows: two patients with IHD,26,74 of the patients receiving hemodialysis,80 one of the patients with chronic kidney disease stage 3–5,83 and one of an outpatient population with a 78% incidence of diabetes.86 Thus, these studies were conducted in population that were still very likely to include patients with LE-ASO/PAD (false-negative LE-ASO/PAD), even if the exclusion criterion was set at ABI ≤0.9. Therefore, the reason for failure in these five studies is considerably similar to that in the two studies.27,45 The reason why ABI and/or (false-negative) LE-ASO/PAD is a stronger indicator of prognosis is given in the following section.
Prognostic significance of ABI is much stronger than that of baPWV without sufficient exclusion of LE-ASO/PAD
In the hemodialysis cohort that did not exclude patients with LE-ASO/PAD, it was already demonstrated that a high baPWV (the top quartile of baPWV ≥23.6 m/s) lost prognostic significance after multivariate adjustment including ABI as one of the covariates using the Cox model, even though a high baPWV showed significance in the Kaplan–Meier analysis.46 This is because ABI, which assesses LE-ASO/PAD as a more severe disease, is a considerably stronger prognostic indicator than baPWV. In the same study, baPWV showed independent prognostic significance after the exclusion of patients with an ABI of <0.9. Nevertheless, patients with a borderline ABI of 0.90–0.99 and patients with a high ABI of ≥1.3 also showed independent prognostic significance. Furthermore, patients with a low normal ABI of 1.00–1.09 showed a statistical tendency or a close value as a prognostic indicator (P=0.113 for ACM, P=0.086 for CCVM after adjustment).46
In another study of hemodialysis patients evaluating the prognostic significance of ABI but not using baPWV,120 patients with an abnormal ABI of <0.9 and those with a borderline ABI of 0.90–0.99 showed the worst prognosis. However, in the present study, those with a high ABI of ≥1.3 and even those with a low normal ABI of 1.00–1.09 showed significantly worse prognosis than those with a reference ABI of 1.10–1.29 (in those with a low normal ABI of 1.00–1.09, an HR of 1.92% and 95% CI of 1.02–3.59 for ACM and an HR of 2.82% and 95% CI of 1.22–6.54 for CCVM after multivariate adjustment using the Cox proportional hazards model). In the report published in 2003, Ono et al120 mentioned that those with an ABI of 0.9–1.1 as well as those with an abnormal ABI should be carefully monitored in the hemodialysis population.
Moreover, in a study of a hemodialysis cohort that evaluated the prognostic predictability of ABI in ACM, the best cutoff ABI of 1.1 was demonstrated by a receiver operating characteristic (ROC) curve analysis (area under the ROC curve to predict mortality, 0.79; sensitivity, 0.90; specificity, 0.62).121 This significance was maintained after multivariate analysis using the Cox model. In the studies that evaluated the diagnostic ability of ABI compared to imaging modalities or clinical symptoms in patients on hemodialysis, an ABI threshold of 1.01–1.10 was mainly reported.122–124 Ohtake et al123 suggested raising the ABI cutoff to 1.1 in patients on hemodialysis. In a cohort of patients with diabetes, significant HRs and P-values in prognostic predictability (ACM and CCVM) were reportedly similar between those with an abnormal ABI of ≤0.9 and those with a borderline ABI of 0.91–0.99 (both HRs of about 2.0, significant) compared to those with normal ABI of 1.00–1.4.125 Moreover, in a study of a cohort with multiple cardiovascular risk factors and a history of CVDs, those with a borderline ABI of 0.91–0.99 showed significantly higher HRs in ACM (HR 2.27, P=0.005) and CCVM (HR 3.47, P=0.003) than those with a normal ABI of 1.00–1.4.126
Among the studies of patients with IHD, one report showed the independent prognostic predictability of a borderline ABI of 0.91–0.99,127 whereas another study demonstrated the best cutoff ABI of 1.057 as an independent prognostic predictor.128 In contrast, in the Hisayama study involving a general population, those with abnormal ABI of ≤0.9 clearly showed independent prognostic predictability in CCVE (HR 2.40, P=0.02). However, those with a borderline ABI of 0.91–0.99 did not show any difference compared to those with a normal ABI of 1.0–1.4, and the result was virtually the same even in the Kaplan–Meier analysis.129 All the ABI values in the study mentioned earlier were measured using VP. The information described earlier indicated that there are frequent cases in which those with LE-ASO/PAD still exist after excluding those with an ABI of ≤0.9 and that a borderline or low normal ABI has stronger prognostic significance than baPWV depending on the cohort or at least has confounding power to weaken the prognostic significance of baPWV in the multivariate analysis. Moreover, it is also plausible that the existence of the patients with false-negative LE-ASO/PAD weakens the prognostic significance of baPWV even if ABI is not included in the multivariate model, because the prognostic risk of such a patient cannot be appropriately assessed by baPWV even if the higher baPWV is used.117 Simply, in other words, the existence of the false-negative LE-ASO/PAD weakens the prognostic significance of baPWV (and also CAVI) anyway regardless of the ABI included in the multivariate model or not.
Appropriate settings when evaluating baPWV and CAVI
Therefore, to set the exclusion criteria of LE-ASO/PAD to appropriately assess baPWV as a prognostic indicator, the cutoff ABI of ≤0.9 is sometimes insufficient, or it would be necessary to increase the ABI value in such a case. This is one of the limitations when using baPWV (and also CAVI). As such, among the seven studies that failed to show the prognostic significance of baPWV,26,27,45,74,80,83,86 if the exclusion criteria of LE-ASO/PAD were defined to include the upstroke time (UT)130 and/or percent mean arterial pressure (%MAP),131 the baPWV success rate would be higher. This might be the same for CAVI. In contrast, several studies showed the independent prognostic significance of baPWV and CAVI without the clarified exclusion criteria of LE-ASO/PAD or without LE-ASO/PAD exclusion. For this reason, some studies might have excluded LE-ASO/PAD using the cutoff ABI of ≤0.9, but it might not be just precisely described in the articles (it is very likely in three articles of reference number of 56, 68, and 111 for some reasons). These studies might also be performed in the cohort with a low frequency of LE-ASO/PAD even if LE-ASO/PAD was not excluded. It is also plausible that the independent prognostic predictability of baPWV or CAVI was proven incidentally in the relationship of the covariates included in the multivariate model.
In reality, two baPWV56,68 and one CAVI111 studies, which were defined as not clarifying exclusion criteria of LE-ASO/PAD, were considered most likely to exclude those with LE-ASO/PAD and/or ABI of ≤0.9. It is almost certain according to the data of ABI (ABI=1.13±0.00 [standard error], the number of patients was 338),56 the context of the patient exclusion criteria and the end point,111 and the information of other studies of their institutions.58,68 Therefore, reanalysis was performed. Among the baPWV articles, the success rate of the articles in the presence of these criteria (82.8% [24/29]) was significantly higher than that of the articles in the absence of these criteria (53.7% [22/41]; P=0.020). In the multivariate logistic regression analysis (Table S1), exclusion of LE-ASO/PAD emerged as an independent predictor of the successful prognostic prediction (P=0.022). The P-value of baPWV improved (from 0.083 to 0.059).
CAVI
Main findings
The success rate tended to be lower in studies of CAVI than in those of baPWV (40% vs 65.7%, P=0.083). Moreover, all four studies of a hemodialysis cohort failed to show prognostic significance.24,25,108,116 In three of the four studies, LE-ASO/PAD was not excluded.24,106,114 The prognostic significance of CAVI was also definitely weakened by uncertainty or absence of the exclusion criteria of LE-ASO/PAD. The relatively small number of the participants in the hemodialysis studies might also affect the outcome. However, among the studies clarifying the exclusion criteria of LE-ASO/PAD, the success rate was 57.1% (4/7), which was lower than that for studies of baPWV (75.9%), although this was not statistically significant. Moreover, four of the six studies that showed the independent prognostic significance of CAVI implied that the statistical power was not very strong from the aspect of P-values despite the studied cohorts not being very small (P=0.039 for 1,003 patients; P=0.029 for 300 patients; P=0.049 or<0.05 for 626 patients; P=0.04 for 400 patients).109,111–113 Furthermore, the largest study of CAVI involving 2,106 Caucasian participants with metabolic syndromes failed. In this study, there were no clarified criteria of LE-ASO/PAD. Nevertheless, the subjects were middle aged (54±6 years), and those with the previous history of CVDs were excluded. Thus, the prevalence of LE-ASO/PAD was considered low in this cohort. In the present study, CAVI showed statistical significance in the Kaplan–Meier analysis and the univariate Cox model. However, in the multivariate analysis, this significance was lost, and age and gender were selected as the independent prognostic predictors.110 These phenomena are similar to that of the hemodialysis study by Kato et al.25 In Kato’s study, the statistical tendency of CAVI was lost after the adjustment of age, gender, and diabetes in the multivariate Cox proportional hazards model.
Therefore, considering the whole information described earlier, the prognostic power of CAVI may be weaker than that of baPWV. The possible reasons are described in the following section.
Factors possibly affecting prognostic predictability of CAVI
CAVI is a product of PWV adjusted by blood pressure, and its concept is derived from the stiffness parameter β.14 In adopting the concept of the stiffness parameter β on heart–ankle PWV (haPWV), the equation is as follows: β= CAVI = 2ρ• PP−1• Ln(SBP/DBP)• haPWV,2 where ρ is the blood density, PP is the pulse pressure, and Ln is a natural logarithm.14 As a result of this method, CAVI is considered less dependent on blood pressure than baPWV or independent from blood pressure.14,132 However, the appropriateness of using only brachial blood pressure as the representative of the haPWV measuring path, as well as the independence of blood pressure, has been controversial.132–141 A few authors in these studies clearly denied the blood pressure independence of CAVI.135,138,139 To calculate haPWV, the pulse transit times between the brachial–ankle and the heart–brachial are required. The equation of haPWV is as follows: haPWV = Lha/Tha = Lha/(Tba + Thb), where Lha is the length between the heart and the ankle, Tha is the pulse transit time between the heart and the ankle, Tba is the pulse transit time between the brachium and the ankle, and Thb is the pulse transit time between the heart and the brachium.14 However, measuring Tha or Thb is virtually impossible. Therefore, it is substituted with the time interval between the onset of the second heart sound and that of the dicrotic notch (DN) on the brachial pulse wave form (Thb ≒ T¢hb = TII–DN). Nevertheless, one study reported that this method using volume plethysmography could induce a 50% reduction in CAVI.142 The timings of the second sound and the DN themselves could be falsely determined depending on the patient’s condition, especially in cases of valvular heart diseases. Furthermore, the blood density ρ is considered constant in the VS device, but this is not always constant in vivo. Kato et al25 pointed out the change in blood density of patients on hemodialysis.143 Moreover, we must recognize the risk of using the brachial blood pressure of the upper extremity (UE)-ASO in those on hemodialysis. Patients on hemodialysis reportedly have falsely elevated ABI to a certain extent because of UE-ASO on the contralateral side of the hemodialysis access, and its frequency was reportedly about 10% in the Japanese hemodialysis patients.117,144 In all four hemodialysis cohort studies that failed to show the prognostic significance of CAVI, no difference in CAVI was found between those with and without the primary end point (before adjustment). This might be caused by the absence of exclusion criteria of LE-ASO/PAD in three of the studies. Nevertheless, a decreased brachial blood pressure due to UE-ASO and error in the pulse transit time (T¢hb and Tba) might have partly affected the results. We must also recognize that a false measurement of ABI and baPWV in the cases of UE-ASO in patients on hemodialysis would affect the prognostic predictability of baPWV.117
With respect to blood pressure and ABI measurement, we must recognize that there are a few minor differences between VP and VS. VP synchronizes the timing of SBP determination to make it simultaneous. In contrast, a blood pressure measurement using VS is basically performed through the sequential method (from the right side to the left side). Thus, for determining SBPs in the arms and ankles, the difference in measurement time is considered more likely to occur with VS than with VP. In the statement document of the American Heart Association published in 2012 regarding the measurement and interpretation of ABI, two studies reported that the left ABI measured using the Doppler method was significantly lower (0.03) than the right ABI.5 In a meta-analysis of the risks of inter-arm blood pressure differences, a significantly increased relative risk of inter-arm difference (ie, difference in SBP ≥10 mmHg) was demonstrated in the sequential method compared to the simultaneous method.145 The difference in the risk of the inter-arm blood pressure difference mentioned earlier may affect the individual ABI and the prevalence of LE-ASO/PAD (it could also change the excluded patients) and may affect the brachial blood pressure to be used in the CAVI equation. Furthermore, as a fundamental issue, we may have to ensure that the blood pressure measured by the oscillometric method is used in the CAVI equation of VS. Blood pressures measured by the oscillometric method are reportedly lower than those measured by the invasive method (internal arterial pressure).146–148 This implies that, even if it is theoretically correct to use the brachial blood pressure as a representative value of the systemic arteries in the CAVI equation, because it is an oscillometric method anyway, a discrepancy inevitably exists regardless of the device used. It should also be recognized that the invasive method is not always perfect.
Difference between CAVI and baPWV
Various factors that would affect the prognostic significance of CAVI were discussed earlier. The correlation between baPWV and haPWV, which is a parameter in the CAVI equation, is reportedly very high in healthy male individuals of the general population (r=0.92 for baPWV and haPWV in the right side, n=135; mean, 59 years old).149 In contrast, the blood pressure dependence of CAVI is reportedly weaker than that of baPWV.14,132 Moreover, several studies have shown superior associations with other atherosclerotic parameters of CAVI to that of baPWV.150,151 However, we may have to recognize that the characteristics of PWV, which is considered to natively possess prognostic significance,6–8,18–21 might be affected by various factors in the measurement and the equation of CAVI as pointed out earlier. Furthermore, we suppose that CAVI is superior to baPWV as an index of arterial wall stiffness. Nevertheless, we may also have to recognize that the superiority for quantifying arterial wall stiffness itself is a different issue from superiority as a prognostic predictor. We may also have to recognize a study that showed the superiority of baPWV to CAVI in terms of reproducibility in the Caucasian population, although the statistical difference was not described.152
Perspective
Necessity of more prognostic studies of CAVI
Three meta-analyses of the prognostic significance of baPWV have already been published,18–21 and the cutoff baPWV of 18 m/s is largely consistent.3,21,22 No published meta-analysis has examined the prognostic significance of CAVI probably due to the shortage of reports. Thus, further studies are required. The large-scale CAVI-J study that aims to validate the prognostic significance of CAVI is currently in progress, and its results are pending.153 A few large longitudinal studies in Western countries are also in progress. According to the MARK study in Spain, CAVI was significantly and positively associated with an index of physical functional quality of life (standardized physical component: the higher, the better), ABI was also significantly and positively associated (both after multivariate adjustment), and baPWV was not correlated.154 Moreover, baPWV and CAVI showed similar correlations with carotid atherosclerosis indices.155 This result is similar to that of the Japanese hemodialysis study.143 In the comparison of cfPWV and CAVI according to the Advanced Approach to Arterial Stiffness study of 18 European countries, age–gender adjustment of cfPWV but not CAVI was higher in the patients with metabolic syndrome than those without.141,156 A similar result was also reported in Japan. In the present study, baPWV but not CAVI was significantly higher in the patients with metabolic syndromes than those without among the middle-aged health checkup population.157 It will be interesting to note whether the same difference in study results as that obtained in the Japanese cohorts is demonstrated.136,154,156
Necessity of rigorous patient exclusion criteria
When using baPWV and CAVI, especially in a study cohort consisting of patients with severe conditions, the patient exclusion criteria should be more rigorous; at least, LE-ASO/PAD definition of ABI ≤0.9 should be used. Arrhythmia and aortic valve disease should be used as well.117 In addition, UE-ASO should be considered in patients receiving hemodialysis.117,144 Especially, regarding the exclusion criteria of LE-ASO/PAD in the study of prognostic significance, as the first step, we can set the best ABI cutoff by ROC curve analysis in the prognostic prediction. The analysis of baPWV and CAVI can be permitted only in patients with an ABI that exceeds the best cutoff. When using a simple cutoff such as ABI ≤0.9 or ABI ≤0.99, the heart rate-adjusted UT130 and %MAP86,131 should also be included in the criteria.158 Moreover, the use of a higher baPWV and CAVI on either side is favorable.22,119 Especially, when using the mean or designated side of those indices, the masked LE-ASO/PAD must be thoroughly excluded.
Adopting the concept of stiffness parameter β and other methods of blood pressure adjustment
The concept of stiffness β and CAVI14 can be applied to other devices that measure PWV and blood pressure if we neglect the “a” and “b” constants, which are used to convert the slope and the coordinate of the CAVI equation in VS. We can also use a general constant for the blood density ρ. In fact, a few studies have adopted this idea using VP.159 However, this is not exactly the same as the CAVI measured by VS. Nevertheless, a baPWV-derived CAVI, the brachial–ankle vascular index, is comparable to the baPWV, and this conversion is quite easy to make. Therefore, a reanalysis comparing both indices in the previously published studies would be interesting. If necessary, haPWV can also be measured by changing the VP settings.149 Regarding CAVI, Spronck et al138 suggested a novel method of adjusting blood pressure. Steppan et al135 also suggested “arterial stiffness index,” which is the product of PWV divided by PP (PWV/PP), as an effective method to adjust the influence of blood pressure on PWV. One study indicated a strong linear relationship between baPWV and the sum of four-limb PP.160 Applying those concepts to these devices would also be easy.
Necessity of prospective large-scale study
Finally, this study discussed the independent prognostic predictability of baPWV and CAVI after adjustment by the multivariate Cox proportional hazards model. The more important ability is the additive predictive value on conventional risk factors (reclassification improvement in risk stratification), which was demonstrated in a few studies of baPWV and CAVI.47,111 This was also demonstrated in the latest meta-analysis of baPWV using individual participant data.20 Further studies are expected to confirm the superior index including this factor. Furthermore, no study to date directly compared prognostic predictability of baPWV and cfPWV in a same study population. Therefore, a prospective large-scale study is warranted to simultaneously investigate baPWV, CAVI, cfPWV, and other arterial stiffness indices in the prognostic significance.
Limitations
There are several limitations to the interpretation of the results. First, because the number of articles was insufficient, the multivariate logistic analysis showed only the statistical tendency for the significance of baPWV after redefining the studies. Moreover, the analysis also demonstrated the most powerful effect of the number of the participants in each study. This implies that the success of the prognostic prediction strongly relies upon the quality of the study itself. However, it should be recognized that the statistical tendency for baPWV and exclusion criteria of LE-ASO/PAD emerged in the number of currently available articles. The results also imply that the reproducibility of baPWV as a prognostic predictor is superior to that of CAVI in the various clinical conditions. Moreover, the fact that baPWV already showed results similar to those for cfPWV in the meta-analyses would be consistent with the results of this study as a whole. Furthermore, we should recognize that only 40% of the studies proved the prognostic significance of CAVI. Second, publication bias was not considered in this study. As such, denying the existence of unpublished studies that could affect the statistical results is difficult. Nevertheless, the ratio of articles that clarified the exclusion criteria of LE-ASO/PAD was similar in the baPWV and CAVI studies. The success rates of the baPWV and CAVI studies declined in the absence of clarified exclusion criteria of LE-ASO/PAD. Moreover, the ratio of the articles studying the prognostic significance of these parameters did not differ significantly (baPWV 70/1,800; CAVI 15/550). Therefore, the prognostic studies of baPWV and CAVI were published without strong bias. Third, this study did not consider other criteria of the patient exclusion such as arrhythmia and aortic valve diseases. However, the description and the definition of the patient exclusion criteria are diverse among each study and sometimes uncertain. Thus, it was impossible to quantitatively include these factors in the statistical analysis. Nevertheless, the major limitation when using baPWV and CAVI is the presence of LE-ASO/PAD. Therefore, this factor was representatively included in the analysis. Fourth, this study did not consider the difference of covariates entered into the Cox multivariate model in each study. It is possible that the success or failure of prognostic prediction of baPWV and CAVI are induced by missing covariates or inappropriate adjustment. However, most articles researched in this study were peer reviewed. Therefore, the incidence of the inappropriate multivariate analysis would be low. Fifth, this study considered seven parameters potentially included in the multivariate logistic analysis. There might be other important factors that should have been included. Nevertheless, the number of the successful studies in the prognostic prediction was 52. Therefore, it was difficult to increase the number of parameters anyway. Finally, PubMed was the only database used in this study. However, PubMed is a widely used database worldwide, so most of the English-written articles related to the theme of this study are included.
Conclusion
This study demonstrated that the number of study participants and dialysis population were the independent determinants of the successful prognostic prediction in the baPWV and CAVI articles. This study also showed that baPWV tended to be superior to CAVI in the prognostic prediction. Moreover, the exclusion criteria of LE-ASO/PAD also affected the prognostic predictive success of both indices. Therefore, for the appropriate use of these indices, thorough LE-ASO/PAD exclusion is essential. In addition, a large-scale prospective study to simultaneously research the prognostic significance of these indices is warranted.
Acknowledgment
The author appreciates the researchers and participants of the preceding studies.
Disclosure
Dai Ato is a former employee of Fukuda Colin (formerly Omron Colin, Nippon Colin) Co., Ltd. Fukuda Colin is a distributor of VP (form PWV/ABI, BP-203RPE series). Dai Ato wrote this article as an academic activity based on the guaranteed right of freedom in academia for the Japanese (Article 23) and on the supreme law provided in Article 98 of the Constitution of Japan. Dai Ato reports no other conflicts of interest in this work.
References
Bramwell JC, Hill AV. Velocity of transmission of the pulse-wave: and elasticity of arteries. Lancet. 1922;199(5149):891–892. | ||
Yao ST, Hobbs JT, Irvine WT. Ankle pressure measurement in arterial disease of the lower extremities. Br J Surg. 1968;55(11):859–860. | ||
Vlachopoulos C, Xaplanteris P, Aboyans V, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241(2):507–532. | ||
Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66(3):698–722. | ||
Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–2909. | ||
Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. | ||
Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636–646. | ||
Zhong Q, Hu MJ, Cui YJ, et al. Carotid-Femoral Pulse Wave Velocity in the Prediction of Cardiovascular Events and Mortality: An Updated Systematic Review and Meta-Analysis. Angiology. 2018;69(7):617–629. | ||
Suzuki E, Kashiwagi A, Nishio Y, et al. Increased arterial wall stiffness limits flow volume in the lower extremities in type 2 diabetic patients. Diabetes Care. 2001;24(12):2107–2114. | ||
Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25(3):359–364. | ||
Yambe T, Kovalev YA, Milyagina IA, et al. A Japanese-Russian collaborative study on aging and atherosclerosis. Biomed Pharmacother. 2004;58(Suppl 1):S91–S94. | ||
Orlova I, Kuz’mina AE, Barinova IV, Iarovaia EB, Ageev FT. Assessment of major artery stiffness: new perspectives of noninvasive diagnosis of coronary atherosclerosis. Ter Arkh. 2009;81(4):8–13. | ||
Yambe T, Yoshizawa M, Saijo Y, et al. Brachio-ankle pulse wave velocity and cardio-ankle vascular index (CAVI). Biomed Pharmacother. 2004;58(Suppl 1):S95–S98. | ||
Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13(2):101–107. | ||
Tomiyama H, Koji Y, Yambe M, et al. Brachial -- ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome. Circ J. 2005;69(7):815–822. | ||
Yamamoto N, Yamanaka G, Ishikawa M, et al. Cardio-ankle vascular index as a predictor of cognitive impairment in community-dwelling elderly people: four-year follow-up. Dement Geriatr Cogn Disord. 2009;28(2):153–158. | ||
Ato D, Takami T. Brachial-Ankle Pulse Wave Velocity, Mortality, and Cardiovascular Events. J Cardiovasc Disord. 2015;2(1):1009. | ||
Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: a systematic review and meta-analysis. Hypertension. 2012;60(2):556–562. | ||
Tomiyama H, Matsumoto C, Shiina K, Yamashina A. Brachial-Ankle PWV: Current Status and Future Directions as a Useful Marker in the Management of Cardiovascular Disease and/or Cardiovascular Risk Factors. J Atheroscler Thromb. 2016;23(2):128–146. | ||
Ohkuma T, Ninomiya T, Tomiyama H, et al. Brachial-Ankle Pulse Wave Velocity and the Risk Prediction of Cardiovascular Disease: An Individual Participant Data Meta-Analysis. Hypertension. 2017;69(6):1045–1052. | ||
Ohkuma T, Tomiyama H, Ninomiya T, et al. Proposed Cutoff Value of Brachial-Ankle Pulse Wave Velocity for the Management of Hypertension. Circ J. 2017;81(10):1540–1542. | ||
Yamashina A, Kario K, Kohara K. Guidelines for noninvasive vascular function test (JCS); 2013. Available from: http://www.j-circ.or.jp/guideline/pdf/JCS2013_yamashina_h.pdf. Accessed October 5, 2018. | ||
Shimamoto K, Ando K, Fujita T. Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. Hypertens Res. 2014;37(4):253–390. | ||
Kato A, Takita T, Furuhashi M, Kumagai H, Hishida A. A small reduction in the ankle-brachial index is associated with increased mortality in patients on chronic hemodialysis. Nephron Clin Pract. 2010;114(1):c29–c37. | ||
Kato A, Takita T, Furuhashi M, Maruyama Y, Miyajima H, Kumagai H. Brachial-ankle pulse wave velocity and the cardio-ankle vascular index as a predictor of cardiovascular outcomes in patients on regular hemodialysis. Ther Apher Dial. 2012;16(3):232–241. | ||
Otsuka K, Fukuda S, Shimada K, et al. Serial assessment of arterial stiffness by cardio-ankle vascular index for prediction of future cardiovascular events in patients with coronary artery disease. Hypertens Res. 2014;37(11):1014–1020. | ||
Kusunose K, Sato M, Yamada H, et al. Prognostic Implications of Non-Invasive Vascular Function Tests in High-Risk Atherosclerosis Patients. Circ J. 2016;80(4):1034–1040. | ||
Gohbara M, Iwahashi N, Sano Y, et al. Clinical Impact of the Cardio-Ankle Vascular Index for Predicting Cardiovascular Events After Acute Coronary Syndrome. Circ J. 2016;80(6):1420–1426. | ||
Kusunose K, Yamada H. Noninvasive Vascular Function Tests - Long Journey for Predicting Cardiovascular Events. Circ J. 2016;80(6):1321–1322. | ||
Shimizu T, Yoshihisa A, Kanno Y, et al. Relationship of hyperuricemia with mortality in heart failure patients with preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2015;309(7):H1123–H1129. | ||
Lin CY, Leu JG, Fang YW, Tsai MH. Association of interleg difference of ankle brachial index with overall and cardiovascular mortality in chronic hemodialysis patients. Ren Fail. 2015;37(1):88–95. | ||
Tokitsu T, Yamamoto E, Hirata Y, et al. Clinical significance of pulse pressure in patients with heart failure with preserved left ventricular ejection fraction. Eur J Heart Fail. 2016;18(11):1353–1361. | ||
Suzuki H, Inoue T, Dogi M, Kikuta T, Takenaka T, Okada H. Role of Pulse Wave Velocity in Patients with Chronic Kidney Disease Stages 3-5 on Long-Term Follow-Up. Pulse. 2015;2(1–4):1–10. | ||
Huang JC, Chen SC, Su HM, Chang JM, Hwang SJ, Chen HC. Performance of the Framingham risk score in patients receiving hemodialysis. Nephrology. 2013;18(7):510–515. | ||
Ohtake T, Ishioka K, Honda K, et al. Impact of coronary artery calcification in hemodialysis patients: Risk factors and associations with prognosis. Hemodial Int. 2010;14(2):218–225. | ||
Miura T, Minamisawa M, Ueki Y, et al. Impressive predictive value of ankle-brachial index for very long-term outcomes in patients with cardiovascular disease: IMPACT-ABI study. PLoS One. 2017;12(6):e0177609. | ||
Minamisawa M, Miura T, Motoki H, et al. Geriatric Nutritional Risk Index Predicts Cardiovascular Events in Patients at Risk for Heart Failure. Circ J. 2018;82(6):1614–1622. | ||
Iimori S, Mori Y, Akita W, et al. Effects of sevelamer hydrochloride on mortality, lipid abnormality and arterial stiffness in hemodialyzed patients: a propensity-matched observational study. Clin Exp Nephrol. 2012;16(6):930–937. | ||
Yoshihisa A, Suzuki S, Yamaki T, et al. Impact of adaptive servo-ventilation on cardiovascular function and prognosis in heart failure patients with preserved left ventricular ejection fraction and sleep-disordered breathing. Eur J Heart Fail. 2013;15(5):543–550. | ||
Sugimoto T, Yamamoto K, Takizawa K, et al. Assessment of pulse wave velocity as a marker of post-operative cardiovascular risk in off-pump coronary artery bypass grafting patients. Kyobu Geka. 2010;63(7):531–535. | ||
Ichikawa K, Sakuragi S, Nishihara T, et al. Influence of arterial stiffness on cardiovascular outcome in patients without high blood pressure. Heart. 2018;104(4):318–323. | ||
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. | ||
Yoshida M, Mita T, Yamamoto R, et al. Combination of the Framingham risk score and carotid intima-media thickness improves the prediction of cardiovascular events in patients with type 2 diabetes. Diabetes Care. 2012;35(1):178–180. | ||
Tokitsu T, Yamamoto E, Hirata Y, et al. Clinical significance of pulse pressure in patients with coronary artery disease. Int J Cardiol. 2015;190:299–301. | ||
Lau KK, Wong YK, Chan YH, et al. Prognostic implications of surrogate markers of atherosclerosis in low to intermediate risk patients with type 2 diabetes. Cardiovasc Diabetol. 2012;11(1):101. | ||
Kitahara T, Ono K, Tsuchida A, et al. Impact of brachial-ankle pulse wave velocity and ankle-brachial blood pressure index on mortality in hemodialysis patients. Am J Kidney Dis. 2005;46(4):688–696. | ||
Ninomiya T, Kojima I, Doi Y, et al. Brachial-ankle pulse wave velocity predicts the development of cardiovascular disease in a general Japanese population: the Hisayama Study. J Hypertens. 2013;31(3):477–483. | ||
Maeda Y, Inoguchi T, Etoh E, et al. Brachial-ankle pulse wave velocity predicts all-cause mortality and cardiovascular events in patients with diabetes: the Kyushu Prevention Study of Atherosclerosis. Diabetes Care. 2014;37(8):2383–2390. | ||
Kuroiwa Y, Miyano I, Nishinaga M, et al. Association between level of brachial-ankle pulse wave velocity and onset of activities of daily living impairment in community-dwelling older individuals. Geriatr Gerontol Int. 2015;15(7):840–847. | ||
Seo HJ, Ki YJ, Han MA, Choi DH, Ryu SW. Brachial-ankle pulse wave velocity and mean platelet volume as predictive values after percutaneous coronary intervention for long-term clinical outcomes in Korea: A comparable and additive study. Platelets. 2015;26(7):665–671. | ||
Chang LH, Lin HD, Kwok CF, et al. The combination of the ankle brachial index and brachial ankle pulse wave velocity exhibits a superior association with outcomes in diabetic patients. Intern Med. 2014;53(21):2425–2431. | ||
Sheng CS, Li Y, Li LH, et al. Brachial-ankle pulse wave velocity as a predictor of mortality in elderly Chinese. Hypertension. 2014;64(5):1124–1130. | ||
Katakami N, Osonoi T, Takahara M, et al. Clinical utility of brachial-ankle pulse wave velocity in the prediction of cardiovascular events in diabetic patients. Cardiovasc Diabetol. 2014;13(1):128. | ||
Ki YJ, Choi DH, Lee YM, Lim L, Song H, Koh YY. Predictive value of brachial-ankle pulse wave velocity for long-term clinical outcomes after percutaneous coronary intervention in a Korean cohort. Int J Cardiol. 2014;175(3):554–559. | ||
Kim J, Song TJ, Song D, et al. Brachial-ankle pulse wave velocity is a strong predictor for mortality in patients with acute stroke. Hypertension. 2014;64(2):240–246. | ||
Kawai T, Ohishi M, Onishi M, et al. Prognostic impact of regional arterial stiffness in hypertensive patients. Heart Vessels. 2015;30(3):338–346. | ||
Nagai K, Shibata S, Akishita M, et al. Efficacy of combined use of three non-invasive atherosclerosis tests to predict vascular events in the elderly; carotid intima-media thickness, flow-mediated dilation of brachial artery and pulse wave velocity. Atherosclerosis. 2013;231(2):365–370. | ||
Takashima N, Turin TC, Matsui K, et al. The relationship of brachial-ankle pulse wave velocity to future cardiovascular disease events in the general Japanese population: the Takashima Study. J Hum Hypertens. 2014;28(5):323–327. | ||
Yoon HE, Shin DI, Kim SJ, et al. Brachial-ankle pulse wave velocity predicts decline in renal function and cardiovascular events in early stages of chronic kidney disease. Int J Med Sci. 2013;10(11):1430–1436. | ||
Ishisone T, Koeda Y, Tanaka F, Sato K, Nagano M, Nakamura M. Comparison of utility of arterial stiffness parameters for predicting cardiovascular events in the general population. Int Heart J. 2013;54(3):160–165. | ||
Kawai T, Ohishi M, Onishi M, et al. Cut-off value of brachial-ankle pulse wave velocity to predict cardiovascular disease in hypertensive patients: a cohort study. J Atheroscler Thromb. 2013;20(4):391–400. | ||
Han JY, Choi DH, Choi SW, et al. Predictive value of brachial-ankle pulse wave velocity for cardiovascular events. Am J Med Sci. 2013;346(2):92–97. | ||
Munakata M, Konno S, Miura Y, Yoshinaga K; J-TOPP Study Group. Prognostic significance of the brachial-ankle pulse wave velocity in patients with essential hypertension: final results of the J-TOPP study. Hypertens Res. 2012;35(8):839–842. | ||
Chen SC, Chang JM, Liu WC, et al. Brachial-ankle pulse wave velocity and rate of renal function decline and mortality in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(4):724–732. | ||
Inoue T, Ogawa T, Ishida H, Ando Y, Nitta K. Aortic arch calcification evaluated on chest X-ray is a strong independent predictor of cardiovascular events in chronic hemodialysis patients. Heart Vessels. 2012;27(2):135–142. | ||
Orlova I, Kuz’mina AE, Masenko VP, Iarovaia EB, Ageev FT. Effect of arterial stiffness on development of cardio-vascular complications in ischemic heart disease. Kardiologiia. 2009;49(12):11–17. | ||
Nakamura M, Yamashita T, Yajima J, et al. Brachial-ankle pulse wave velocity as a risk stratification index for the short-term prognosis of type 2 diabetic patients with coronary artery disease. Hypertens Res. 2010;33(10):1018–1024. | ||
Turin TC, Kita Y, Rumana N, et al. Brachial-ankle pulse wave velocity predicts all-cause mortality in the general population: findings from the Takashima study, Japan. Hypertens Res. 2010;33(9):922–925. | ||
Miyano I, Nishinaga M, Takata J, et al. Association between brachial-ankle pulse wave velocity and 3-year mortality in community-dwelling older adults. Hypertens Res. 2010;33(7):678–682. | ||
Meguro T, Nagatomo Y, Nagae A, et al. Elevated arterial stiffness evaluated by brachial-ankle pulse wave velocity is deleterious for the prognosis of patients with heart failure. Circ J. 2009;73(4):673–680. | ||
Matsuoka O, Otsuka K, Murakami S, et al. Arterial stiffness independently predicts cardiovascular events in an elderly community -- Longitudinal Investigation for the Longevity and Aging in Hokkaido County (LILAC) study. Biomed Pharmacother. 2005;59(Suppl 1):S40–S44. | ||
Wang X, Dang A. Prognostic Value of Brachial-Ankle Pulse Wave Velocity in Patients With Takayasu Arteritis With Drug-Eluting Stent Implantation. Arthritis Care Res. 2015;67(8):1150–1157. | ||
Chen SC, Lee WH, Hsu PC, et al. Association of Brachial-Ankle Pulse Wave Velocity With Cardiovascular Events in Atrial Fibrillation. Am J Hypertens. 2016;29(3):348–356. | ||
Park KH, Han SJ, Kim HS, et al. Impact of Framingham risk score, flow-mediated dilation, pulse wave velocity, and biomarkers for cardiovascular events in stable angina. J Korean Med Sci. 2014;29(10):1391–1397. | ||
Kuwahara M, Hasumi S, Mandai S, et al. Rate of ankle-brachial index decline predicts cardiovascular mortality in hemodialysis patients. Ther Apher Dial. 2014;18(1):9–18. | ||
Orlova IA, Nuraliev EY, Yarovaya EB, Ageev FT. Prognostic value of changes in arterial stiffness in men with coronary artery disease. Vasc Health Risk Manag. 2010;6:1015–1021. | ||
Tanaka M, Ishii H, Aoyama T, et al. Ankle brachial pressure index but not brachial-ankle pulse wave velocity is a strong predictor of systemic atherosclerotic morbidity and mortality in patients on maintenance hemodialysis. Atherosclerosis. 2011;219(2):643–647. | ||
Amemiya N, Ogawa T, Otsuka K, Ando Y, Nitta K. Comparison of serum albumin, serum C-reactive protein, and pulse wave velocity as predictors of the 4-year mortality of chronic hemodialysis patients. J Atheroscler Thromb. 2011;18(12):1071–1079. | ||
Chen SC, Chang JM, Tsai JC, et al. A new systolic parameter defined as the ratio of brachial pre-ejection period to brachial ejection time predicts overall and cardiovascular mortality in hemodialysis patients. Hypertens Res. 2010;33(5):492–498. | ||
Morimoto S, Yurugi T, Aota Y, et al. Prognostic significance of ankle-brachial index, brachial-ankle pulse wave velocity, flow-mediated dilation, and nitroglycerin-mediated dilation in end-stage renal disease. Am J Nephrol. 2009;30(1):55–63. | ||
Nagata Y, Miura S, Suematsu Y, et al. Association between major adverse cardiovascular events and brachial-ankle pulse wave velocity and a difference in blood pressure between arms after percutaneous coronary intervention. Exp Clin Cardiol. 2014. | ||
Lee HS, Kim HL, Kim H, et al. Incremental Prognostic Value of Brachial-Ankle Pulse Wave Velocity to Single-Photon Emission Computed Tomography in Patients with Suspected Coronary Artery Disease. J Atheroscler Thromb. 2015;22(10):1040–1050. | ||
Chen SC, Chang JM, Tsai JC, et al. A systolic parameter defined as the ratio of brachial pre-ejection period to brachial ejection time predicts cardiovascular events in patients with chronic kidney disease. Circ J. 2010;74(10):2206–2210. | ||
Wei SY, Huang JC, Chen SC, Chang JM, Chen HC. Unequal Arterial Stiffness With Overall and Cardiovascular Mortality in Patients Receiving Hemodialysis. Am J Med Sci. 2016;351 (2):187–193. | ||
Iino R, Yokoyama N, Konno K, Naito K, Isshiki T. Impact of combined assessment of coronary artery calcium score, carotid artery plaque score, and brachial-ankle pulse wave velocity for early coronary revascularization in patients with suspected coronary artery disease. Int Heart J. 2012;53(3):154–159. | ||
Li YH, Lin SY, Sheu WH, Lee IT. Relationship between percentage of mean arterial pressure at the ankle and mortality in participants with normal ankle-brachial index: an observational study. BMJ Open. 2016;6(3):e010540. | ||
Sugamata W, Nakamura T, Uematsu M, et al. The combined assessment of flow-mediated dilation of the brachial artery and brachial-ankle pulse wave velocity improves the prediction of future coronary events in patients with chronic coronary artery disease. J Cardiol. 2014;64(3):179–184. | ||
Song Y, Xu B, Xu R, et al. Independent and Joint Effect of Brachial-Ankle Pulse Wave Velocity and Blood Pressure Control on Incident Stroke in Hypertensive AdultsNovelty and Significance. Hypertension. 2016;68(1):46–53. | ||
Ikura K, Hanai K, Oka S, et al. Brachial-ankle pulse wave velocity, but not ankle-brachial index, predicts all-cause mortality in patients with diabetes after lower extremity amputation. J Diabetes Investig. 2017;8(2):250–253. | ||
Kawai T, Ohishi M, Takeya Y, et al. Adiponectin single nucleotide polymorphism is a genetic risk factor for stroke through high pulse wave pressure: a cohort study. J Atheroscler Thromb. 2013;20(2):152–160. | ||
Chen SC, Chang JM, Tsai YC, et al. Association of interleg BP difference with overall and cardiovascular mortality in hemodialysis. Clin J Am Soc Nephrol. 2012;7(10):1646–1653. | ||
Mimura T, Takenaka T, Kanno Y, Moriwaki K, Okada H, Suzuki H. Vascular compliance is secured under angiotensin inhibition in non-diabetic chronic kidney diseases. J Hum Hypertens. 2008;22(1):38–47. | ||
Ahn KT, Jeong JO, Jin SA, et al. Brachial-ankle PWV for predicting clinical outcomes in patients with acute stroke. Blood Press. 2017;26(4):204–210. | ||
Kim EJ, Choi MJ, Lee JH, et al. Extracellular Fluid/Intracellular Fluid Volume Ratio as a Novel Risk Indicator for All-Cause Mortality and Cardiovascular Disease in Hemodialysis Patients. PLoS One. 2017;12(1):e0170272. | ||
Tabata N, Sueta D, Yamashita T, et al. Relationship between asymptomatic intra-cranial lesions and brachial-ankle pulse wave velocity in coronary artery disease patients without stroke. Hypertens Res. 2017;40(4):392–398. | ||
Saji N, Murotani K, Shimizu H, et al. Increased pulse wave velocity in patients with acute lacunar infarction doubled the risk of future ischemic stroke. Hypertens Res. 2017;40(4):371–375. | ||
Ueki Y, Miura T, Minamisawa M, et al. The usefulness of brachial-ankle pulse wave velocity in predicting long-term cardiovascular events in younger patients. Heart Vessels. 2017;32(6):660–667. | ||
Aisu H, Saito M, Inaba S, et al. Association of worsening arterial stiffness with incident heart failure in asymptomatic patients with cardiovascular risk factors. Hypertens Res. 2017;40(2):173–180. | ||
Kuo TH, Yang DC, Lin WH, et al. Compliance Index, a Marker of Peripheral Arterial Stiffness, may Predict Renal Function Decline in Patients with Chronic Kidney Disease. Int J Med Sci. 2015;12(7):530–537. | ||
Kajimoto T, Sawamura MS, Hayashi RD, Oya T, Hirao RA, Kouhara H. High efficient and cost-effective screening method for diabetic cardiovascular risk. Diabetol Metab Syndr. 2014;6(1):51. | ||
Woo JS, Kim W, Jang HH, Kim JB, Kim WS, Kim KS. Effect of platelet reactivity, endothelial function, and inflammatory status on outcomes in patients with stable angina pectoris on clopidogrel therapy. Am J Cardiol. 2014;113(5):786–792. | ||
Park HW, Kang MG, Kim K, et al. Prognostic value of brachial-ankle pulse wave velocity in patients with non-ST-elevation myocardial infarction. Coron Artery Dis. 2017;28(8):642–648. | ||
Lu YC, Lyu P, Zhu HY, et al. Brachial-ankle pulse wave velocity compared with mean arterial pressure and pulse pressure in risk stratification in a Chinese population. J Hypertens. 2018;36(3):528–536. | ||
Tokitsu T, Yamamoto E, Oike F, et al. Clinical significance of brachial-ankle pulse-wave velocity in patients with heart failure with preserved left ventricular ejection fraction. J Hypertens. 2018;36(3):560–568. | ||
Hwang IC, Jin KN, Kim HL, et al. Additional prognostic value of brachial-ankle pulse wave velocity to coronary computed tomography angiography in patients with suspected coronary artery disease. Atherosclerosis. 2018;268:127–137. | ||
Wang LL, Luo Q, Zhu BX, Zhou FF. Brachial-Ankle Pulse Wave Velocity Could Be a Predictor of Mortality in Patients on Peritoneal Dialysis. Perit Dial Int. 2018;38(3):215–219. | ||
Taniguchi Y, Kitamura A, Shinozaki T, et al. Trajectories of arterial stiffness and all-cause mortality among community-dwelling older Japanese. Geriatr Gerontol Int. 2018;18(7):1108–1113. | ||
Yamaguchi S, Gohda T, Gotoh H, et al. Factors associated with cardiovascular death and events in patients with end stage renal disease. Nihon Jinzo Gakkai Shi. 2013;55(2):159–166. | ||
Sato Y, Nagayama D, Saiki A, et al. Cardio-Ankle Vascular Index is Independently Associated with Future Cardiovascular Events in Outpatients with Metabolic Disorders. J Atheroscler Thromb. 2016;23(5):596–605. | ||
Laucevičius A, Ryliškytė L, Balsytė J, et al. Association of cardio-ankle vascular index with cardiovascular risk factors and cardiovascular events in metabolic syndrome patients. Medicina. 2015;51(3):152–158. | ||
Satoh-Asahara N, Kotani K, Yamakage H, et al. Cardio-ankle vascular index predicts for the incidence of cardiovascular events in obese patients: a multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS). Atherosclerosis. 2015;242(2):461–468. | ||
Chung SL, Yang CC, Chen CC, Hsu YC, Lei MH. Coronary Artery Calcium Score Compared with Cardio-Ankle Vascular Index in the Prediction of Cardiovascular Events in Asymptomatic Patients with Type 2 Diabetes. J Atheroscler Thromb. 2015;22(12):1255–1265. | ||
Kubota Y, Maebuchi D, Takei M, et al. Cardio-Ankle Vascular Index is a predictor of cardiovascular events. Artery Res. 2011;5(3):91–96. | ||
Yoshihisa A, Takiguchi M, Shimizu T, et al. Cardiovascular function and prognosis of patients with heart failure coexistent with chronic obstructive pulmonary disease. J Cardiol. 2014;64(4):256–264. | ||
Sawai A, Yasuda Y, Suzuki S, et al. Impact of non-invasive cardiovascular screening programs as a predictor of cardiovascular events among asymptomatic chronic kidney disease patients. Clin Exp Nephrol. 2016;20(3):416–424. | ||
Harada M, Tsukada W, Tsukada O, Hashimoto K, Kamijo Y. The Optimal Cut-off Value of Ankle Brachial Index for Screening Cardiovascular Disease Risk in Hemodialysis Patients. Shinshu Med J,. 2016;64(3):135–146. | ||
Ato D. Pitfalls in the ankle-brachial index and brachial-ankle pulse wave velocity. Vasc Health Risk Manag. 2018;14:41–62. | ||
Ciccone MM, Bilianou E, Balbarini A, et al. Task force on: ‘Early markers of atherosclerosis: influence of age and sex’. J Cardiovasc Med. 2013;14(10):757–766. | ||
Munakata M. Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: recent evidence and clinical applications. Curr Hypertens Rev. 2014;10(1):49–57. | ||
Ono K, Tsuchida A, Kawai H, et al. Ankle-brachial blood pressure index predicts all-cause and cardiovascular mortality in hemodialysis patients. J Am Soc Nephrol. 2003;14(6):1591–1598. | ||
Otani Y, Otsubo S, Kimata N, et al. Effects of the ankle-brachial blood pressure index and skin perfusion pressure on mortality in hemodialysis patients. Intern Med. 2013;52(21):2417–2421. | ||
Ogata H, Kumata-Maeta C, Shishido K, et al. Detection of peripheral artery disease by duplex ultrasonography among hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(12):2199–2206. | ||
Ohtake T, Oka M, Ikee R, et al. Impact of lower limbs’ arterial calcification on the prevalence and severity of PAD in patients on hemodialysis. J Vasc Surg. 2011;53(3):676–683. | ||
Tsuyuki K, Kohno K, Ebine K, et al. Usefulness of Exercise-Ankle Brachial Pressure Index Test and Its Diagnostic Criteria in Patients on Maintenance Hemodialysis. J Japanese College Angiol. 2015;55(2):21–25. | ||
Natsuaki C, Inoguchi T, Maeda Y, et al. Association of borderline ankle-brachial index with mortality and the incidence of peripheral artery disease in diabetic patients. Atherosclerosis. 2014;234(2):360–365. | ||
Tanaka S, Kaneko H, Kano H, et al. The predictive value of the borderline ankle-brachial index for long-term clinical outcomes: An observational cohort study. Atherosclerosis. 2016;250:69–76. | ||
Lee SH, Choi SH, Kim EK, et al. Borderline ankle-brachial index is associated with poor short-term clinical outcome after coronary artery intervention. Atherosclerosis. 2016;249:186–190. | ||
Kim HL, Seo JB, Chung WY, Zo JH, Kim MA, Kim SH. Prognostic value of the ankle-brachial index in patients undergoing drug-eluting stent implantation. J Atheroscler Thromb. 2015;22(1):27–37. | ||
Kojima I, Ninomiya T, Hata J, et al. A low ankle brachial index is associated with an increased risk of cardiovascular disease: the Hisayama study. J Atheroscler Thromb. 2014;21(9):966–973. | ||
Sheng CS, Li Y, Huang QF, Kang YY, Li FK, Wang JG. Pulse Waves in the Lower Extremities as a Diagnostic Tool of Peripheral Arterial Disease and Predictor of Mortality in Elderly Chinese. Hypertension. 2016;67(3):527–534. | ||
Hashimoto T, Ichihashi S, Iwakoshi S, Kichikawa K. Combination of pulse volume recording (PVR) parameters and ankle-brachial index (ABI) improves diagnostic accuracy for peripheral arterial disease compared with ABI alone. Hypertens Res. 2016;39(6):430–434. | ||
Shirai K, Song M, Suzuki J, et al. Contradictory effects of β1- and α1- adrenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI)--CAVI independent of blood pressure. J Atheroscler Thromb. 2011;18(1):49–55. | ||
Tomiyama H, Yamashina A. Central blood pressure--a possible latent factor affecting CAVI. J Atheroscler Thromb. 2011;18(8):720; author reply 721–722. | ||
Shirai K, Takahashi M. Author’s Reply to: Central Blood Pressure: A Possible Latent Factor Affecting CAVI. J Atheroscler Thromb. 2011;18(8):721–722. | ||
Steppan J, Sikka G, Hori D, et al. Seeking a blood pressure-independent measure of vascular properties. Hypertens Res. 2016;39(1):27–38. | ||
Maliha G, Townsend RR. A study of the VaSera arterial stiffness device in US patients. J Clin Hypertens. 2017;19(7):661–668. | ||
Lim J, Pearman ME, Park W, Alkatan M, Machin DR, Tanaka H. Impact of blood pressure perturbations on arterial stiffness. Am J Physiol Regul Integr Comp Physiol. 2015;309(12):R1540–R1545. | ||
Spronck B, Avolio AP, Tan I, Butlin M, Reesink KD, Delhaas T. Arterial stiffness index beta and cardio-ankle vascular index inherently depend on blood pressure but can be readily corrected. J Hypertens. 2017;35(1):98–104. | ||
Segers P. A lesson in vigilance: pressure dependency of a presumed pressure-independent index of arterial stiffness. J Hypertens. 2017;35(1):33–35. | ||
Shirai K, Shimizu K, Takata M, Suzuki K. Independency of the cardio-ankle vascular index from blood pressure at the time of measurement. J Hypertens. 2017;35(7):1521–1523. | ||
Grillo A, Salvi P. Cardio-ankle vascular index and carotid-femoral pulse wave velocity: limits and strengths. J Hypertens. 2018;36(4):759–764. | ||
Obata Y, Mizogami M, Singh S, et al. Ejection time: influence of hemodynamics and site of measurement in the arterial tree. Hypertens Res. 2017;40(9):811–818. | ||
Ueyama K, Miyata M, Kubozono T, et al. Noninvasive indices of arterial stiffness in hemodialysis patients. Hypertens Res. 2009;32(8):716–720. | ||
Takizawa C, Ito M, Iwasa M, et al. [Clinical significance of ABI by measuring bilateral arms in hemodialysis patients]. Japanese Association of Cardiovascular Intervention and Therapeutic (1884-0027)5, Suppl.I Page736(2013.05). Japanese. | ||
Verberk WJ, Kessels AG, Thien T. Blood pressure measurement method and inter-arm differences: a meta-analysis. Am J Hypertens. 2011;24(11):1201–1208. | ||
Van Bergen FH, Weatherhead DS, Treloar AE, Dobkin AB, Buckley JJ. Comparison of indirect and direct methods of measuring arterial blood pressure. Circulation. 1954;10(4):481–490. | ||
Gourdeau M, Martin R, Lamarche Y, Tétreault L. Oscillometry and direct blood pressure: a comparative clinical study during deliberate hypotension. Can Anaesth Soc J. 1986;33(3 Pt 1):300–307. | ||
Ribezzo S, Spina E, di Bartolomeo S, Sanson G. Noninvasive techniques for blood pressure measurement are not a reliable alternative to direct measurement: a randomized crossover trial in ICU. Scientific World J. 2014;2014:353628. | ||
Tsushima M, Kyotani S, Nakano S, et al. [Diagnosis of early stages of atherosis and sclerosis of carotid artery and risk factors. The pulse wave velocity(PWV), ankle-brachial index(ABI), and the intima-media complex thickness(IMT) in inhabitants]. JJCDP. 2002;37:117–124. Japanese. | ||
Takaki A, Ogawa H, Wakeyama T, et al. Cardio-ankle vascular index is superior to brachial-ankle pulse wave velocity as an index of arterial stiffness. Hypertens Res. 2008;31(7):1347–1355. | ||
Ichihara A, Yamashita N, Takemitsu T, et al. Cardio-ankle vascular index and ankle pulse wave velocity as a marker of arterial fibrosis in kidney failure treated by hemodialysis. Am J Kidney Dis. 2008;52(5):947–955. | ||
Endes S, Caviezel S, Dratva J, et al. Reproducibility of oscillometrically measured arterial stiffness indices: Results of the SAPALDIA 3 cohort study. Scand J Clin Lab Invest. 2015;75(2):170–176. | ||
Miyoshi T, Ito H, Horinaka S, Shirai K, Higaki J, Orimio H. Protocol for Evaluating the Cardio-Ankle Vascular Index to Predict Cardiovascular Events in Japan: A Prospective Multicenter Cohort Study. Pulse. 2017;4(Suppl 1):11–16. | ||
García-Ortiz L, Recio-Rodríguez JI, Mora-Simón S, et al. Vascular structure and function and their relationship with health-related quality of life in the MARK study. BMC Cardiovasc Disord. 2016;16:95. | ||
Gomez-Sanchez L, Garcia-Ortiz L, Patino-Alonso MC, et al. The Association Between the Cardio-ankle Vascular Index and Other Parameters of Vascular Structure and Function in Caucasian Adults: MARK Study. J Atheroscler Thromb. 2015;22(9):901–911. | ||
Topouchian J, Labat C, Gautier S, et al. Effects of metabolic syndrome on arterial function in different age groups: the Advanced Approach to Arterial Stiffness study. J Hypertens. 2018;36(4):824–833. | ||
Fukui T, Abe Y, Yasuda T, Yoshitaka S. [Comparison of the Measurements of Brachial-Ankle Pulse Wave Velocity (baPWV) and Cardio-ankle Vascular Index (CAVI) as an Examination for Aortic Stiffness]. Ningen Dock. 2008;23:70–76. Japanese. | ||
Ato D, Sawayama T. Factors associated with high brachial-ankle pulse wave velocity in non-hypertensive and appropriately treated hypertensive patients with atherosclerotic risk factors. Vasc Health Risk Manag. 2017;13:383–392. | ||
Kim T, Lee CS, Lee SD, et al. Impacts of comorbidities on the association between arterial stiffness and obstructive sleep apnea in the elderly. Respiration. 2015;89(4):304–311. | ||
Zheng Y, Li Z, Shu H, Liu M, Chen Z, Huang J. Relationship between sum of the four limbs’ pulse pressure and brachial-ankle pulse wave velocity and atherosclerosis risk factors in Chinese adults. Biomed Res Int. 2015;2015:434516–7. |
Supplementary material
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.