Back to Journals » International Journal of Women's Health » Volume 12
Bowel Endometriosis: Current Perspectives on Diagnosis and Treatment
Authors Habib N, Centini G , Lazzeri L , Amoruso N, El Khoury L, Zupi E, Afors K
Received 6 August 2019
Accepted for publication 29 November 2019
Published 29 January 2020 Volume 2020:12 Pages 35—47
DOI https://doi.org/10.2147/IJWH.S190326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Nassir Habib,1 Gabriele Centini,2 Lucia Lazzeri,2 Nicola Amoruso,2 Lionel El Khoury,3 Errico Zupi,2 Karolina Afors4
1Department of Obstetrics and Gynaecology, Beaujon Hospital-University of Paris, Clichy Cedex 92110, France; 2Department of Molecular and Developmental Medicine, University of Siena, Ospedale Santa Maria alle Scotte, Siena 53100, Italy; 3Department of Colorectal Surgery-Delafontaine Hospital, Saint Denis 93200, France; 4Department of Obstetrics and Gynaecology, Whittington Hospital, London, UK
Correspondence: Karolina Afors
Email [email protected]
Abstract: Endometriosis is a chronic condition primarily affecting young women of reproductive age. Although some women with bowel endometriosis may be asymptomatic patients typically report a myriad of symptoms such as alteration in bowel habits (constipation/diarrhoea) dyschezia, dysmenorrhoea and dyspareunia in addition to infertility. To date, there are no clear guidelines on the evaluation of patients with suspected bowel endometriosis. Several techniques have been proposed including transvaginal and/or transrectal ultrasonography, magnetic resonance imaging, and double-contrast barium enema. These different imaging modalities provide greater information regarding presence, location and extent of endometriosis ensuring patients are adequately informed whilst also optimizing preoperative planning. In cases where surgical management is indicated, surgery should be performed by experienced surgeons, in centres with access to multidisciplinary care. Treatment should be tailored according to patient symptoms and wishes with a view to excising as much disease as possible, whilst at the same time preserving organ function. In this review article current perspectives on diagnosis and management of bowel endometriosis are discussed.
Keywords: endometriosis, bowel endometriosis, segmental resection, recurrence, infertility
Background
Endometriosis is a common benign gynaecological disease defined by the presence of endometrium glands outside the uterine cavity. It is frequently diagnosed in the third decade of life, affecting 10–12% women of reproductive age.1 The gold standard for diagnosis of endometriosis is visual inspection by laparoscopy. An experienced surgeon, familiar with the disease process and its varying clinical presentation, should perform the laparoscopy so as to safeguard that cases are not missed or overlooked. This ensures an accurate diagnosis is made in a timely manner with the best opportunity for a positive health outcome.2–4
Deep endometriosis (DE) is defined as subperitoneal invasion by lesions exceeding 5 mm in depth. Disease involving the bowel can be associated with severe pain.5–7 DE can be found at multiple locations within the pelvis, but more frequently remain localized to the posterior compartment where it can involve the ureters, the torus uterinum, the uterosacral ligaments, the bowel, and the vaginal wall.
Bowel endometriosis typically presents as a single nodule, with a diameter larger than 1 cm, commonly infiltrating the muscularis of the bowel and the surrounding structures.8–10 Bowel involvement accounts for 5% to 12% of the women presenting with the disease, with the rectum and sigmoid involved in up to 90% of all intestinal lesions.4,11
Symptoms of bowel endometriosis can be non-specific consisting of dysmenorrhoea and dyspareunia. More specific bowel-related symptoms such as diarrhea, constipation, dyschezia and rarely bowel obstruction depend on disease localization, size of nodule and depth of involvement of the bowel wall.12 However, any pelvic symptoms, specifically cyclical in nature should raise the suspicion of endometriosis. In some instances of bowel endometriosis, women remain asymptomatic, with 5% of the larger lesions remaining symptom free and in whom surgical resection is probably not indicated.4
Symptoms may improve with medical treatment; however, deep infiltrating lesions are less likely to resolve. There remains a high chance of symptom relapse following cessation of medical management. Equally, prolonged medical treatment can be associated with side effects and is not suitable for all, in particular, those wishing to conceive.13
The natural progression of endometriosis has never been well defined it is not clearly understood how endometriosis progresses. There is no clear evidence of typical small lesions evolving into cystic or deep infiltrating lesions, but logically, large nodules must have developed over time.14 Equally, it is not unusual to observe small lesions that regress.15 Clinically, intestinal deep infiltrating endometriosis does not appear to progress rapidly if surgical treatment is delayed, and regarding recurrence, it is unclear whether this constitutes disease recurrence, or in fact, represents residual lesions following incomplete resection.2,16 Typically, deep endometriosis lesions progress slowly although rapid progression can occur.
More recently alternative hypotheses have been suggested such as deregulation of genes, which can result in aberrant placement of stem cells. Equally immune cells, adhesion molecules, and pro-inflammatory cytokines can alter the peritoneal environment leading to adhesion and proliferation of ectopic endometrial cells. In addition, studies have demonstrated a possible role of oxidative stress and reactive oxygen species which appear to trigger an inflammatory process that may contribute to the pathogenesis of endometriosis.1,17 DE is an estrogen-dependent disease; therefore, two phenomena can lead to spontaneous regression or symptom improvement, pregnancy, and menopause. Nevertheless, post-menopausal deep endometriosis does exist and pregnancy can be complicated by the presence of endometriosis.18
DE symptoms are not only related to the lesion itself but also to the associated fibrotic reaction causing pelvic adhesions and anatomical distortion that can persist even after the lesion has become inactive (Figure 1).
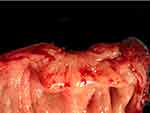 |
Figure 1 Fibrosis of the muscular layer of the anterior wall of the sigmoid caused by a DE nodule. |
The preoperative workup including physical examination and imaging, mainly high resolution transvaginal ultrasound and/or MRI, is mandatory to define the extent of the lesion, depth of invasion of the muscularis, the circumference involved, the number of lesions and the distance from the anal sphincter to guide surgical decision-making and provide appropriate counseling to the patient.19,20 Surgical treatment can be associated with considerable morbidity of which the patient should be made aware.
Unfortunately, to date, there exist no guidelines with high level of evidence specifying which lesions should be operated on, when this is indicated and which standardized surgical technique is recommended.
Counseling
DE is a chronic disease affecting young women of childbearing age. Affected patients need to learn to cope with both the physical and psychological impact of this disease throughout their lifetime, whilst also pursuing the most appropriate management. Studies have demonstrated that patients with endometriosis often present with significant psychopathological comorbidities such as anxiety and depression, which can often amplify the severity of pain experienced.21 Psychological assessment should be considered in those patients deemed at risk in order to provide adequate psychological support.22
Treatment should be individualized for each patient, and management tailored according to patient’s needs and medical history, taking into consideration specific demographics such as age, symptoms, pregnancy desire, and previous medical or surgical treatment. When counseling a patient for the first time, symptom evaluation plays a pivotal role in guiding clinical decision-making.
Bowel endometriosis is generally associated with involvement of the uterosacral ligaments with or without involvement of the torus uterinum and vaginal wall. In its initial stages it is commonly associated with deep dyspareunia; however, as the disease progresses symptoms can deteriorate and in more severe cases bowel obstruction can occur (Figure 2).
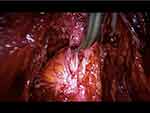 |
Figure 2 Shaving of a rectosigmoid nodule involving the uterosacral ligaments and retracting the bowel. |
The chronic nature of the disease exposes patients to symptom relapse and reintervention and in the absence of adjuvant medical treatment can reach up to 50% in 5 years.11 Decision for surgical intervention should be considered cautiously in asymptomatic patients or in whom symptomatic DIE is adequately controlled with medical management due to the associated peri-operative risks and possibility of disease recurrence in the future. Symptoms can be controlled initially with medication unless symptoms of bowel obstruction occur, or pain becomes intolerable.1,23
Preoperative clinical and imaging assessment is essential to stage the disease accurately obtaining precise information regarding disease progression to correctly guide decision-making regarding the pros and cons of both medical and surgical management. Taking into account the complications associated with surgery.
Surgical treatment is effective in improving pain especially in instances of bowel obstruction.11,24–26 However, it can be associated with significant complications, which can be as high as 22% in case of multi-organ involvement. The radicality of the surgery is associated with lower recurrence, but a higher risk of complications.27
Complete excision of disease, whilst preserving organ function remains the definitive objective; however, this is not always feasible and a trend towards individualized and less aggressive surgery is emerging.28,29
Patients must be informed preoperatively about possible complications and the potential need for a protective stoma. A stoma is normally recommended in cases of low rectal resection for nodules located at less than 5 cm from the anal margin, and/or when more than one lumen is opened with suture lines lying in close proximity to one another, potentially increasing the risk of fistula formation. A stoma is required in 10% to 14% of the cases undergoing bowel resection for deep infiltrating bowel endometriosis.30
A tailored approach should take into consideration patients wishes and specific demographics, notably patient’s age, severity of symptoms and desire for pregnancy. Surgery should be carefully and appropriately timed as laparoscopic eradication of the disease is associated with a higher pregnancy rate within 2 years.
Although surgery is indicated when pain is the main symptom, assisted reproductive technology should be considered if infertility is the main patient objective.31 Clear information regarding patient’s ovarian reserve with a complete assessment of the couple’s fertility potential should be considered preoperatively in order to optimize chances of conceiving following surgical management.32
Clinical Assessment
A detailed patient history taken by an experienced clinician is crucial when dealing with endometriosis. The most frequently described symptom is that of pain, which is typically cyclical and chronic in nature. Bowel DE generally produces cyclical pain and specific bowel-related symptoms such as dyschezia, constipation, and rarely rectal bleeding, in addition to generalized symptoms such as deep dyspareunia and dysmenorrhea.33,34
Despite there being no clear correlation between the extent of the disease and severity of pain, symptoms suggestive of posterior compartment disease involvement such as deep dyspareunia should be considered a red flag signal for further evaluation by examination.35
Pelvic bimanual examination following a detailed patient history is a low cost and effective diagnostic tool in detecting deep infiltrating endometriosis. Findings such as a fixed retroverted uterus, fibrotic nodule of the parametrium, uterosacral ligaments, anterior vesicovaginal septum, or more often, the torus uterinum and rectovaginal septum can be detected. The pain elicited on palpation allows the clinician to assess clinically the extent of endometriotic infiltration and localization of the disease.
Currently, there are no reliable non-invasive biomarker tests available in clinical practice that diagnose endometriosis and using any non-invasive tests should only be undertaken in a research setting.36,37
Imaging
Accurate and timely diagnosis of pelvic endometriosis is crucial to guarantee patients are given the best treatment strategies. The most commonly used imaging techniques to identify and characterize endometriosis lesions are Transvaginal sonography (TVS) and magnetic resonance imaging (MRI).
Nowadays, TVS is well accepted and widely available with a low relative cost that should be considered as a first-line imaging technique providing detailed dynamic images of the pelvis with minimal discomfort for the patients.
MRI is generally used as a second-line diagnostic tool in the evaluation of DE and the extent of disease in particular focusing on specific organ involvement and depth of infiltration. Other diagnostic procedures such as rectal sonography, barium enema, or computed tomography urography may be indicated to assess specific organ function following an initial diagnosis of bowel or ureteral stenosis.
Transvaginal Ultrasound
The rectum and recto-sigmoid are the most frequently involved sites of bowel endometriosis accounting for 70% to 88% of all cases. Implants of endometrial gland and stroma typically involve the bowel wall from the serosa inwards. Endometriosis deposits can extend to involve the muscularis propria and submucosa only rarely involving the mucosa itself. Lesions vary from microscopic foci to larger nodules and are often surrounded by smooth muscle hyperplasia and fibrosis.38
TVS enables all of the layers of the bowel to be clearly identified. The rectal serosa appears as a thin, hypoechogenic line covered by the rectal submucosa and mucosa which is visualized as a hyperechogenic rim covering the rectal smooth muscle layer.39 Bowel endometriosis nodules appear on ultrasound as linear or nodular retroperitoneal hypoechoic thickening of the muscular layer with irregular borders with few vessels seen on power Doppler penetrating into the intestinal wall distorting its normal structure (Figure 3).40–43 The three diameters of each lesion should be recorded. A 3D volume calculation permits for accurate measurement and evaluation of the DIE lesion in different planes.
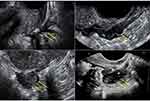 |
Figure 3 Ultrasound appearance of different nodules of deep infiltrating endometriosis of the bowel. |
During TVS the location of bowel lesions should also be evaluated in terms of distance from the anal verge. The uterosacral ligaments can be used as a reference point to discriminate between lower and upper rectal lesions. Utero-sacral ligaments generally appear as a virtual crossing with the insertion of these ligaments on the cervix delineating a plane under the peritoneum of the pouch of Douglas (POD), corresponding laterally to the parametria and medially to the recto-vaginal septum.
In case of low rectal lesions, the distance from the anus can be assessed by transrectal sonography positioning the tip of the probe on the lowest aspect of the endometriotic lesion and measuring the length of the probe.
In addition, the number of lesions should also be evaluated. Multifocal lesions are defined as the presence of deep lesions within 2 cm of the main lesions or multiple endometriotic lesions affecting the same segment. Whilst, multicentric lesions are defined as satellite nodules located greater than 2 cm from the main lesion.44,45 Both disease localization and number of lesions play a pivotal role in the preoperative workup to assess risk of complication and to provide adequate counseling for the patient.
Previous studies showed that transvaginal sonography, performed by experienced sonographers, reported high sensitivity and specificity for the prediction of bowel endometriosis.39,46,47 However, TVS has low accuracy in diagnosing infiltration of the mucosal layer.38 Transrectal ultrasound is a valuable tool for detecting rectal endometriosis specifically endometriotic infiltration of the muscularis layer it is, however, less accurate in assessing submucosal and mucosal layer involvement.48
Neither transvaginal nor transrectal sonography assists surgeons in deciding whether to perform segmental or discoid resection; however, it can provide important information to predict complication rates and to plan appropriate surgical management.
To further evaluate deep infiltrating bowel lesions, TVS can be used with contrast medium such as in rectal water-contrast transvaginal ultrasonography (RWC-TVS).
RWC-TVS is performed using a flexible 25 Fr catheter inserted into the rectal lumen up to 20 cm from the anus. Saline solution is then instilled in the rubber balloon of the catheter under ultrasound control. This water contrast allows the capture of high-definition images of the rectal wall and its layers, allowing a dynamic evaluation of endometriotic lesion and rectal stenosis. Bergamini et al demonstrated that this method had comparable accuracy to transrectal sonography and barium enema in the preoperative assessment of low intestinal endometriosis.49
MRI
MRI is widely used for imaging of endometriotic lesions owing to its high diagnostic accuracy. MRI is a highly efficient and accurate technique, with a sensitivity of 88%, specificity of 98%, PPV of 95%, and NPV of 96% coupled with a diagnostic accuracy of 96%.50 The presence of blood (iron) inside the nodule undoubtedly aids in identifying disease localization.
Bowel lesions are mainly fibromuscular, with occasional foci of T1- and T2-weighted hyperintensity. The use of contrast media allows for improved definition and distinction between the lesion itself and normal bowel wall. Diagnostic criteria for rectal invasion on MRI include colorectal wall thickening with anterior triangular attraction of the rectum toward the torus uterinum or asymmetric wall thickening of the lower third of sigmoid colon. These diagnostic findings are similar to those obtained using TVS; however, MRI is better suited to discriminate between multifocal lesions and for identifying higher lesions, located above the rectosigmoid junction, which cannot be visualized by TVS due to the limited field-of-vision.
The challenge of MRI, thanks to its multiplanar capabilities and outstanding contrast resolution, is to evaluate the entire pelvis and abdomen to accurately detect associated lesions.51 Some conditions, however, can reduce the quality and sensitivity of magnetic resonance images. One of the most important conditions is bowel peristalsis, especially in women undergoing MRI to determine the presence of DIE of the intestine. Peristalsis blurs the bowel contours and adjacent organs and may simulate bowel thickening or mask small lesions.
Double-Contrast Barium Enema
Double-contrast barium enema (DCBE) has shown promising results when carried out by expert radiologists in the preoperative evaluation of women with clinically suspected intestinal DE. The technique requires a low-residue diet for 1 day before the examination, administration of drugs to empty the colon, and exposure to X-rays. The presence of DE is diagnosed on DCBE when the bowel lumen is narrowed at any level from the sigmoid to the anus (extrinsic mass effect) in association with crenulation of the mucosa, spiculation of contour, or both causing lumen stenosis. The accuracy was reported at nearly 90% (sensitivity 88% and specificity 93%), with a positive predictive value of 97%.52
However, DCBE does not allow direct visualization of the lesion itself and is generally used to evaluate the degree of stenosis after TVS or MRI raises suspicion and to aid surgical decision-making on possible bowel resection.
Surgical Treatment
The surgical treatment of deep infiltrating endometriosis is a complex procedure and requires wide knowledge of the disease process and should be managed in a multidisciplinary team led by a specialist gynecologist.
Laparoscopy is the ideal tool for this kind of surgery. It allows precision and complete assessment of the peritoneal cavity, and when indicated, radical yet economical treatment conserving organ function is possible in the hands of an expert surgeon.
The rectosigmoid is the most frequent part of the bowel affected by deep infiltrating endometriosis. Over the years different surgical techniques have been described in the literature ranging from rectal shaving and discoid resection to segmental resection.
The final decision as to whether or not to perform a bowel resection is typically only made during surgery; however, complete preoperative staging should be carried out to ensure a balanced discussion, weighing up the risk of complications and the outcomes allowing patients to make an informed decision.
When surgery is planned, the optimum goal is a complete resection of all visible lesions whilst preserving pelvic organ function. The greater the radicality towards the disease the higher the risk of complications and in these instances the patient’s wishes should be respected and a more conservative approach adopted.27,53–56 All surgical techniques, therefore, should be individualized and attempts made to preserve and restore organ function, as patients are likely to require further surgical intervention in the future.
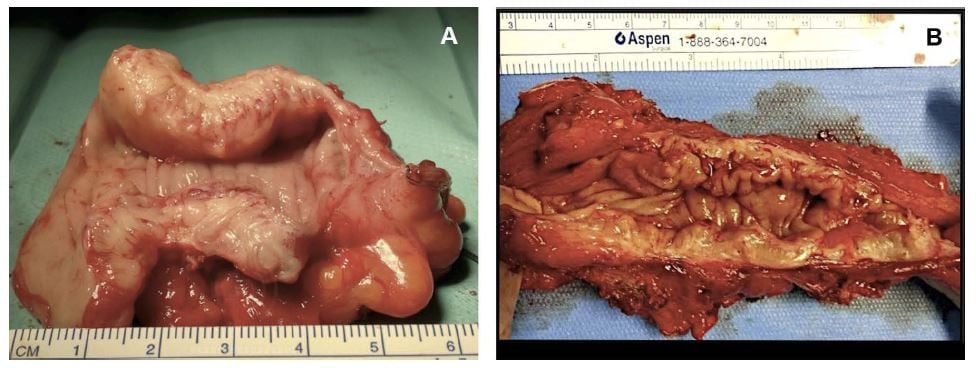
Careful evaluation for a bowel resection should be conducted in cases of obstructive bowel symptoms. Stenosis of greater than 50%, multifocal lesions or involvement of more than 50% of the bowel circumference are indication for bowel resection.57 The localization of the lesion also plays an important role as the lower the level of disease the greater the risk of complications. A lesion of the sigmoid can be resected without significant sequelae and negating the need for a stoma; however, a lesion located lower than 5 cm from the anal margin should be avoided (Figure 4).
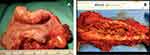 |
Figure 4 (A) Economical sigmoid resection limited to the nodule obstructing the bowel lumen; (B) wide bowel resection for multiple lesions. |
DE is a benign disease and should be treated accordingly. It is preferred practice to preserve vascularization and blood supply to the bowel opting for trans-mesorectal excision rather than a total-mesorectal resection.55,58 Segmental resection is associated with positive results in terms of symptoms relief and quality of life, with low recurrence rate; however, it is associated with a higher risk of complications (up to 18%) such as anastomosis dehiscence, rectovaginal fistulae, peritonitis, and pelvic abscess.27,59
A stoma should be considered when the anastomosis is 5 cm or less from the anal margin, if the anastomosis is defective, or if two lumens are sutured and the suturing lines are facing one another, in order to decrease the risk of anastomosis dehiscence and fistulae.57
Rectal shaving may be considered for nodules less than 3 cm in diameter, with a depth of infiltration of less than 7 mm, and less than 50% of the bowel circumference involvement.33
Being economical allows bowel vascularization and innervation to be preserved, and as a consequent maintain bowel function.57,60 It is associated with lower complications rate in terms of fistulae occurrence, but a higher risk of incomplete resection with persistent disease in around 50% of the cases.1,33,59
Vercellini et al reported a recurrence rate of 20% at 1-year follow-up when using this technique, with the need for adjuvant therapy in around 50% of the patients because of symptoms of recurrent pain. Furthermore, additional surgery was required in 25% of the patients within 5 years.61
Discoid anterior rectal resection is an alternative to segmental resection when the shaving is incomplete because of deep infiltration of the bowel wall or in case of awkward localization for bowel resection (Figure 1). The risk of recurrence after this technique has been reported as between 1.8% and 8% with a risk of complication of approximately 3.62,63
Afors et al compared clinical outcomes and recurrence rates comparing three different surgical techniques (shaving, discoid and segmental resection). This study demonstrated that in the shaving group there was a significantly higher rate of medium-term symptom recurrence of dysmenorrhea and dyspareunia. The shaving group was also associated with a significantly higher rate of reintervention for recurrent DE lesions compared with the segmental resection group. Interestingly postoperative complication rates remained similar regardless of whether shaving, discoid or segmental resection was performed.63
Also, Minelli et al compared efficacy, risk of complications and rate of reintervention amongst non-radical surgery, radical without colorectal surgery and radical surgery with bowel treatment in 1363 women with stage IV endometriosis. Colorectal surgery was associated with significantly increased intraoperative blood loss and operating time, an overall nearly threefold higher risk of intraoperative complications, with a significantly higher risk of complications within the first week and in the first month after surgery.64
The main risk factors for recurrent disease reported in the literature suggest incomplete surgery; however, Busacca et al defined this as persistent disease and not recurrence, young age, stage III-IV disease, and delay in achieving pregnancy for those patients wishing to conceive.65,66 For those patients wishing to fall pregnant following surgery, accurate counseling must be offered with the recommendation given to conceive as soon as possible. For the patient without a desire to conceive, hormonal treatment is advised, in order to decrease the risk of relapse.54,65–67
Although surgical techniques attempt to excise all macroscopic appearance of endometriosis, it is not a guarantee of disease-free margins even if large segment of bowel are resected. Histological analysis of colorectal resection specimens demonstrated positive margins in up to 19% of the cases. Interestingly, no correlation was found between the presence of positive margins and worsening symptoms or rate of recurrence.68,69
Equally, there is no evidence to suggest that residual lesions can become symptomatic; however, perhaps medical treatment following surgery can prevent the possible progression of endometriosis.70
Medical Therapy
Medical treatment should be considered first-line therapy in all patients with bowel endometriosis who are not surgical candidates to control endometriosis-related symptoms and to avoid repeated surgery. Women opting for medical therapy should be counseled and advised that treatment often needs to be continued for a long period, until pregnancy desire or menopause. However, equally, it should be considered as a temporary solution for the management of pain symptoms and can not eradicate the disease.71
DE is composed of three components endometrial, muscle and fibrotic tissue. Medical therapy has an effect on ectopic endometrial mucosa and the smooth muscle fibers infiltrated by it. Hormonal treatment primarily acts suppressing the metabolic activity of ectopic endometrium and consequently reducing chronic inflammation, which is responsible for secondary fibrosis and activation of muscle tissue. Hormonal therapy may also directly affect fibrotic tissue formation, and it has been suggested that progestogens can alter fibrosis remodeling, due to their anti-inflammatory properties.71
Medical therapy has also been shown to improve symptoms of pain by resolving cyclical inflammation and the effects of progestogens on progesterone receptors in ectopic tissue. The effect on constipation is small and likely related to a decrease in nodule size with improvement of stenosis of the affected bowel tract. It is known, however, that constipation can originate from the presence of nodules and fibrosis but also due to altered nerve innervation, which cannot be restored by surgery. It is therefore important to counsel patients appropriately advising that there are no guarantees that symptoms of constipation will improve regardless of whether a more aggressive approach is adopted.1
Nowadays, there are several medications available to manage bowel endometriosis that actually aim to reduce circulating hormones including inducing a pseudo-menopause or pseudo-pregnancy state.
First-line therapy comprises long-term use of oral contraceptive which is associated with significant reduction in pain and nodule size with good patient satisfaction.66,72
Vercellini et al compared a monophasic estrogen–progesterone combination (ethinyl-E2 0.01 mg + cyproterone acetate 3 mg) and norethindrone acetate 2.5 mg/d and found no statistically significant difference in pain relief and satisfaction. Known side effects are weight gain, decreased libido, bloating, vaginal dryness, headache and mood changes; in addition to, glucocorticoid and antimineralocorticoid activities of estrogens that can increase cardiovascular risk factors (thromboembolism and hypertension).73
To maximize the effect of OC nowadays it has been well established that they should be administered continuously or in a tricycling pattern to optimize symptom control and reduce recurrence. Seracchioli et al reported a significant lower recurrence rate following endometrioma excision comparing cyclic (14%) versus continuous (8%) administration of OC.74
Progestins are also used in long-term continuous therapy to induce endometrial atrophy. Progestins are generally well tolerated and should be proposed as a possible alternative.75 There most common adverse effects (1–10% of population) are bleeding problems, headache, altered or depressed mood, breast discomfort, abdominal pain, acne, and weight gain.
Dienogest is the only progestogen approved in Europe, Japan, Australia, and Singapore for long-term treatment of endometriosis and its use has been attributed to reduction in pain, nodule size and quality of life improvement.76–78 A recent prospective Korean study of 40 women of reproductive-age highlights a possible association between long-term dienogest treatment (2 mg/d for at least 12 months) and significant bone demineralization, that occurs in 75% of the patients occurring mainly in the first 6 months of therapy.79
Norethindrone acetate (NETA) 2.5 mg/d has also been proposed as a possible alternative, showing significant improvement in pain and cyclical symptoms related to menses; it shares with dienogest similar adverse effects, except its effect on bone, and has a more favorable cost analysis.80
Progestins can also be administered trans-vaginally to reduce associated side-effects as has been proposed for other therapies.81
Levonorgestrel-releasing intrauterine devices have also been considered as treatment for symptomatic endometriosis but studies have demonstrated conflicting results. Fedele et al found improvements of pain and reduction in nodule size in women affected by rectovaginal endometriosis, in contrast, other studies have shown limited improvement in symptoms, in particular dyspareunia. They also appeared to be ineffective in preventing endometrioma development or recurrence. The most common side effects are menstrual abnormalities, amenorrhea, dysmenorrhea, oligomenorrhea, headaches, and acne.82–84
GnRH agonists act by prolonged activation of GnRH receptors responsible for desensitization and consequently suppression of gonadotropin secretion, inducing pseudo-menopausal state. Experts studied leuprolide acetate 3.75 mg monthly depot formulation, which was associated with marked improvement in moderate to severe pain symptoms but with rapid recurrence following drug discontinuation.85 Furthermore, this form of treatment is associated with major side effects due to an induced hypogonadism state, such as hot flushes, decreased libido, breakthrough bleeding, vaginal dryness, irritability, fatigue, headache, depression, changes in skin texture, and bone mineral depletion and as such is not recommended for greater than 6 to 12 months.85
Bowel Endometriosis and Infertility
It is recognized that infertility and pelvic pain are two characteristic manifestations of endometriosis. In particular, 30% of the patients affected by endometriosis are infertile and 30% to 40% infertile women are affected by endometriosis.86
Ectopic implants of endometrial cells produce intraperitoneal menstruation and create a state of inflammation with biochemical alterations of peritoneal fluid; this factor is responsible of poor oocyte quality, reduced sperm motility and impaired interaction between sperm and oocyte.
A study of Jørgensen et al, conducted on 94 women undergoing diagnostic laparoscopy for infertility, supports this hypothesis: of 48 cytokines tested in peritoneal fluid, four of them, MCP-1, IL-8, HGF, and SCGF-ß, exhibited significantly higher concentrations in patients with endometriosis compared to those patients without disease, while IL-13 had a lower concentration in patients with endometriosis.87 Other hypotheses have been proposed: endometrial abnormalities responsible for implantation failure altered interaction between fimbria and oocyte and following utero‐tubal transportation of the embryo, peritoneal mesothelium damage creating adhesion sites for endometrial cells and the presence of adhesions distorting pelvic anatomy.88–91
It is difficult to clearly define how intestinal endometriosis alone impacts on infertility as posterior DIE often co-exists with the presence of other ectopic implants, such as adenomyosis, anterior DIE, peritoneal endometriosis, and endometriomas. Furthermore, studies conducted have various limitations: the majority of them are retrospective; analyze non-homogeneous groups, with different extension of lesions, presence of concurrent diseases affecting fertility and lastly different surgical and medical strategies.92
Harb et al showed a significant reduction in pregnancy rates and implantation rates in women with severe endometriosis (Stage III/IV) who underwent assisted reproductive technology (ART) treatment.93
There is no agreed consensus regarding surgical management of bowel endometriosis in cases of concurrent infertility. Nor is it clear which specific technique should be adopted over the other. A Cochrane reviewfrom2014 showed that laparoscopic surgery in cases of mild and moderate disease was associated with an increased live birth or ongoing pregnancy rate (OR 1.94) and increased clinical pregnancy rate (OR 1.89) compared with diagnostic laparoscopy alone.94
Adamson and Pasta have demonstrated that in women affected by severe DIE an aggressive surgical strategy is associated with an increased spontaneous pregnancy rate with a differential gain in pregnancy of 39%95 A review of Darai et al identified a potential benefit of surgery on fertility outcomes for women with colorectal endometriosis; in detail, spontaneous pregnancy rate after surgery was 31.4% whereas MAR-associated pregnancy rate following bowel surgery was 19.8%, underlining the relatively limited contribution of MAR in this specific population.96
A symbolic study was conducted by Stepniewka et al in 2009 on 155 women affected by DIE associated with infertility for at least 1 year undergoing surgery; they concluded that bowel endometriosis had a negative effect on the capacity to conceive.97 The reproductive outcomes and time required to conceive were better if bowel resection was performed and there was a lower risk of recurrence of disease.
Darai et al emphasized the importance of laparoscopy when performing endometriosis surgery for infertility. They reported a spontaneous pregnancy rate of 13.3% and 0% in the laparoscopic and laparotomic groups, respectively.98 In this study, the cumulative pregnancy rates had a plateau after 24 months, emphasizing the importance of timed surgery prior to conception. In line with the previous studies, Centini et al reported a spontaneous pregnancy rate of 38% following laparoscopic excision with a plateau after 18 months.99
Ballester et al studied the influence of ICSI-IVF in women affected by bowel endometriosis associated with infertility. Their prospective study on 75 patients showed a beneficial impact of IVF on pregnancy rates with percentage of conception that rose after every cycle of ART (29.3%, 52.9%, and 68.6%). Adenomyosis seemed to be the main negative factor infertility outcomes in ICSI-IVF: pregnancy rates in patients with colorectal endometriosis with or without adenomyosis were 19% and 82.4%, respectively; also, age greater than 35 and AMH serum levels lower than 2 ng/mL were associated with significantly lower pregnancy rates.32
Barri et al compared IVF and surgery to treat infertility associated with endometriosis; they found the combined strategy of surgery followed by IVF, in women who did not conceive after 1 year of spontaneous attempts post-surgery, had the highest chance of conception (65.8%) when compared with surgery (54.2%) or IVF alone (32.2%).100
IVF should be proposed as the first strategy after surgery only when patients have additional infertility factors. In all the other cases, ART should be recommended to infertile patients who fail to conceive spontaneously within 12 months after surgery for patients less than 35 years old and within only 6 months for older women.
Lastly, it is important to recognise that endometriosis seems to have a negative impact not only on women’s ability to conceive but also on pregnancy outcomes. A study by Exacoustos et al, conducted on 101 women who became pregnant with a residual DIE nodule of ≥2 cm following previous surgery for endometriosis showed a significantly higher risk of hypertension, placental abruption, placenta praevia, preterm birth, caesarean section, hemoperitoneum, and postpartum hysterectomy.101 Similarly, Nirgianakis et al noted that even following surgical excision of DIE the risk of pregnancy complications such as placenta praevia, gestational hypertonia, and intrauterine growth restriction remained higher compared to those women without endometriosis.102
Conclusion
In conclusion, deep infiltrating bowel endometriosis is a challenging condition significantly affecting not only quality of life but also future fertility outcomes in young women. Unfortunately, the literature lacks high-level evidence in order to draw any clear conclusions mainly due to the heterogeneous data collected. Surgery is clearly indicated for symptomatic painful disease, and in the hands of a specialist is associated with positive outcomes in terms of symptom relief and quality of life. Minimal invasive surgery should be the preferred option of choice with different surgical techniques utilized for nodule excision, ranging from more conservative excision by means of rectal shaving to discoid resection or segmental bowel resection. The choice of technique used is based on the experience of the surgeon and the characteristics of the lesion taking into account the size, depth, circumference of bowel wall infiltration, number of lesions and the distance from the anal margin. These characteristics must be defined precisely with an accurate preoperative work-up.
Whilst less aggressive surgery is associated with higher recurrence rates and reduced fertility rates, surgery, for DIE is complex and prone to complications. As such surgical management of bowel endometriosis should be performed in specialized centres by experienced surgeons with the appropriate skill set. Care should be individualized according to disease severity whilst also safeguarding patient’s requests and wishes and improving painful symptoms and health-related quality of life for women.
Patients need not choose between either medical or surgical management alone, but consider a combined approach based on prolonged medical therapy with occasional surgical treatment as required. The rationale for medical therapy prior to surgery is to determine whether this alone can improve patient’s symptoms, potentially negating the need for surgical intervention. Medical treatment can equally be reinstated following surgery to help reduce disease recurrence depending on each woman’s individual desire for pregnancy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alabiso G, Alio L, Arena S, et al. How to manage bowel endometriosis: the ETIC approach. J Minim Invasive Gynecol. 2015;22(4):517–529. doi:10.1016/j.jmig.2015.01.021
2. Koh CE, Juszczyk K, Cooper MJW, Solomon MJ. Management of deeply infiltrating endometriosis involving the rectum. Dis Colon Rectum. 2012;55(9):925–931. doi:10.1097/DCR.0b013e31825f3092
3. Darai E, Bazot M, Rouzier R, Houry S, Dubernard G. Outcome of laparoscopic colorectal resection for endometriosis. Curr Opin Obstet Gynecol. 2007;19(4):308–313. doi:10.1097/GCO.0b013e328216f6bc
4. Roman H, Ness J, Suciu N, et al. Are digestive symptoms in women presenting with pelvic endometriosis specific to lesion localizations? A preliminary prospective study. Hum Reprod. 2012;27(12):3440–3449. doi:10.1093/humrep/des322
5. Meuleman C, Tomassetti C, D’Hoore A, et al. Surgical treatment of deeply infiltrating endometriosis with colorectal involvement. Hum Reprod Update. 2011;17(3):311–326. doi:10.1093/humupd/dmq057
6. Nassif J, Trompoukis P, Barata S, Furtado A, Gabriel B, Wattiez A. Management of deep endometriosis. Reprod Biomed Online. 2011;23(1):25–33. doi:10.1016/j.rbmo.2010.08.012
7. Cornillie FJ, Oosterlynck D, Lauweryns JM, Koninckx PR. Deeply infiltrating pelvic endometriosis: histology and clinical significance. Fertil Steril. 1990;53(6):978–983. doi:10.1016/s0015-0282(16)53570-5
8. Anaf V, Simon P, El Nakadi I, et al. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod. 2002;17(7):1895–1900. doi:10.1093/humrep/17.7.1895
9. Anaf V, El Nakadi I, Simon P, et al. Preferential infiltration of large bowel endometriosis along the nerves of the colon. Hum Reprod. 2004;19(4):996–1002. doi:10.1093/humrep/deh150
10. Siquara De Sousa AC, Capek S, KK A, Spinner RJ. Neural involvement in endometriosis: review of anatomic distribution and mechanisms. Clin Anat. 2015;28(8):1029–1038. doi:10.1002/ca.22617
11. De Cicco C, Corona R, Schonman R, Mailova K, Ussia A, Koninckx P. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118(3):285–291. doi:10.1111/j.1471-0528.2010.02744.x
12. Koninckx PR, Meuleman C, Oosterlynck D, Cornillie FJ. Diagnosis of deep endometriosis by clinical examination during menstruation and plasma CA-125 concentration. Fertil Steril. 1996;65(2):280–287. doi:10.1016/S0015-0282(16)58086-8
13. Centini G, Lazzeri L, Dores D, et al. Chronic pelvic pain and quality of life in women with and without endometriosis. J Endometr Pelvic Pain Disord. 2013;5:1. doi:10.5301/je.5000148
14. Canis M, Bourdel N, Houlle C, Gremeau AS, Botchorishvili R, Matsuzaki S. Endometriosis may not be a chronic disease: an alternative theory offering more optimistic prospects for our patients. Fertil Steril. 2016;105(1):32–34. doi:10.1016/j.fertnstert.2015.09.009
15. Hans Evers JLH. Is adolescent endometriosis a progressive disease that needs to be diagnosed and treated? Hum Reprod. 2013;28(8):2023. doi:10.1093/humrep/det298
16. Ianieri MM, Mautone D, Ceccaroni M. Recurrence in deep infiltrating endometriosis: a systematic review of the literature. J Minim Invasive Gynecol. 2018;25(5):786–793. doi:10.1016/j.jmig.2017.12.025
17. Laganà AS, Vitale SG, Salmeri FM, et al. Unus pro omnibus, omnes pro uno: a novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med Hypotheses. 2017;103:10–20. doi:10.1016/j.mehy.2017.03.032
18. Alio L, Angioni S, Arena S, et al. Endometriosis: seeking optimal management in women approaching menopause. Climacteric. 2019;22(4):329–338. doi:10.1080/13697137.2018.1549213
19. Hudelist G, English J, Thomas AE, Tinelli A, Singer CF, Keckstein J. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37(3):257–263. doi:10.1002/uog.8858
20. Scardapane A, Lorusso F, Bettocchi S, et al. Deep pelvic endometriosis: accuracy of pelvic MRI completed by MR colonography. Radiol Med. 2013;118(2):323–338. doi:10.1007/s11547-012-0850-6
21. Vitale SG, La Rosa VL, Rapisarda AMC, Laganà AS. Impact of endometriosis on quality of life and psychological well-being. J Psychosom Obstet Gynecol. 2017;38(4):317–319. doi:10.1080/0167482X.2016.1244185
22. Laganà AS, La Rosa VL, Rapisarda AMC, et al. Anxiety and depression in patients with endometriosis: impact and management challenges. Int J Womens Health. 2017;9:323–330. doi:10.2147/IJWH.S119729
23. Vannuccini S, Reis FM, Coutinho LM, Lazzeri L, Centini G, Petraglia F. Surgical treatment of endometriosis: prognostic factors for better quality of life. Gynecol Endocrinol. 2019;1–5. doi:10.1080/09513590.2019.1616688
24. Dousset B, Leconte M, Borghese B, et al. Complete surgery for low rectal endometriosis: long-term results of a 100-case prospective study. Ann Surg. 2010;251(5):887–895. doi:10.1097/SLA.0b013e3181d9722d
25. Roman H, Bridoux V, Tuech JJ, et al. Bowel dysfunction before and after surgery for endometriosis. Am J Obstet Gynecol. 2013;209(6):524–530. doi:10.1016/j.ajog.2013.04.015
26. Dunselman GAJ, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412. doi:10.1093/humrep/det457
27. Fanfani F, Fagotti A, Gagliardi ML, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril. 2010;94(2):444–449. doi:10.1016/j.fertnstert.2009.03.066
28. Zupi E, Lazzeri L, Centini G. Deep endometriosis: less is better. J Endometr Pelvic Pain Disord. 2015;7:1. doi:10.5301/je.5000206
29. Afors K, Murtada R, Centini G, et al. Employing laparoscopic surgery for endometriosis. Women’s Heal. 2014;10:4. doi:10.2217/whe.14.28
30. Kondo W, Branco AW, Trippia CH, Ribeiro R, Zomer MT. Retrocervical deep infiltrating endometriotic lesions larger than thirty millimeters are associated with an increased rate of ureteral involvement. J Minim Invasive Gynecol. 2013;20(1):100–103. doi:10.1016/j.jmig.2012.09.012
31. Sun W, Stegmann BJ, Henne M, Catherino WH, Segars JH. A new approach to ovarian reserve testing. Fertil Steril. 2008;90(6):2196–2202. doi:10.1016/j.fertnstert.2007.10.080
32. Ballester M, d’Argent EM, Morcel K, Belaisch-Allart J, Nisolle M, Darai E. Cumulative pregnancy rate after ICSI-IVF in patients with colorectal endometriosis: results of a multicentre study. Hum Reprod. 2012;27(4):1043–1049. doi:10.1093/humrep/des012
33. Remorgida V, Ragni N, Ferrero S, Anserini P, Torelli P, Fulcheri E. How complete is full thickness disc resection of bowel endometriotic lesions? A prospective surgical and histological study. Hum Reprod. 2005;20(8):2317–2320. doi:10.1093/humrep/dei047
34. Ferrero S, Abbamonte LH, Anserini P, Remorgida V, Ragni N. Future perspectives in the medical treatment of endometriosis. Obstet Gynecol Surv. 2005;60(12):817–826. doi:10.1097/01.ogx.0000189153.87365.dc
35. Vercellini P, Frontino G, Pietropaolo G, Gattei U, Daguati R, Crosignani PG. Deep endometriosis: definition, pathogenesis, and clinical management. J Am Assoc Gynecol Laparosc. 2004;11(2):153–161. doi:10.1016/S1074-3804(05)60190-9
36. Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–160. doi:10.1097/AOG.0000000000002675
37. Nisenblat V, Prentice L, Bossuyt PMM, Farquhar C, Hull ML, Johnson N. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;7:CD012281. doi:10.1002/14651858.CD012281
38. Koninckx PR, Ussia A, Adamyan L, Wattiez A, Donnez J. Deep endometriosis: definition, diagnosis, and treatment. Fertil Steril. 2012;98(3):564–571. doi:10.1016/j.fertnstert.2012.07.1061
39. Hudelist G, Tuttlies F, Rauter G, Pucher S, Keckstein J. Can transvaginal sonography predict infiltration depth in patients with deep infiltrating endometriosis of the rectum? Hum Reprod. 2009;24(5):1012–1017. doi:10.1093/humrep/dep014
40. Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48(3):318–332. doi:10.1002/uog.15955
41. Bazot M, Thomassin I, Hourani R, Cortez A, Darai E. Diagnostic accuracy of transvaginal sonography for deep pelvic endometriosis. Ultrasound Obstet Gynecol. 2004;24(2):180–185. doi:10.1002/uog.1108
42. Exacoustos C, Malzoni M, Di Giovanni A, et al. Ultrasound mapping system for the surgical management of deep infiltrating endometriosis. Fertil Steril. 2014;102(1):143–150.e2. doi:10.1016/j.fertnstert.2014.03.043
43. Abrao MS, Goncalves MO da C, Dias JAJ, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007;22(12):3092–3097. doi:10.1093/humrep/dem187
44. Belghiti J, Thomassin-Naggara I, Zacharopoulou C, et al. Contribution of computed tomography enema and magnetic resonance imaging to diagnose multifocal and multicentric bowel lesions in patients with colorectal endometriosis. J Minim Invasive Gynecol. 2015;22(5):776–784. doi:10.1016/j.jmig.2015.02.019
45. Rossi L, Palazzo L, Yazbeck C, et al. Can rectal endoscopic sonography be used to predict infiltration depth in patients with deep infiltrating endometriosis of the rectum? Ultrasound Obstet Gynecol. 2014;43(3):322–327. doi:10.1002/uog.12535
46. Holland TK, Cutner A, Saridogan E, Mavrelos D, Pateman K, Jurkovic D. Ultrasound mapping of pelvic endometriosis: does the location and number of lesions affect the diagnostic accuracy? A multicentre diagnostic accuracy study. BMC Womens Health. 2013;13:43. doi:10.1186/1472-6874-13-43
47. Piketty M, Chopin N, Dousset B, et al. Preoperative work-up for patients with deeply infiltrating endometriosis: transvaginal ultrasonography must definitely be the first-line imaging examination. Hum Reprod. 2009;24(3):602–607. doi:10.1093/humrep/den405
48. Koga K, Osuga Y, Yano T, et al. Characteristic images of deeply infiltrating rectosigmoid endometriosis on transvaginal and transrectal ultrasonography. Hum Reprod. 2003;18(6):1328–1333. doi:10.1093/humrep/deg243
49. Bergamini V, Ghezzi F, Scarperi S, Raffaelli R, Cromi A, Franchi M. Preoperative assessment of intestinal endometriosis: a comparison of transvaginal sonography with water-contrast in the rectum, transrectal sonography, and barium enema. Abdom Imaging. 2010;35(6):732–736. doi:10.1007/s00261-010-9610-z
50. Bazot M, Darai E, Hourani R, et al. Deep pelvic endometriosis: MR imaging for diagnosis and prediction of extension of disease. Radiology. 2004;232(2):379–389. doi:10.1148/radiol.2322030762
51. Manganaro L, Vittori G, Vinci V, et al. Beyond laparoscopy: 3-T magnetic resonance imaging in the evaluation of posterior cul-de-sac obliteration. Magn Reson Imaging. 2012;30(10):1432–1438. doi:10.1016/j.mri.2012.05.006
52. Faccioli N, Foti G, Manfredi R, et al. Evaluation of colonic involvement in endometriosis: double-contrast barium enema vs. magnetic resonance imaging. Abdom Imaging. 2010;35(4):414–421. doi:10.1007/s00261-009-9544-5
53. Roman H, Vassilieff M, Gourcerol G, et al. Surgical management of deep infiltrating endometriosis of the rectum: pleading for a symptom-guided approach. Hum Reprod. 2011;26(2):274–281. doi:10.1093/humrep/deq332
54. Dubernard G, Piketty M, Rouzier R, Houry S, Bazot M, Darai E. Quality of life after laparoscopic colorectal resection for endometriosis. Hum Reprod. 2006;21(5):1243–1247. doi:10.1093/humrep/dei491
55. Wattiez A, Puga M, Albornoz J, Faller E. Surgical strategy in endometriosis. Best Pract Res Clin Obstet Gynaecol. 2013;27(3):381–392. doi:10.1016/j.bpobgyn.2012.12.003
56. Vercellini P, Barbara G, Buggio L, Frattaruolo MP, Somigliana E, Fedele L. Effect of patient selection on estimate of reproductive success after surgery for rectovaginal endometriosis: literature review. Reprod Biomed Online. 2012;24(4):389–395. doi:10.1016/j.rbmo.2012.01.003
57. Bassi MA, Podgaec S, Dias JAJ, D’Amico Filho N, Petta CA, Abrao MS. Quality of life after segmental resection of the rectosigmoid by laparoscopy in patients with deep infiltrating endometriosis with bowel involvement. J Minim Invasive Gynecol. 2011;18(6):730–733. doi:10.1016/j.jmig.2011.07.014
58. Ballester M, Chereau E, Dubernard G, Coutant C, Bazot M, Darai E. Urinary dysfunction after colorectal resection for endometriosis: results of a prospective randomized trial comparing laparoscopy to open surgery. Am J Obstet Gynecol. 2011;204(4):
59. Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod. 2010;25(8):1949–1958. doi:10.1093/humrep/deq135
60. Mohr C, Nezhat FR, Nezhat CH, Seidman DS, Nezhat CR. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. J Soc Laparoendosc Surg. 2005;9(1):16–24.
61. Vercellini P, Crosignani PG, Abbiati A, Somigliana E, Vigano P, Fedele L. The effect of surgery for symptomatic endometriosis: the other side of the story. Hum Reprod Update. 2009;15(2):177–188. doi:10.1093/humupd/dmn062
62. Mabrouk M, Raimondo D, Altieri M, et al. Surgical, clinical, and functional outcomes in patients with rectosigmoid endometriosis in the gray zone: 13-year long-term follow-up. J Minim Invasive Gynecol. 2018. doi:10.1016/j.jmig.2018.08.031
63. Afors K, Centini G, Fernandes R, et al. Segmental and discoid resection are preferential to bowel shaving for medium-term symptomatic relief in patients with bowel endometriosis. J Minim Invasive Gynecol. 2016;23:7. doi:10.1016/j.jmig.2016.08.813
64. Minelli L, Ceccaroni M, Ruffo G, et al. Laparoscopic conservative surgery for stage IV symptomatic endometriosis: short-term surgical complications. Fertil Steril. 2010;94(4):1218–1222. doi:10.1016/j.fertnstert.2009.08.035
65. Busacca M, Chiaffarino F, Candiani M, et al. Determinants of long-term clinically detected recurrence rates of deep, ovarian, and pelvic endometriosis. Am J Obstet Gynecol. 2006;195(2):426–432. doi:10.1016/j.ajog.2006.01.078
66. Seracchioli R, Mabrouk M, Manuzzi L, et al. Post-operative use of oral contraceptive pills for prevention of anatomical relapse or symptom-recurrence after conservative surgery for endometriosis. Hum Reprod. 2009;24(11):2729–2735. doi:10.1093/humrep/dep259
67. Article O, Zupi E, Lazzeri L, Centini G. Endometriosis and pain: postsurgical alternative treatment in patients desiring pregnancy. J Endometr Pelvic Pain Disord. 2015;7(3):95–99. doi:10.5301/je.5000226
68. Roman H, Hennetier C, Darwish B, et al. Bowel occult microscopic endometriosis in resection margins in deep colorectal endometriosis specimens has no impact on short-term postoperative outcomes. Fertil Steril. 2016;105(2):423–9.e7. doi:10.1016/j.fertnstert.2015.09.030
69. Nirgianakis K, McKinnon B, Imboden S, Knabben L, Gloor B, Mueller MD. Laparoscopic management of bowel endometriosis: resection margins as a predictor of recurrence. Acta Obstet Gynecol Scand. 2014;93(12):1262–1267. doi:10.1111/aogs.12490
70. Mabrouk M, Spagnolo E, Raimondo D, et al. Segmental bowel resection for colorectal endometriosis: is there a correlation between histological pattern and clinical outcomes? Hum Reprod. 2012;27(5):1314–1319. doi:10.1093/humrep/des048
71. Vercellini P, Buggio L, Somigliana E. Role of medical therapy in the management of deep rectovaginal endometriosis. Fertil Steril. 2017;108(6):913–930. doi:10.1016/j.fertnstert.2017.08.038
72. Ferrari S, Persico P, Di Puppo F, et al. Continuous low-dose oral contraceptive in the treatment of colorectal endometriosis evaluated by rectal endoscopic ultrasonography. Acta Obstet Gynecol Scand. 2012;91(6):699–703. doi:10.1111/j.1600-0412.2012.01366.x
73. Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375–1387. doi:10.1016/j.fertnstert.2005.03.083
74. Seracchioli R, Mabrouk M, Frasca C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertil Steril. 2010;93(1):52–56. doi:10.1016/j.fertnstert.2008.09.052
75. Ruan X, Seeger H, Mueck AO. The pharmacology of dienogest. Maturitas. 2012;71(4):337–344. doi:10.1016/j.maturitas.2012.01.018
76. Leonardo-Pinto JP, Benetti-Pinto CL, Cursino K, Yela DA. Dienogest and deep infiltrating endometriosis: the remission of symptoms is not related to endometriosis nodule remission. Eur J Obstet Gynecol Reprod Biol. 2017;211:108–111. doi:10.1016/j.ejogrb.2017.02.015
77. Harada M, Osuga Y, Izumi G, et al. Dienogest, a new conservative strategy for extragenital endometriosis: a pilot study. Gynecol Endocrinol. 2011;27(9):717–720. doi:10.3109/09513590.2010.533800
78. Yela DA, Kajikawa P, Donati L, Cursino K, Giraldo H, Benetti-pinto CL. Deep infiltrating endometriosis treatment with dienogest: a pilot study. J Endometr Pelvic Pain Disord. 2015;7(1):33–37. doi:10.5301/je.5000202
79. Seo J-W, Lee D-Y, Yoon B-K CD. Effects of long-term postoperative dienogest use for treatment of endometriosis on bone mineral density. Eur J Obstet Gynecol Reprod Biol. 2017;212:9–12. doi:10.1016/j.ejogrb.2017.03.011
80. Ferrero S, Camerini G, Ragni N, Venturini PL, Biscaldi E, Remorgida V. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25(1):94–100. doi:10.1093/humrep/dep361
81. Tosti C, Vannuccini S, Troìa L, et al. Long-term vaginal danazol treatment in fertile age women with adenomyosis. J Endometr Pelvic Pain Disord. 2017;9(1):39–43. doi:10.5301/je.5000270
82. Fedele L, Bianchi S, Zanconato G, Portuese A, Raffaelli R. Use of a levonorgestrel-releasing intrauterine device in the treatment of rectovaginal endometriosis. Fertil Steril. 2001;75(3):485–488. doi:10.1016/s0015-0282(00)01759-3
83. Ferrero S, Tramalloni D, Venturini PL, Remorgida V. Vaginal danazol for women with rectovaginal endometriosis and pain symptoms persisting after insertion of a levonorgestrel-releasing intrauterine device. Int J Gynaecol Obstet. 2011;113(2):116–119. doi:10.1016/j.ijgo.2010.11.015
84. Chen Y-J, Hsu T-F, Huang B-S, Tsai H-W, Chang Y-H, Wang P-H. Postoperative maintenance levonorgestrel-releasing intrauterine system and endometrioma recurrence: a randomized controlled study. Am J Obstet Gynecol. 2017;216(6):
85. Roman H, Saint Ghislain M, Milles M, et al. Improvement of digestive complaints in women with severe colorectal endometriosis benefiting from continuous amenorrhoea triggered by triptorelin. A prospective pilot study. Gynecol Obstet Fertil. 2015;43(9):575–581. doi:10.1016/j.gyobfe.2015.07.001
86. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398. doi:10.1056/NEJMcp1000274
87. Jorgensen H, Hill AS, Beste MT, et al. Peritoneal fluid cytokines related to endometriosis in patients evaluated for infertility. Fertil Steril. 2017;107(5):1191–1199.e2. doi:10.1016/j.fertnstert.2017.03.013
88. Hurst BS, Shimp KE, Elliot M, Marshburn PB, Parsons J, Bahrani-Mostafavi Z. Molecular evaluation of proliferative-phase endometrium may provide insight about the underlying causes of infertility in women with endometriosis. Arch Gynecol Obstet. 2014;289(5):1119–1124. doi:10.1007/s00404-013-3103-6
89. Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75(1):1–10. doi:10.1016/s0015-0282(00)01630-7
90. Kissler S, Hamscho N, Zangos S, et al. Diminished pregnancy rates in endometriosis due to impaired uterotubal transport assessed by hysterosalpingoscintigraphy. BJOG. 2005;112(10):1391–1396. doi:10.1111/j.1471-0528.2005.00676.x
91. Van Langendonckt A, Casanas-Roux F, Dolmans M-M DJ. Potential involvement of hemoglobin and heme in the pathogenesis of peritoneal endometriosis. Fertil Steril. 2002;77(3):561–570. doi:10.1016/s0015-0282(01)03211-3
92. Zupi E, De Felice G, Conway F, et al. From endometriosis to pregnancy: which is the “road-map”? J Endometr Pelvic Pain Disord. 2017;9(4):252–262. doi:10.5301/jeppd.5000307
93. Harb HM, Gallos ID, Chu J, Harb M, Coomarasamy A. The effect of endometriosis on in vitro fertilisation outcome: a systematic review and meta-analysis. BJOG. 2013;120(11):1308–1320. doi:10.1111/1471-0528.12366
94. Duffy JMN, Arambage K, Correa FJS, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031. doi:10.1002/14651858.CD011031.pub2
95. Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2010;94(5):1609–1615. doi:10.1016/j.fertnstert.2009.09.035
96. Bendifallah S, Roman H, Mathieu d’Argent E, et al. Colorectal endometriosis-associated infertility: should surgery precede ART? Fertil Steril. 2017;108(3):525–531.e4. doi:10.1016/j.fertnstert.2017.07.002
97. Stepniewska A, Pomini P, Bruni F, et al. Laparoscopic treatment of bowel endometriosis in infertile women. Hum Reprod. 2009;24(7):1619–1625. doi:10.1093/humrep/dep083
98. Darai E, Lesieur B, Dubernard G, Rouzier R, Bazot M, Ballester M. Fertility after colorectal resection for endometriosis: results of a prospective study comparing laparoscopy with open surgery. Fertil Steril. 2011;95(6):1903–1908. doi:10.1016/j.fertnstert.2011.02.018
99. Centini G, Afors K, Murtada R, et al. Impact of laparoscopic surgical management of deep endometriosis on pregnancy rate. J Minim Invasive Gynecol. 2016;23:1. doi:10.1016/j.jmig.2015.09.015
100. Barri PN, Coroleu B, Tur R, Barri-Soldevila PN, Rodriguez I. Endometriosis-associated infertility: surgery and IVF, a comprehensive therapeutic approach. Reprod Biomed Online. 2010;21(2):179–185. doi:10.1016/j.rbmo.2010.04.026
101. Exacoustos C, Lauriola I, De FG, Frusca T, Zupi E. Complications during pregnancy in patients with Deep Infiltrating Endometriosis (DIE). J Minim Invasive Gynecol. 2015;22(6S):S169. doi:10.1016/j.jmig.2015.08.629
102. Nirgianakis K, Gasparri ML, Radan A-P, et al. Obstetric complications after laparoscopic excision of posterior deep infiltrating endometriosis: a case-control study. Fertil Steril. 2018;110(3):459–466. doi:10.1016/j.fertnstert.2018.04.036
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
