Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Borealin Promotes Tumor Growth and Metastasis by Activating the Wnt/β-Catenin Signaling Pathway in Hepatocellular Carcinoma
Authors Chen B , Gu Y, Shen H, Liu Q, Wang H, Li Y, Liu X, Liu Y, Du Q, Sun H, Liao X
Received 27 August 2021
Accepted for publication 24 January 2022
Published 11 March 2022 Volume 2022:9 Pages 171—188
DOI https://doi.org/10.2147/JHC.S336452
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Ahmed Kaseb
Baiyang Chen,1,* Yang Gu,2,* Hui Shen,3 Qiangsheng Liu,4 Hongbo Wang,1 Yabo Li,1 Xifan Liu,1 Yu Liu,1 Qinghao Du,1 Huapeng Sun,1 Xiaofeng Liao1
1Department of General Surgery, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, People’s Republic of China; 2Department of Hepatobiliary and Pancreas, The First People’s Hospital of Jingmen, Jingmen, Hubei, People’s Republic of China; 3Department of Oncology, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, People’s Republic of China; 4Department of Anesthesiology, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaofeng Liao; Huapeng Sun, Department of General Surgery, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, People’s Republic of China, Email [email protected]; [email protected]
Background and Aims: Hepatocellular carcinoma (HCC) is a common malignant disease with high morbidity and mortality throughout the world. While Borealin is a putative oncogene that is dysregulated in multiple tumors, its exact role in HCC remains less investigated.
Methods: Quantitative reverse transcription polymerase chain reaction (qRT-PCR) and immunohistochemistry (IHC) assays were employed to examine the relative amount of Borealin. Gene set enrichment analysis (GSEA) and other bioinformatic analyses were implemented to probe into the potential functions of Borealin. The biological roles and mechanisms of Borealin in the tumorigenesis and development of HCC were further evaluated using a battery of functional assays in vivo and in vitro.
Results: Borealin was enhanced in the HCC tissue samples and hepatoma cells when compared with the nontumor tissues and normal liver cells. Higher Borealin expression was positively linked with advanced pathological phenotypes and inferior overall survival. The overexpression of Borealin promoted the cells’ abilities on proliferation, invasion and epithelial–mesenchymal transition (EMT) in vitro, facilitated tumor growth and lung metastasis in vivo, whereas the silencing of Borealin inhibited these capabilities in vitro. Furthermore, Borealin interacted with β-catenin and further activated the Wnt/β-catenin signaling pathway, which endowed HCC cells with highly aggressive and metastatic capabilities.
Conclusion: Borealin was identified as an oncogene that could promote HCC growth and metastasis by activating the WNT/β-catenin signaling pathway. These findings extended the understanding of Borealin in HCC tumorigenesis and development and highlighted the significance of Borealin in HCC diagnosis and treatment.
Keywords: hepatocellular carcinoma, Borealin, epithelial mesenchymal transition, Wnt/β-catenin
Lay Summary
Our findings verified Borealin as an oncogene clinically, mechanistically and functionally, and it could be an effective molecular indicator and potential therapeutic target for HCC.
Introduction
As one of the most common malignancies in the digestive system, hepatocellular carcinoma is the third cause of cancer-related death globally.1,2 Particularly in Asia, the morbidity and mortality of HCC is still high.3 Viral infections, aflatoxin exposure and alcohol abuse have been implicated in HCC.4–6 With the development of the prevention and treatment of hepatitis B virus in China, the number of hepatitis B patients has decreased in the young age group. Nevertheless, the morbidity of HCC is still high on account of the large accumulation of previously infected patients.7 At present, surgical excision, transcatheter arterial chemoembolization and radiofrequency ablation are the major treatments for HCC patients.8–13 In view of its early stage without obvious symptoms, HCC has been in middle and advanced stage when clinical diagnosis and the surgical resection rate is not high. Liver transplantation is not suitable for the majority of the patients.14 Due to the high recurrence rates of transcatheter arterial chemoembolization and radiofrequency ablation, and the low sensitivity of HCC to radiotherapy and chemotherapy, HCC patients possessed unfavourable prognosis. The median survival time of patients who suffer from HCC is merely 6 months.15 Hence, an intensive comprehension of its sophisticated molecular pathogenesis and the identification of new biomarker of HCC are urgently requisite.16
Borealin, also called as Dasra B and cell division cycle associated 8 (CDCA8), is located in chromosome 1p34.3 (Supplementary file 1 Figure 1). Borealin encodes an essential component of the chromosomal passenger complex (CPC), which is considered as a key orchestrator of orderly mitotic exit and cytokinesis.17–19 The functions of Borealin are mainly confined in CPC and mitosis through facilitating CPC assembling, Aurora B kinase activation, and localization in kinetochores.20,21 Besides, Borealin targets INCENP, Aurora B, Survivin and contributes to proper chromosome segregation and cell division.22,23 Borealin exists in homologues in most vertebrates, as well as in mosquito and D. melanogaster. In Drosophila, the lack of Borealin induces polyploidy, delayed apoptosis and abnormal tissue development.24 In mice, Borealin is differentially expressed in undifferentiated and differentiated embryonic stem cells, and its loss results in embryonic arrest.25,26 Borealin exhibits malignant behaviors in multiple human cancers. For instance, Borealin promotes the estrogen-stimulated breast cancer cell growth.27 In human gastric cancer, nuclear Borealin accumulation is related to poor prognosis.28 Borealin enhances the proliferation of colorectal cancer cells.29 In bladder cancer, Borealin is high expressed and affects its progression.30 Also, silencing of Borealin restrains the progression and stemness in HCC.31 All these findings illustrate that Borealin participates in cancers’ occurrence and progression and it could serve as a function and molecular mechanisms in HCC are yet to be determined. In the current study, we screened out Borealin using bioinformatics and found that it was up-regulated in both HCC cell lines and tissues. High Borealin expression was positively linked with advanced pathological phenotypes and inferior overall survival. Furthermore, high Borealin was an independent risk factor for overall survival in HCC. Functionally, Borealin ablation attenuated cell proliferation, invasion, and EMT in vitro, whereas overexpressed Borealin improved these activities in vitro and accelerated tumor growth and metastasis in vivo. In addition, Borealin interacted with β-catenin and further activated the Wnt/β-catenin signaling pathway to promote cell proliferation, invasion and EMT. Collectively, Borealin was a key regulatory molecule that regulated the Wnt/β-catenin signaling pathway, which endowed HCC cells with highly aggressive and metastatic capacities. Our results illustrated that Borealin act as an oncogene clinically, functionally and mechanistically and it could be an effective molecular indicator and potential therapeutic target for hepatocellular carcinoma patients.
Materials and Methods
HCC Patients and Clinical Specimens
The tumor and paired adjacent non-tumor tissues were gathered and well preserved from 80 primary HCC patients who performed hepatectomy at Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science from 2013 to 2015. Before Surgery, people did not receive any neo-adjuvant therapy. Written informed consent was obtained from all patients. Our study was known and supported by the Human Subjects Protection Committee of Xiangyang Central Hospital. The study was conducted in accordance with the Declaration of Helsinki.
Cell Lines and Cell Culture
Hepatoma cell lines (Huh7, HCCLM3, Hep3B, HepG2 and SK-hep1) and normal human liver cell-line HL-7702 (L02) appeared in our study were purchased from Chinese Academy of Sciences (Shanghai, China). Dulbecco’s modified Eagle’s medium furnished with 10% fetal bovine serum (Gibco) was used to foster cells. Cells were cultured in cell incubator with 5% carbon dioxide at 37°C.
RNA Isolation, Reverse Transcription and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was isolated from cells and tissues by TRIzol reagent (Invitrogen). Then, PrimeScript RT reagent Kit (Takara) was employed to carry out reverse transcription to generate complementary DNA (cDNA). RT-qPCR was performed using a CFX connect Real-Time PCR detection system. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), known as a key enzyme in the glycolytic pathway, was selected as an internal standard gene due to its high and constant expression in almost all tissue cells. And it could provide accurately normalized data in HCC tissues. The 2−ΔΔCt method was employed to analyse the relative expression of target genes (ct = threshold cycle). The primers employed were listed in Supplementary file 2 Table 1.
Small Interfering RNAs, Lentiviral Vectors, Plasmid and Cell Transfection
The small interfering RNAs specifically targeting Borealin (named Si-Borealin#1, #2, #3), control small interfering RNA (named Si-control), pcDNA3.1/Borealin plasmid (named Borealin), and control vector plasmid (named vector) were entirely designed and processed by GeneCopoeia company (Guangzhou, China). SiRNAs and plasmids were transfected into cells using Genmute reagent (SignaGen) and Lipofectamine 3000 reagent (Invitrogen) as per the manufacturer’s protocols, respectively.
Proliferation Assay
We employed the cell Counting Kit-8 (CCK8) to measure cell proliferative potential. Seventy-two hours after transfection, cells were completely collected and plated in 96-well plates. Each plate was put in 4–6 × 104 cells. At the 0, 24, 48, 72 h, total 0.01 mL of CCK8 reagent was added to each well and incubated at 37°C for 2 hours. After incubation, we measured the absorbance (450 nm) of these cells.
Colony Formation assay
Colony formation assay was used to measure cell potential ability of colony formation. About 103 cells were planted into each 6-well plate. And 4% paraformaldehyde and 0.1% crystal violet solution were used to fix and stain the colonies after 14 days. Images of the wells were obtained via a camera.
Cell Apoptosis Analysis
Flow cytometry (FACS, USA) was used to analyze the cell apoptosis condition. Cells were collected 48 hours after transfection and diluted in cold buffer. Afterwards, cells were stained with 5 μL cold propidium iodide solution and 10 μL FITC for 5 minutes and then tested.
Transwell Migration and Invasion Assays
Cell migration and invasion assay were employed by 24-well plates and chambers (BD BioSciences). In terms of migration assays, the upper chambers were planted with about 30,000 cells in Dulbecco’s modified Eagle’s medium. The lower chambers were filled with Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. In terms of invasion assays, matrigel (BD Biosciences) was added to the chamber at a 1:6 dilution. Twenty-four hours later, cells on the outer surface of the membrane were fixed and stained by 4% paraformaldehyde and 0.1% crystal violet solution, respectively, and then counted via microscope.
Immunohistochemistry (IHC)
All experimental steps were strictly conducted according to the protocol of UltraSensitiveTM SP kits (Maixin, China). Tissue samples were fixed using 4% paraformaldehyde, then wrapped in paraffin and segmented at 4 μm. The slices were deparaffinized, rehydrated and heated in boiling citrate buffer solution. Then, the slices were immersed in 3% hydrogen peroxide solution. The slices were blocked in bovine serum albumin and further incubated with the primary antibody. The slices were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody, and detected it by the standard substrate recognition of HRP. Afterwards, slices were stained with hematoxylin and dehydrated by categorized alcohols and xylene. Antibodies applied in our study were listed in Supplementary file 2 Table 2.
Immunofluorescence (IF) Staining
Cells were planted on the glass coverslips in 6-well plates. After 24–48 hours, cells were fixed with cold 4% paraformaldehyde for 20 min, infiltrated by 0.5% Triton X-100 for 20 min, treated with 10% bovine serum albumin for 30 min, and then incubated with the corresponding primary at 4°C overnight and secondary antibody for 1 h at 37°C. After counterstained with 4′,6-diamino-2-phenyl-indole, cells were imaged by fluorescence microscopy. Antibodies applied in our study were listed in Supplementary file 2 Table 2.
Western Blotting (WB)
Tissues were homogenized, and cells were lysed using RIPA buffer containing protease and phosphatase inhibitors. After measuring the quantity using a bicinchoninic acid assay, an equivalent volume protein samples were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then electrotransferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% skimmed milk at room temperature for 1 hour and incubated with primary antibodies at 4°C overnight. Membranes were washed and then incubated with secondary antibody at room temperature for 1 h. Enhanced chemiluminescence solutions were employed to get the exposure result. Lamin B1 and GAPDH served as internal control. Antibodies applied in our research were listed in Supplementary file 2 Table 2.
In vivo Experiments and Hematoxylin–Eosin (HE) Staining
Animal experimental protocols were performed in accordance with the National Institute of Health guidelines for the Care and Use of Laboratory Animals. Four-week-old male nude mice (BALB/c) were obtained from the Animal Center affiliated to the Chinese Academy of Medical Sciences (Beijing, China). Before experiments, we conducted HCCLM3 cells stably transfected with Borealin and vector, respectively. For xenograft experiments, HCCLM3 cells (5x105 cells in 100 µL phosphate buffered solution) stably transfected with Borealin and vector were then subcutaneously injected into mice armpits. Every five days, we calculated length and width of subcutaneous tumors using a vernier caliper. Six weeks later, we euthanized the mice and preserved the solid tumor. The tumor volume was metered via the following formula: length * width2/2. For metastasis assays, 105 treated cells in 100 µL phosphate buffered solution were injected into mice by tail vein, and they were euthanized seven weeks later. The mice lungs were excised and kept for hematoxylin–eosin (HE) staining experiments. The mice lungs were fixed in 4% paraformaldehyde then were dehydrated, embedded in paraffin, and cut into slices. Slices were finally stained with hematoxylin and eosin. Metastatic lung tumors were observed and counted via microscopy.
Immunoprecipitation
All experimental steps were strictly conducted according to the protocol of ACE rProtein A/G Magnetic IP/Co-IP Kit (ACE, China). Cells were collected using RIPA buffer containing protease and phosphatase inhibitors. The protein lysates were obtained from the supernatant through centrifugation at 45,000 r.p.m. for 45 min at 4°C. About 20 ul of cell lysis supernatant was collected and used as input group. The antibodies were added to the magnetic beads at room temperature for 1 h. After that, the protein extracts were added to the above reagents incubated at 4°C overnight to capture the specific antibody captured proteins. The magnetic beads were collected by placing the tube in the magnetic separator and further washed with buffer to remove all of the non-specifically bounded proteins. The bounded proteins were eluted from the beads with corresponding elution buffer for Western blot analysis.
Gene Set Enrichment Analysis (GSEA)
Based on the median value of Borealin expression levels in 371 TCGA RNA-seq datasets downloaded from UCSC xena, HCC samples were defined as Borealin-high (≥7.471) group and Borealin-low (<7.471) group. The 371 HCC sample data were then subjected to GSEA software to analyse the biological processes or signaling pathways of Borealin based on Molecular Signatures Database, a collection of annotated gene sets for use with GSEA software. Thresholds for significance were determined by permutation analysis (1000 permutations). P value <0.01 and false discovery rate <0.05 were considered statistically significant.
Bioinformatic Analysis
To compare the differential expression of Borealin in the HCC tissues and other tumor tissues, oncomine (https://www.oncomine.org) and uclan (http://ualcan.path.uab.edu/) were used in our study. GSE64041 and GSE25097 databases from GEO (https://www.ncbi.nlm.nih.gov/geo/) were employed to analyze the relative Borealin expression. The Kaplan–Meier curves associated with Borealin were retrieved from Kaplan–Meier Plotter (http://www.kmplot.com/). Via GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/), we, respectively, selected top 250 DEGs in GSE64041 and GSE25097 databases. Kaplan–Meier survival, Cox’s proportional hazards model, as well as multivariate analysis were conducted to screen out the target gene Borealin using R software (https://bioconductor.org/biocLite.R). In Kaplan–Meier survival and Cox’s proportional hazards model, p value < 0.001 were considered as extremely significant. Metascape (http://metascape.org/), an online tool for gene annotation and visualization, was employed for gene ontology, Hallmark analysis, KEGG Pathway and Canonical Pathways analysis to further clarify the potential role of DEGs. P value <0.05 was considered as the cutoff criterion.
Statistics
Data are presented as the mean ± standard deviation (SD) from three independent experiments. GraphPad Prism 6 software (La Jolla, USA) was adopted to perform Student’s t-test, χ2 test, Fisher’s exact tests or analysis of variance. P value <0.05 was considered to be significant.
Results
Bioinformatic Analysis of Two HCC Datasets (GSE64041 and GSE25097) Screened Out Our Target Gene Borealin
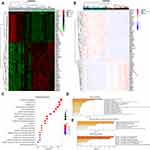
We downloaded two message RNA expression datasets GSE64041 and GSE25097 from Gene Expression Omnibus (GEO) hepatocellular carcinoma datasets.32,33 With the help of GEO2R, an online tool in GEO, we selected the top 250 differential expressed genes (DEGs) in the two databases, respectively. After the intersection of the 250 DEGs, 81 DEGs were discovered and displayed via clustering analysis (Figure 1A and B, Supplementary file 2: Table 3). To further investigate the biological function and related signaling pathways, the 81 DEGs were analyzed using R software and Metascape (Supplementary file 1 Figure 2).34 Based on gene ontology (GO) analysis, the 81 DEGs were found to mainly participate in chromosome segregation, mitotic spindle, extracellular matrix and spindle (Figure 1C and D). Hallmark analysis confirmed that the 81 DEGs mainly enriched in the G2M checkpoint, spermatogenesis and epithelial-mesenchymal transition (EMT). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and canonical pathway enrichment analysis signified that the DEGs were principally enriched in cell cycle, PID CXCR4 pathway and other pathways (Figure 1E). To identify the crucial genes interrelated to HCC patients’ survival from 81 DEGs, the Cox’s proportional hazards regression model and Kaplan–Meier analysis were employed via R software according to the clinical information based on the TCGA Liver hepatocellular carcinoma database.35,36 In Cox’s proportional hazards regression model, 28 genes were found to be related to the survival of HCC patients when the P value was defined to be less than 0.001 (Supplementary file 2: Table 4). Through Kaplan–Meier analysis, 11 genes were observed to be associated with HCC patients’ survival when the P value was defined to be less than 0.001 (Supplementary file 2: Table 5). Nine genes including Borealin, TOP2A, ANLN, DNASE1L3, BUB1, HJURP, RACGAP1, PTTG1 and EZH2 in the intersection of Kaplan–Meier analysis and Cox’s proportional hazards regression model when P < 0.001 was considered as extremely significant. Subsequently, nine genes were analyzed via multivariate Cox, Borealin aroused our interest and was further selected as our target gene (P = 0.021, Table 1).
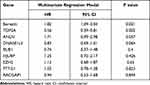 |
Table 1 The Multivariate Cox Analysis of the Nine Genes |
Borealin Was Upregulated in the HCC Tissues and Was Associated with Aggressive Clinical Pathological Phenotypes and Poor Prognosis
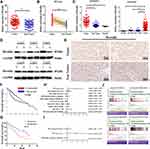
To identify whether Borealin was involved in HCC, we detected its mRNA expression level in 80 paired tissue samples from patients with HCC. The results showed that HCC tumor tissues exhibited higher Borealin expression than the paired adjacent nontumor tissue samples (Figure 2A). Consistent with our findings, Borealin expression was enhanced in the tumor samples compared with the non-tumor samples in the TCGA liver cancer dataset, GSE64041 and GSE25097 (Figure 2B and C). As revealed by Uclan and Oncomine data mining analyses, Borealin was upregulated in most of the cancers, thereby suggesting its oncogenic function (Supplementary file 1 Figure 3). Western blotting (WB) and IHC staining analyses verified the higher protein level of Borealin in HCC tissues than in non-tumor tissues (Figure 2D and E).
To better explore the clinical role of Borealin, we collected and analyzed the 80 HCC patients’ clinical information and found that higher expression of Borealin was linked to various aggressive clinical pathological phenotypes including multifocal tumor, larger tumor size, portal vein tumor thrombus (PVTT) and advanced Barcelona Clinic Liver Cancer stage (Table 2). Other features, such as age, gender, AFP, HBV infection and liver cirrhosis showed no obvious correlation with Borealin levels. Furthermore, the Kaplan–Meier cures associated with Borealin suggested that its expression had vital impact on the survival time of HCC patients in TCGA (Figure 2F). Patients with lower Borealin possessed longer overall survival time than patients with high Borealin expression (Figure 2G). In general, we concluded that Borealin expression was enhanced in the HCC tissues and cells, and its high expression was associated with poor prognosis in HCC. Univariate analysis showed that high Borealin expression, PVTT, and BCLC stage were related to overall survival. Further multivariate analysis indicated that high Borealin was an independent risk factor for overall survival in HCC (Figure 2H and I). These results suggest that high Borealin expression is crucial in hepatic carcinogenesis and could be used as a prognostic indicator for HCC patients in the clinic.
 |
Table 2 Patients Clinical Characteristics |
HCC patients were classified into high- and low-Borealin groups according to the median mRNA expression of Borealin in the TCGA-LIHC dataset. Subsequent gene set enrichment analysis (GSEA) was performed in 371 TCGA RNA-seq datasets with high (≥7.471) or low (<7.471) Borealin mRNA expression to investigate its biological function.37 The results indicated that high Borealin expression was involved in multiple biological functions and crucial signal pathways including cell apoptosis, adherens junction, mitotic spindle, spermatogenesis, glycolysis, the Wnt signaling pathway and cancer-related pathways, which implied that up-regulated Borealin may participate in the acquisition of an aggressive phenotype (Figure 2J and Supplementary file 1 Figure 4).
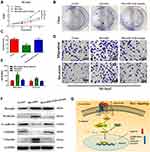
Borealin Promoted Hepatoma Cells Proliferation, Colony Formation and Inhibited Apoptosis in vitro
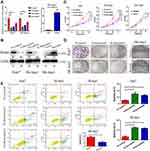
To further clarify the biological role of Borealin in the behavior of the cells, we first examined the expression of Borealin in the HCC cell lines. As shown in Supplementary file 1 Figure 5, Borealin exhibited higher expression in five hepatoma cell lines in comparison with the immortalized liver cell-line L02. Huh7 and SK-hep1 cells were chosen to establish Borealin-silenced and Borealin-overexpressed models. Three short interference RNAs (named Si-Borealin#1, #2, and #3) were synthesized and employed to silence Borealin in the HCC cells. The empty vector and Borealin overexpression vector were transfected into SK-hep1 cells (named Vector and Borealin respectively). The transfection efficiency was measured using qRT-PCR and WB assays 24 hours after the transfection (Figure 3A and B). Given the differences in the decreased mRNA and protein levels of Borealin, Si-Borealin#1 and Si-Borealin#2 were selected for further experiments. Knockdown of Borealin significantly restrained the proliferation and colony formation abilities of the hepatoma cells. Overexpressed Borealin promoted growth and colony formation in SK-hep1 cells (Figure 3C and D). The subsequent cell apoptosis analysis displayed augmented proportion of apoptotic cells in the Borealin knockdown group when compared with the control group. In comparison with the control group, Borealin overexpression reduced the apoptosis rates in SK-hep1 cells (Figure 3E). Generally speaking, Borealin participated in the proliferation, colony formation and cell apoptosis of hepatoma cells in vitro.
Borealin Promoted Hepatoma Cells Migration, Invasion and EMT in vitro
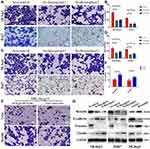
Given the crucial role in migration and invasion, we then studied the involvement of Borealin in the motility of hepatoma cells through cell migration and invasion experiments. On the basis of migratory and invasive cell counts, decreased Borealin was found to drastically restrain the cell migration and invasion capabilities of SK-hep1 and Huh7 cells (Figure 4A–D). Overexpressed Borealin promoted the migration and invasion abilities of SK-hep1 hepatoma cells (Figure 4E and F). We subsequently focused on the relationship between Borealin and EMT.38–40 Lower Borealin was observed to augment the expression of epithelial indicators (E-cadherin and claudin-1) and decrease mesenchymal marker (Vimentin). Higher expressed Borealin increased the vimentin level and reduced the E-cadherin and claudin-1 levels (Figure 4G and Supplementary file 1 Figure 6). Taken together, these findings strongly suggest that Borealin is involved in migration, invasion and EMT in vitro.
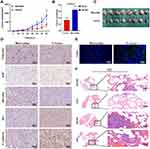
Borealin Promoted Tumor Growth and Metastasis in vivo
To probe the effects of Borealin in vivo, we established the subcutaneous and metastatic tumor models. The nude mice were divided into two groups (n = 8 per group). For the subcutaneous tumor model, HCCLM3 cells were stably transfected with the Borealin overexpression vector or control vector, which were injected into the right armpit of the nude mice. The results showed that the Borealin overexpression group exhibited higher tumor growth rates, heavier tumor weight and larger final tumor volumes when compared with the control group (Figure 5A–C). The IHC staining results revealed that overexpressed Borealin increased the Ki67 proliferation index and vimentin level, decreased the positive rates for Bax and E-cadherin (Figure 5D). Furthermore, Borealin decreased the apoptosis of the hepatoma cells by TUNEL apoptosis assays (Figure 5E). In the metastatic tumor model, the treated cells were injected into the mice through the tail vein to access the changes in the invasive behaviors of the hepatoma cells. When Borealin was overexpressed, the number and size of metastatic nodules in the lungs of mice were dramatically increased when compared with the control group. Hematoxylin–eosin (HE) staining further confirmed that (Figure 5F and Supplementary file 1 Figure 7). In summary, it is reasonable to believe that Borealin increased the growth and metastasis of HCC tumors in vivo and in vitro.
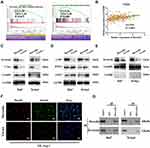
Borealin Interacted with β-Catenin and Activated the Wnt/β-Catenin Signaling Pathway in the HCC Cells
To better understand the mechanism of Borealin in HCC, we sought for signaling pathways that might be manipulated by Borealin. According to previous GSEA results, Borealin was thought to act on the Wnt/β-catenin signaling pathway (Figure 6A). In normal liver cells, the Wnt/β-catenin signaling pathway was generally inactive, and β-catenin was phosphorylated and subsequently degraded. Nevertheless, the reactivation of the Wnt/β-catenin signaling pathway, a key regulator of tumor progression, tumor invasion and EMT has been shown in hepatoma cells.41–43 Tissue expression data (371 tumor tissues and 50 non-tumor tissues) from TCGA indicated significant correlation between the expression of Borealin and CTNNB1 (Pearson R = 0.37, Figure 6B). To find out whether Borealin could regulate the Wnt/β-catenin signaling pathway, we measured the related molecules’ expression. WB analysis signified enhanced β-catenin, c-Myc, cyclin-D1, and LGR5 expression and decreased RNF43 and AXIN2 expression in the Borealin overexpression group when compared with the control group in the hepatoma cells (Figure 6C and D, Supplementary file 1 Figure 8). WB analysis of cell nucleoprotein revealed upregulated nuclear β-catenin in the HCC cells containing Borealin plasmids (Figure 6E). Further immunofluorescence showed a redistribution of cytoplasmic β-catenin to the nucleus upon transfection of the Borealin plasmids which was consistent with our WB findings (Figure 6F). Immunoprecipitation and WB assays suggested that β-catenin immunoprecipitated with Borealin in Huh7 and SK-hep1 cells. Hence, β-catenin might be a Borealin interacting protein (Figure 6G). Our findings suggest that the Borealin interacted with β-catenin and activated the Wnt/β-catenin signaling pathway in hepatoma cells.
The Wnt/β-Catenin Signaling Pathway Was Responsible for Borealin in Regulating Hepatoma Cells Proliferation, Invasion and EMT
To further explore the contributions of β-catenin in Borealin-mediated hepatoma cell growth and metastasis, we further transfected β-catenin interference (Si-β-catenin) into Borealin overexpressed SK-hep1 cells. CCK-8 combined with colony formation assays showed that si-β-catenin recovered the influence of Borealin on cell growth and clone formation in SK-hep1 cells (Figure 7A and B). Si-β-catenin significantly rescued Borealin overexpression-inhibited ability on cell apoptosis (Figure 7C). Borealin-mediated facilitation of migration and invasion was partially reversed when cotransfected with Borealin and si-β-catenin in the HCC cells (Figure 7D and E). Concomitant Borealin and si-β-catenin counteracted the effect of Borealin on β-catenin, E-cadherin, Claudin and Vimentin expression in the HCC cells (Figure 7F). Briefly, these findings demonstrated that Borealin affected cell behaviors via activating the Wnt/β-catenin signaling pathway.
Discussion
Hepatocellular carcinoma, one of the major malignancies possessed high incidence and mortality in China. According to the data released by the World Health Organization, more than 750,000 people have died of HCC around the world.44–46 Surgical treatment and interventional therapy have achieved certain clinical effects. Due to the high recurrence of HCC, patients with advanced tumors possessed unsatisfactory therapeutic effect.47 Hence, it is vital for us to clarify the underlying molecular mechanism of HCC initiation and progression.48
Through bioinformatic analysis of the GSE64041 and GSE25097, we screened out our target gene Borealin and found it to be up-regulated in HCC. The GSEA results indicated that the high expression of Borealin was related to spermatogenesis, cell apoptosis, adherens junction, mitotic spindle, and the Wnt signaling pathway. As a vital component of the CPC, Borealin is indispensable for chromosome segregation during cell division. Borealin also regulates cell growth, differentiation and apoptosis.49 In tumor cells, Borealin may regulate tumor progression via the regulation of mitosis, crossover chromosome separation and division. Borealin is a putative oncogene and has been reported to be upregulated in many types of cancer tissues (eg, prostate cancer and cutaneous melanoma).50,51 However, the concrete functional roles of Borealin in tumor initiation and progression are still unclear. In our study, we first confirmed the higher expression of Borealin in the tumor tissues than corresponding nontumor tissues both at the mRNA and protein levels. Then, the clinical data emphasized that higher Borealin was particularly associated with aggressive characteristics and shorter survival time. Univariate and multivariate analyses indicated that high Borealin was an independent risk factor for overall survival in HCC. Furthermore, Borealin promoted hepatoma cells proliferation, invasion, as well as EMT in vivo and in vitro.
The Wnt/β-catenin signaling pathway plays crucial roles in animal development. It also regulates cell proliferation, differentiation, apoptosis, migration, genetic stability and instability to maintain adult tissue homeostasis. The aberrant regulation of the Wnt/β-catenin signaling pathway is associated with multiple diseases, including fibrosis, metabolic diseases, neurodegenerative disorders and cancers.52–56 The Wnt/β-catenin signaling pathway is involved in HCC tumorigenesis and progression.57–59 The immunoprecipitation and WB assays suggested that β-catenin immunoprecipitated with Borealin. Then we transfected the Borealin-overexpressed cells with Si-β-catenin, and observed that it partially reversed the clone formation, invasion and EMT induced by Borealin. These results suggest that Borealin promoted the development of HCC by interacting with Borealin and regulating the Wnt/β-catenin signaling pathway.
Our study has offered substantial evidences to indicate that Borealin facilitated HCC growth and metastasis by activating the Wnt/β-catenin signaling pathway and it may therefore pave the way to a novel approach to treatment strategies for advanced HCC patients (Figure 7).
Abbreviations
HCC, hepatocellular carcinoma; mRNA, messenger RNA; WB, Western blotting; IHC, immunohistochemistry; IF, immunofluorescence; CDCA8, cell division cycle associated 8; CPC, chromosomal passenger complex; qRT-PCR, quantitative reverse transcription polymerase chain reaction; cDNA, complementary DNA; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; CCK8, cell counting kit 8; SiRNA, small interference RNA; LIHC, liver hepatocellular carcinoma; GEO, Gene Expression Omnibus; PVTT, portal vein tumor thrombus; BCLC, Barcelona Clinic Liver Cancer; EMT, Epithelial–mesenchymal transition; PVDF, polyvinylidene fluoride; GSEA, gene set enrichment analysis; DEGs, differential expressed genes; SD, standard deviation.
Data Sharing Statement
The data used to support the findings of this study are included within the article and Supplementary files.
Ethical Approval
This study was approved by the Ethical Committee of Xiangyang Central Hospital.
Informed Consent
The human study was known and supported by the Human Subjects Protection Committee of Xiangyang Central Hospital. Animal experiments were approved and supervised by the Animal Ethics Committee of Xiangyang Central Hospital. Written informed consent was obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We thank Quanyan Liu, Deliang Guo and Pengpeng Liu for their technical support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This study was supported by the key scientific research project of Xiangyang Central Hospital (Award number: 2021ZD02) and Xiangyang Scientific and Technological Project (Award number: 2021YL07).
Disclosure
All authors declare no conflicts of interest for this work.
References
1. Hay SI, Abajobir AA, Abate KH, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1260–1344.
2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Venook AP, Papandreou C, Furuse J, et al. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. doi:10.1634/theoncologist.2010-S4-05
4. de Martel C, Maucort-Boulch D, Plummer M, et al. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62(4):1190–1200. doi:10.1002/hep.27969
5. McKillop IH, Schrum LW. Role of alcohol in liver carcinogenesis. Semin Liver Dis. 2009;29(2):222–232. doi:10.1055/s-0029-1214377
6. Ferreira RG, Cardoso MV, Furtado KMD, et al. Epigenetic alterations caused by aflatoxin b1: a public health risk in the induction of hepatocellular carcinoma. Transl Res. 2019;204:51–71. doi:10.1016/j.trsl.2018.09.001
7. Dibisceglie AM, Rustgi VK, Hoofnagle JH, et al. Hepatocellular-carcinoma. Ann Intern Med. 1988;108(3):390–401. doi:10.7326/0003-4819-108-3-390
8. Galle PR, Tovoli F, Foerster F, et al. The treatment of intermediate stage tumours beyond TACE: from surgery to systemic therapy. J Hepatol. 2017;67(1):173–183. doi:10.1016/j.jhep.2017.03.007
9. Kumar Y, Sharma P, Bhatt N, et al. Transarterial therapies for hepatocellular carcinoma: a comprehensive review with current updates and future directions. Asian Pac J Cancer Prev. 2016;17(2):473–478. doi:10.7314/APJCP.2016.17.2.473
10. Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223(2):331–337. doi:10.1148/radiol.2232010775
11. Graf D, Vallböhmer D, Knoefel WT, et al. Multimodal treatment of hepatocellular carcinoma. Eur J Intern Med. 2014;25(5):430–437. doi:10.1016/j.ejim.2014.03.001
12. Parikh ND, Marshall VD, Singal AG, et al. Survival and cost-effectiveness of sorafenib therapy in advanced hepatocellular carcinoma: an analysis of the SEER-medicare database. Hepatology. 2017;65(1):122–133. doi:10.1002/hep.28881
13. Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604.
14. Neuberger J. Liver transplantation. Semin Liver Dis. 2009;29(1):1. doi:10.1055/s-0029-1192051
15. Trojan J, Zangos S, Schnitzbauer AA. Diagnosis and treatment of hepatocellular carcinoma. Der Onkologe. 2013;19(10):893–902. doi:10.1007/s00761-013-2552-7
16. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. doi:10.1053/j.gastro.2007.04.061
17. Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8(10):798–812. doi:10.1038/nrm2257
18. Carmena M, Wheelock M, Funabiki H, et al. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat Rev Mol Cell Biol. 2012;13(12):789–803. doi:10.1038/nrm3474
19. Sampath SC, Ohi R, Leismann O, et al. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118(2):187–202. doi:10.1016/j.cell.2004.06.026
20. Schuldt A. Borealin shines light on spindle dynamics. Nat Cell Biol. 2004;6(8):692. doi:10.1038/ncb0804-692
21. Gassmann R, Carvalho A, Henzing AJ, et al. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol. 2004;166(2):179–191. doi:10.1083/jcb.200404001
22. Jeyaprakash AA, Klein UR, Nigg EA, et al. Structure and function of survivin- borealin-INCENP core complex in mitosis. Acta Crystallogr A. 2007;63:S127–S128.
23. Klein UR, Nigg EA, Gruneberg U. Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of borealin, survivin, and the N-terminal domain of INCENP. Mol Biol Cell. 2006;17(6):2547–2558. doi:10.1091/mbc.e05-12-1133
24. Hanson KK, Kelley AC, Bienz M. Loss of Drosophila borealin causes polyploidy, delayed apoptosis and abnormal tissue development. Development. 2005;132(21):4777–4787. doi:10.1242/dev.02057
25. Zhang QJ, Lin G, Gu YF, et al. Borealin is differentially expressed in ES cells and is essential for the early development of embryonic cells. Mol Biol Rep. 2009;36(3):603–609. doi:10.1007/s11033-008-9220-9
26. Dai C, Miao CX, Xu XM, et al. Transcriptional activation of human CDCA8 gene regulated by transcription factor NF-Y in embryonic stem cells and cancer cells. J Biol Chem. 2015;290(37):22423–22434. doi:10.1074/jbc.M115.642710
27. Bu YH, Shi LB, Yu DH, et al. CDCA8 is a key mediator of estrogen-stimulated cell proliferation in breast cancer cells. Gene. 2019;703:1–6.
28. Chang JL, Chen TH, Wang CF, et al. Borealin/Dasra B is a cell cycle-regulated chromosomal passenger protein and its nuclear accumulation is linked to poor prognosis for human gastric cancer. Exp Cell Res. 2006;312(7):962–973. doi:10.1016/j.yexcr.2005.12.015
29. Wang Y, Zhao Z, Bao XP, et al. Borealin/Dasra B is overexpressed in colorectal cancers and contributes to proliferation of cancer cells. Med Oncol. 2014;31(11). doi:10.1007/s12032-014-0248-5.
30. Bi YQ, Chen S, Jiang JZ, et al. CDCA8 expression and its clinical relevance in patients with bladder cancer. Medicine. 2018;97(34):e11899. doi:10.1097/MD.0000000000011899
31. Jeon T, Ko MJ, Seo YR, et al. Silencing CDCA8 suppresses hepatocellular carcinoma growth and stemness via restoration of ATF3 tumor suppressor and inactivation of AKT/beta-catenin signaling. Cancers. 2021;13(5):1055. doi:10.3390/cancers13051055
32. Lamb JR, Zhang CS, Xie T, et al. Predictive genes in adjacent normal tissue are preferentially altered by sCNV during tumorigenesis in liver cancer and may rate limiting. PLoS One. 2011;6(7):e20090. doi:10.1371/journal.pone.0020090
33. Makowska Z, Boldanova T, Adametz D, et al. Gene expression analysis of biopsy samples reveals critical limitations of transcriptome-based molecular classifications of hepatocellular carcinoma. J Pathol Clin Res. 2016;2(2):80–92. doi:10.1002/cjp2.37
34. Zhou YY, Zhou B, Pache L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1.
35. George B, Seals S, Aban I. Survival analysis and regression models. J Nucl Cardiol. 2014;21(4):686–694. doi:10.1007/s12350-014-9908-2
36. Lira RPC, Antunes-Foschini R, Rocha EM. Survival analysis (Kaplan-Meier curves): a method to predict the future. Arq Bras Oftalmol. 2020;83(2):V–VII. doi:10.5935/0004-2749.20200036
37. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. doi:10.1073/pnas.0506580102
38. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi:10.1038/nrm3758
39. Dominguez C, David JM, Palena C. Epithelial-mesenchymal transition and inflammation at the site of the primary tumor. Semin Cancer Biol. 2017;47:177–184. doi:10.1016/j.semcancer.2017.08.002
40. Karlsson MC, Gonzalez SF, Welin J, et al. Epithelial-mesenchymal transition in cancer metastasis through the lymphatic system. Mol Oncol. 2017;11(7):781–791. doi:10.1002/1878-0261.12092
41. Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7(344):344. doi:10.1126/scisignal.2005189
42. Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi:10.1016/j.cell.2012.05.012
43. Gasior K, Hauck M, Wilson A, et al. A theoretical model of the wnt signaling pathway in the epithelial mesenchymal transition. Theor Biol Med Model. 2017;14(1):19. doi:10.1186/s12976-017-0064-7
44. Bertuccio P, Turati F, Carioli G, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67(2):302–309. doi:10.1016/j.jhep.2017.03.011
45. Wang FS, Fan JG, Zhang Z, et al. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–2108. doi:10.1002/hep.27406
46. Mazzaferro V, Bhoori S, Sposito C, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;Suppl 17(S2):S44–S57. doi:10.1002/lt.22365
47. Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):
48. Chen C, Wang G. Mechanisms of hepatocellular carcinoma and challenges and opportunities for molecular targeted therapy. World J Hepatol. 2015;7(15):1964–1970. doi:10.4254/wjh.v7.i15.1964
49. Bekier ME, Mazur T, Rashid MS, et al. Borealin dimerization mediates optimal CPC checkpoint function by enhancing localization to centromeres and kinetochores. Nat Commun. 2015;6(1). doi:10.1038/ncomms7775.
50. Ci C, Tang B, Lyu DL, et al. Overexpression of CDCA8 promotes the malignant progression of cutaneous melanoma and leads to poor prognosis. Int J Mol Med. 2019;43(1):404–412. doi:10.3892/ijmm.2018.3985
51. Buscheck F, Sulimankhil M, Melling N, et al. Loss of cytoplasmic survivin expression is an independent predictor of poor prognosis in radically operated prostate cancer patients. Cancer Med. 2020;9(4):1409–1418. doi:10.1002/cam4.2773
52. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi:10.1016/j.devcel.2009.06.016
53. Birchmeier W. The Wnt/beta-catenin signaling pathway in development and disease. Pathol Res Pract. 2007;203(5):341.
54. Miao CG, Yang YY, He X, et al. Wnt signaling in liver fibrosis: progress, challenges and potential directions. Biochimie. 2013;95(12):2326–2335. doi:10.1016/j.biochi.2013.09.003
55. Ackers I, Malgor R. Interrelationship of canonical and non-canonical Wnt signalling pathways in chronic metabolic diseases. Diabetes Vasc Dis Res. 2018;15(1):3–13. doi:10.1177/1479164117738442
56. Libro R, Bramanti P, Mazzon E. The role of the Wnt canonical signaling in neurodegenerative diseases. Life Sci. 2016;158:78–88. doi:10.1016/j.lfs.2016.06.024
57. Vilchez V, Turcios L, Marti F, et al. Targeting Wnt/beta-catenin pathway in hepatocellular carcinoma treatment. World J Gastroenterol. 2016;22(2):823–832. doi:10.3748/wjg.v22.i2.823
58. Wang WH, Smits R, Hao HP, et al. Wnt/beta-catenin signaling in liver cancers. Cancers. 2019;11(7):926.
59. Pez F, Lopez A, Kim M, et al. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013;59(5):1107–1117. doi:10.1016/j.jhep.2013.07.001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.