Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Bony Hyperplasia Beneath Atrophic Soft Tissue: A Rare Case of En Coup de Sabre and Literature Review
Authors Ma X , Huang J, Chen Y, Wang X, Long X
Received 3 June 2023
Accepted for publication 25 August 2023
Published 31 August 2023 Volume 2023:16 Pages 2375—2379
DOI https://doi.org/10.2147/CCID.S424231
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Xuda Ma,1,* Jiuzuo Huang,1,* Yu Chen,2 Xiaojun Wang,1 Xiao Long1
1Department of Plastic Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 2Department of Radiology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao Long, Department of Plastic Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, No. 1 Shuaifuyuan, Wangfujing, Dongcheng District, Beijing, 100730, People’s Republic of China, Tel +86 135 1616 6205, Fax +86 10 6915 8730, Email [email protected]
Abstract: In this study, we report a rare case of en coup de sabre with hyperplasia of the left frontal bone beneath skin lesion, which is detected during magnetic resonance imaging screening and preoperative evaluation. A 27-year-old woman with 13-year history of progressive soft tissue depression in the forehead and scalp, and was treated by traditional Chinese herb before the disease went into stationary stage. The patient underwent serial long-pulsed laser treatments and autologous fat grafting with satisfactory outcome. To our knowledge, this is the first time that bony hyperplasia beneath the soft tissue lesion was found in a patient with en coup de sabre.
Keywords: localized scleroderma, morphea, autologous fat grafting, magnetic resonance imaging, computed tomography
Introduction
Localized scleroderma (morphea) is a group of sclerotic autoimmune diseases of the skin. Although the skin is the main organ involved in localized scleroderma, the underlying soft tissues and bones may also be affected with varying degrees of atrophy as the disease progresses. En coup de sabre (ECDS) is a common type of craniofacial localized scleroderma, with a linear, scar-like lesion on the forehead as its typical clinical presentation.1,2
Magnetic resonance imaging (MRI) of the head can help exclude potential brain involvement and was recommended by the European Dermatology Forum S1-guideline.3 The role of head MRI in the assessment of soft and bony tissue involvement is neglected in previous studies. We report a case of ECDS with rare bony hyperplasia beneath the skin lesion detected by MRI and computed tomography (CT) screening, and this lesion was stabilized after long-term traditional Chinese herbal treatment and was improved by multiple laser treatments and autologous fat grafting. Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) and PUMC LoSFAI were used for clinical scoring. We also reviewed and discussed the current assessment of craniofacial LoS. To our knowledge, this is the first time that bony hyperplasia beneath the atrophic soft tissue atrophy was found in a patient with ECDS.
Case Presentation
A 27-year-old woman presented with a 19-year history of soft tissue depression of the left upper eyelid, forehead, and scalp in March 2023. Since the onset, hair loss had gradually appeared in the affected area of the left scalp, and the depth of depression gradually increased, accompanied by skin sclerosis and hyperpigmentation in the affected area. At the age of 8, the patient went to our hospital, and laboratory tests suggested negative antinuclear antibody (ANA) and DNA double-stranded antibody (ds-DNA) IgG. Immunoglobulin blood tests showed that the patient’s IgG, IgM, and IgA were in the normal range. No abnormalities in the patient’s liver and kidney function tests were found. The patient was diagnosed with morphea ECDS and was treated with oral “Fu Su Pian” tablets, a traditional Chinese herb made from Lignum Sappan, Dalbergia Odorifera, Radix Paeoniae Rubra, Cyathula Officinalis Kuan and Astragalus Membranaceus, which has the therapeutic effect of promoting blood circulation. Topical tacrolimus ointment was also used to soften the lesion. No abnormalities were found in the above tests at subsequent annual follow-ups, and the disease gradually went into the stationary phase when the patient was 21 years old. Over the next 6 years, the patient underwent serial long-pulsed laser treatments on the alopecia lesion, resulting in a significant reduction in the hair loss area and a gradual appearance of vellus hair in the center of the lesion.
Surgery
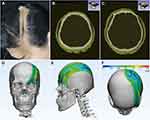
In April 2023, the patient decided to undergo surgical treatment to improve the tissue depression but refused to undergo an excision procedure; thus, autologous fat grafting was chosen for treatment (Figure 1). For screening of brain involvement and preoperative planning, the patient underwent a 3D Dixon MRI examination targeting both the head and facial soft tissues before performing surgery,4 and the MRI suggested that a speckled slightly long T2 signal shadow was visible subcutaneously on the right top frontal area with no obvious abnormal signal on diffusion-weighted imaging (DWI), and there was thinning and disappearance of the scalp fat layer on the left top frontal area, local depression and thinning of the left frontal bone, and bony protrusion of the frontal bone. Further CT and 3D reconstruction revealed a slight thinning of the left parietal bone and the zygomatic process of the left frontal bone and a sphenoidal bony protrusion of the left frontal area (Figure 2). Despite partial resorption of the fat graft, the patient was satisfied with the outcome of the procedure (Figure 1). Preoperative and postoperative clinical scores assessment showed a good surgical outcome (LoSCAT: 8 to 6; PUMC LoSFAI: 15 to 12). The bony protrusion was not surgically managed as the patient had no discomfort and refused to undergo resection.
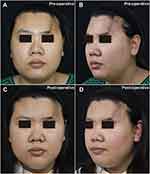 |
Figure 1 Facial photography of the patient. (A) Pre-operative front photo, (B) Pre-operative left 45-degree photo, (C) Post-operative front photo, (D) Post-operative left 45-degree photo. |
Discussion
Facial LoS often causes different degrees of cosmetic or functional damage. The area and depth vary greatly from patient to patient, and the skin, subcutaneous fat, oral mucosa, and brain can be involved. Therefore, an accurate assessment is critical during treatment, and several clinical assessment methods have been developed. LoSCAT is a convenient and easy-to-use clinical evaluation tool that assesses the activity of the disease as well as the degree of damage.5 Nevertheless, in the LoSCAT assessment, the face/scalp is evaluated as a part of the whole-body surface skin and lacks refinement in the face. Therefore, we developed the PUMC LoSFAI, which can be used as a compensatory clinical score for facial condition assessment.6 In addition, Guerrerosantos et al classified Parry-Romberg syndrome into four types according to the overall degree of facial tissue atrophy, which is an important guideline for reconstructive surgery.7 However, there is a lack of comprehensive evaluation methods for facial lesions, which may pose difficulties for condition monitoring and preoperative evaluation for plastic surgery.
Objective evaluation, including MRI, CT, ultrasound, 3D photographs, etc, can overcome the problem of overreliance on physicians’ experience in clinical scoring. The current application of MRI in LoS focuses on two areas: the assessment of deep tissue involvement beneath cutaneous lesions on the trunk and the screening of brain involvement in patients with head and facial LoS because of potential central nervous system problems such as epilepsy.8,9 However, the role of MRI in the assessment of facial lesions is often neglected, especially considering the excellent resolving power of MRI for soft tissue. As shown in this case, MRI is able to provide a good determination of the extent and depth of craniofacial involvement, not only for soft tissue abnormalities but also for abnormal bony changes. CT is very important for the assessment of altered bony structures, especially in patients with severe bony atrophy, and is a necessary preoperative examination for maxillofacial surgery, but radiation limits its use in condition monitoring.10 In most cases, MRI is not the primary method for bony assessment, and CT has unparalleled advantages. Ultrasound is a convenient and economical way to make a clear assessment of soft tissue involvement at the site of the disease and to detect changes in blood flow deep within the lesion.11 However, ultrasonography relies on experienced examiners for consistency and reproducibility. 3D photography is widely used in facial plastic surgery and is a very suitable method for the evaluation of facial LoS, which enables very convenient and economical documentation and measurement of facial lesions.12 It should be noted, however, that the current 3D photographic equipment has difficulty in evaluating lesions within the scalp, and other modalities may be required to assist in severe cases. In addition, 3D devices only record surface information and cannot be used for the assessment of deeper tissues, and other examinations are needed when necessary. Laser speckle contrast imaging13 and infrared thermal photography14 were used to detect abnormal blood flow changes in morphea, and skin elasticity measurements could be used to examine the degree of skin fibrosis,15 yet the application in facial lesions remains to be further validated.
Generally, there is a varying degree of atrophy of the deep subcutaneous fat as well as the bones at the lesions of ECDS.10,16 However, for the first time to our knowledge, abnormal bony hyperplasia beneath the atrophic subcutaneous fat atrophy was found in a patient with ECDS. Unfortunately, despite the detection of the rare bony hyperplasia, we were not able to find the exact cause. The “Fu Su Pian” is common traditional Chinese medicine for morphea in China, and no complications of bone changes have been reported with this drug for decades. One possible explanation is that the skin lesion affected the closure of the cranial sutures, as the bony hyperplasia seems to be distributed along the left coronal suture. The tension on the margins of the left frontal bone by severe atrophy of the superficial soft tissues during closure of the bony suture might be the cause of the hyperplasia. Common surgical treatments for ECDS include autologous fat grafting, local flaps, expanded scapular flap, etc.16 Since the overall tissue deficit in the frontal region can be adequately compensated for with soft tissue reconstructions, it is not necessary to intervene on bone atrophy or hyperplasia from an aesthetic perspective.
In conclusion, we report a rare case of ECDS with bony hyperplasia beneath atrophic skin lesion which is detected by MRI, expanding its application in craniofacial localized scleroderma.
Abbreviations
ECDS, en coup de sabre; MRI, magnetic resonance imaging; CT, computed tomography; ANA, antinuclear antibody; ds-DNA, NA double-stranded antibody; LoSCAT, Localized Scleroderma Cutaneous Assessment Tool; PUMC LoSFAI, Peking Union Medical College LoS facial aesthetic index.
Consent Statements
This study has been approved by the Institutional Review Board of Peking Union Medical College Hospital; approval I-22PJ352. Written informed consent has been obtained from the patient for publication of her photograph and information.
Acknowledgments
The research reported in this article was supported by grants from National Key Research and Development Program of China (2020YFE0201600), Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Science (2020-I2M-C&T-A-004 and 2021-I2M-1-003) and National High Level Hospital Clinical Research Funding of China (2022-PUMCH-A-210, 2022-PUMCH-B-041 and 2022-PUMCH-C-025).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tollefson MM, Witman PM. En coup de sabre morphea and Parry-Romberg syndrome: a retrospective review of 54 patients. J Am Acad Dermatol. 2007;56(2):257–263. doi:10.1016/j.jaad.2006.10.959
2. Mazzilli S, Vollono L, Cosio T, et al. Reflectance confocal microscopy applied to linear (en Coup de Sabre) morphea. Skin Appendage Disord. 2020;6(3):171–174. doi:10.1159/000506748
3. Knobler R, Moinzadeh P, Hunzelmann N, et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: localized scleroderma, systemic sclerosis and overlap syndromes. J Eur Acad Dermatol Venereol. 2017;31(9):1401–1424. doi:10.1111/jdv.14458
4. Liao X, Wang X, Xu Z, et al. Assessment of facial autologous fat grafts using Dixon magnetic resonance imaging. Quant Imaging Med Surg. 2022;12(5):2830–2840. doi:10.21037/qims-21-570
5. Arkachaisri T, Vilaiyuk S, Torok KS, Medsger TA Jr. Development and initial validation of the localized scleroderma skin damage index and physician global assessment of disease damage: a proof-of-concept study. Rheumatology. 2010;49(2):373–381. doi:10.1093/rheumatology/kep361
6. Wang HC, Ling S, Wang X, et al. The development and initial validation of PUMC localized scleroderma facial aesthetic index: a pilot study. Aesthetic Plast Surg. 2021;45(4):1531–1539. doi:10.1007/s00266-020-02111-4
7. Guerrerosantos J, Guerrerosantos F, Orozco J. Classification and treatment of facial tissue atrophy in Parry-Romberg disease. Aesthetic Plast Surg. 2007;31(5):424–434. doi:10.1007/s00266-006-0215-4
8. Schanz S, Fierlbeck G, Ulmer A, et al. Localized scleroderma: MR findings and clinical features. Radiology. 2011;260(3):817–824. doi:10.1148/radiol.11102136
9. Careta MF, Leite Cda C, Cresta F, Albino J, Tsunami M, Romiti R. Prospective study to evaluate the clinical and radiological outcome of patients with scleroderma of the face. Autoimmun Rev. 2013;12(11):1064–1069. doi:10.1016/j.autrev.2013.05.005
10. Hennocq Q, Facchini A, Kverneland B, Bodemer C, Picard A, Khonsari RH. Craniofacial bone atrophy in Parry Romberg syndrome demonstrated using a Bayesian hierarchical model. J Craniomaxillofac Surg. 2019;47(6):909–914. doi:10.1016/j.jcms.2019.03.032
11. Wortsman X, Wortsman J, Sazunic I, Carreno L. Activity assessment in morphea using color Doppler ultrasound. J Am Acad Dermatol. 2011;65(5):942–948. doi:10.1016/j.jaad.2010.08.027
12. Abbas LF, Joseph AK, Day J, et al. Measuring asymmetry in facial morphea via 3-dimensional stereophotogrammetry. J Am Acad Dermatol. 2023;88(1):101–108. doi:10.1016/j.jaad.2022.05.029
13. Chen B, Wang X, Long X, et al. Supportive use of adipose-derived stem cells in cell-assisted lipotransfer for localized scleroderma. Plast Reconstr Surg. 2018;141(6):1395–1407. doi:10.1097/PRS.0000000000004386
14. Ranosz‐Janicka I, Lis‐święty A, Skrzypek‐Salamon A, Brzezińska‐Wcisło L. Detecting and quantifying activity/inflammation in localized scleroderma with thermal imaging. Skin Res Technol. 2019;25(2):118–123. doi:10.1111/srt.12619
15. Andres C, Kollmar A, Mempel M, Hein R, Ring J, Eberlein B. Successful ultraviolet A1 phototherapy in the treatment of localized scleroderma: a retrospective and prospective study. Br J Dermatol. 2010;162(2):445–447. doi:10.1111/j.1365-2133.2009.09438.x
16. Skorochod R, Nesher G, Gronovich Y. Management options for linear scleroderma (“En Coup de Sabre”). Dermatol Surg. 2022;48(10):1038–1045. doi:10.1097/DSS.0000000000003539
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.