Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 8
Body vectoring technique with Radiesse® for tightening of the abdomen, thighs, and brachial zone
Authors Wasylkowski VC
Received 10 October 2014
Accepted for publication 19 February 2015
Published 19 May 2015 Volume 2015:8 Pages 267—273
DOI https://doi.org/10.2147/CCID.S75631
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Valeria Cogorno Wasylkowski
Médico Estético Cosmetic Medicine, Clinic Novosalud, Madrid, Spain
Background: The objective of this study was to investigate the efficacy, safety, and subject satisfaction of the calcium hydroxylapatite-based dermal filler Radiesse® in a novel body vectoring technique to correct skin flaccidity in the thighs, abdomen, and brachial zones.
Methods: Female subjects with self-evaluated flaccidity scores ≥3 on a 6-point scale (0, no flaccidity; 5, very severe flaccidity) in the zones of interest were included. Radiesse was injected according to predesigned vector maps (3 mL per thigh, 1.5 mL per hemiabdomen or brachial zone). Clinical assessments (skin density and thickness) were made by an independent reviewer at an exact position before and 5 weeks after treatment using a cutometer and an ultrascan. Subjects rated skin flaccidity before and 5 weeks after treatment on the 6-point scale and performed a pinch test to self-assess changes in skin thickness. All adverse events were recorded.
Results: Twenty females (aged 28–67 years) were enrolled, contributing 36 treatment zones. Across all zones, 78% of flaccidity measurements improved after treatment. Improvements in skin flaccidity were most common in the thighs (82% of cases). An improvement in skin density versus baseline was observed in the majority across all zones, most frequently in the abdomen (88% of cases). Skin thickness in each zone also improved versus baseline for the majority, most frequently in the thighs (88% of cases). Mean self-assessed flaccidity scores at baseline were 3.6 (thighs), 3.7 (abdomen), and 3.8 (brachial zone), and 2.6, 2.7, and 3.0, respectively, posttreatment. All subjects reported a positive pinch test. In total, 47.0% of subjects had bruising after treatment, which resolved within a week. No serious adverse events were reported.
Conclusion: Using this novel technique, Radiesse had notable results on skin flaccidity, density and thickness in the thighs, abdomen, and brachial zones, and was well tolerated.
Keywords: calcium hydroxylapatite, dermal filler, body vectoring, thighs, brachial zone, abdomen
Introduction
Aging and weight loss have visible effects on the skin. Structural changes to the skin associated with aging, such as changes in fat distribution and loss of collagen and elastic fibers, cumulate in a loss of tone and elasticity and manifest as wrinkles.1,2 Substantial weight loss has also been shown to be associated with decreased levels of skin collagen and elastin that might result in undesirable flaccidity.3
Soft tissue facial augmentation with dermal fillers is a minimally invasive esthetic technique that is growing in popularity.4–6 Several injectable, cross-linked hyaluronic acid-based gel fillers are available that are intended for use as an implant for restoring facial volume loss. The calcium hydroxylapatite-based volumetric filler, Radiesse® (Merz Pharmaceuticals GmbH, Frankfurt, Germany), is formulated to augment volume immediately, and subsequently to stimulate collagen production and thus restore volume in a process that mimics physiological remodeling of the extracellular matrix.7–9 The ability of Radiesse to evoke physiological collagenogenesis is associated with improvements in skin quality, such as skin tightening, and is evidenced by its efficacy for the correction of moderate-to-severe facial wrinkles and folds, particularly nasolabial folds.10,11
The aim of the present study was to investigate the efficacy, safety, and subject satisfaction of the volumetric effect of Radiesse in a novel body vectoring technique for correction of skin flaccidity in the thighs, abdomen, and brachial zones.
Materials and methods
This was a single-arm, prospective clinical study performed at a single center in Spain. Healthy female subjects aged 18–69 years were eligible for inclusion if they had a self-evaluated skin flaccidity rating of 3 or more in the treatment area of interest (thigh, abdomen, or brachial zone) on a 6-point scale from 0 (no flaccidity) to 5 (very severe flaccidity). Exclusion criteria comprised pregnancy, breast feeding, chronic use of nonsteroidal anti-inflammatory drugs, autoimmune conditions, obesity, and a known allergy to lidocaine. Written informed consent was obtained from each subject and the study was performed in line with local guidelines and those that have their origins in the 1975 Declaration of Helsinki. Twenty female subjects aged 28–67 years participated in the study, 25% of whom were current smokers. The 20 subjects provided a combined total of 36 treatment zones. Demographics and baseline characteristics are shown in Table 1.
  | Table 1 Demographics and baseline characteristics |
Visits and assessments
Digital photographs of the three treatment zones were taken before and 5 weeks after treatment. A cutometer MPA 580 (Courage + Khazaka Electronic GmbH, Cologne, Germany) and an ultrascan UC22C 22MHz (Taberna Pro Medicum GmbH, Luneburg, Germany) were used to locate and record skin density, thickness, and flaccidity measurements from an exact point, which was used before and 5 weeks after treatment. Measurements were taken by an independent reviewer. Cutometer and ultrascan measurements were conducted at the following approximate positions: for the thigh, 20 cm below the iliac crest bone, 10 cm from the internal edge; for the abdomen, 3 cm from the navel (left or right); and for the brachial zone, 20 cm from the acromion in a coronal cut of the arm, 12 cm internal.
Subjects were also asked to rate the flaccidity of their skin before treatment using the 6-point scale, and to perform a skin pinch test before treatment to assess skin thickness/density at baseline. Clinical assessments were repeated 5 weeks after treatment.
Study treatment
All eligible subjects received treatment at visit 1 (day 0, baseline). The right hand side of the body was treated first (visit 1); the left hand side was treated following completion of posttreatment assessments of the right hand side (5 weeks after treatment). Follow-up assessments of left hand side treatment occurred a further 5 weeks later.
Prior to treatment, vector maps for the right hand side of the body were designed to inform positioning of needles during administration (Figure 1). Briefly, all vector maps were drawn with the subject in a standing position. For the thigh, an anchor point on the border between the different skin types of the inner and outer thigh was located and two lines drawn. One followed the transition line of the two skin types and the other extended to the middle of the fat of the inner thigh. Several lines were then drawn protruding from these, with one line prolonged to ensure coverage of the entire inner thigh. This positioning approach aimed to lift the fat tissue of the internal side of the leg and improve skin quality. For the abdomen, a fix point to anchor the vectors was found under the ribs and two lines were drawn from this point, one line downward passing next to the navel; the second was drawn at a 45 degree angle to the first. Several protruding lines were drawn to cover almost the entire zone with the aim of correcting navel shape and improving skin quality. For the brachial zone, the aim was to lift the internal side of the arm and improve skin quality. A fix point was found at the deltoid muscle, then one line was drawn 3 cm into the axillary zone and a second was drawn to protrude two thirds into the arm. Several protruding lines were then drawn to cover the whole internal brachial zone. Five weeks after the right hand side of the body was treated, the same approach was used to design vectors for the left hand side ahead of treatment.
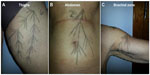  | Figure 1 Examples of a vector map for injection in (A) thighs, (B) abdomen, and (C) brachial zones. |
Radiesse (3 mL) was diluted, in line with the US Food and Drug Administration approval, with 0.6 mL lidocaine (2% without epinephrine). In all, 3 mL Radiesse solution was administered per thigh, 1.5 mL per hemiabdomen, and 1.5 mL per brachial zone. Injections were administered into the deep dermis using a 1 mL Luer-Lok™ syringe and a 27 G ×40 mm needle. For each line of the vector map, 0.05 mL Radiesse solution was injected.
Adverse events
All adverse events were recorded. Subjects rated the pain experienced during treatment on a 10-point scale from 1 (no pain) to 10 (very severe pain).
Results
Skin density and thickness
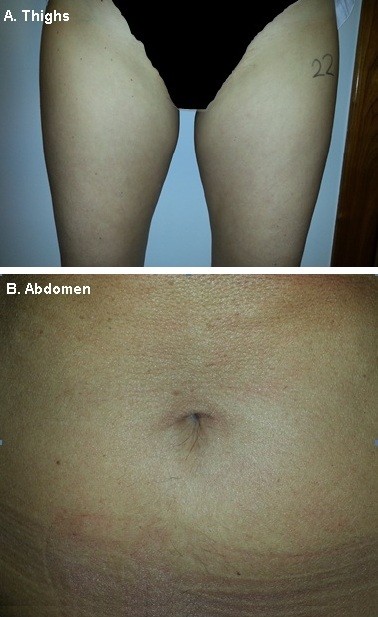
Across all treatment zones, cutometer data showed improvements in skin flaccidity versus baseline in 78% of cases at 5 weeks after treatment (Figure 2), most commonly in the thighs (82% of cases). The mean reduction in flaccidity from baseline to 5 weeks after treatment was also calculated, and was 0.0924 mm, 0.0117 mm, and 0.0814 mm for the brachial zone, thighs, and abdomen, respectively. The overall mean reduction in flaccidity from baseline to 5 weeks after treatment was 0.093 mm. At 5 weeks after treatment, ultrascan measurements were taken at the same positions as those taken at baseline. Improvements in skin density relative to baseline were recorded in the majority of measurements across all treatment zones (Figure 3). The abdomen showed the best treatment response, with improvements versus baseline in 88% of cases. Skin thickness also showed improvement versus baseline in the majority of cases (Figure 3), but most frequently in the thighs (88% of cases). Representative clinical photographs before and after treatment are provided in Figure 4.
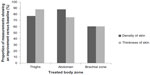  | Figure 3 Proportions of skin density and thickness scores that showed an improvement 5 weeks after treatment relative to baseline. |
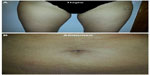  | Figure 4 Clinical photographs of the treatment area before and 5 weeks after treatment for (A) thighs and (B) abdomen (right side, treated; left side, untreated). |
Subject self-assessments
At baseline, mean subject-assessed flaccidity scores for the thighs, abdomen, and brachial zones were 3.6, 3.7, and 3.8, respectively (Figure 5). All scores at baseline were in the range of 3–5. Following treatment, subject-assessed flaccidity scores showed an improvement versus baseline in 27 of the 36 treated zones (75%). The mean self-assessed flaccidity score following treatment was 2.6 for the thighs, 2.7 for the abdomen, and 3.0 for the brachial zones. All subjects (100%) reported a positive pinch test, indicating an improvement in skin thickness.
Safety
Bruising was reported for 16 of the 36 treated zones (44.4%) following treatment. All cases of bruising resolved within 1 week. No serious adverse events were reported. Seventeen subjects (85%) rated the pain they experienced during treatment as 4 on a 10-point scale from 1 (no pain) to 10 (very severe pain).
Discussion
This single-arm, prospective clinical study demonstrated that the body vectoring technique using Radiesse induced notable reductions in skin flaccidity, increased skin density, and increased skin thickness in three body zones. Subjects were satisfied with their treatment, with the majority recording an improvement in skin flaccidity via self-evaluation and all subjects recording a positive pinch test. All three treatment zones showed a good response in terms of efficacy and subject satisfaction, particularly at the abdomen and thighs. The treatment was well tolerated, with any treatment-related bruising resolving within a week.
While body vectoring is a relatively novel application of Radiesse, its use in facial soft tissue augmentation is established4 and it has been demonstrated to have a good safety and efficacy profile.10,12–14 A vectoring approach has also been documented as an effective method for restoring facial proportions.15 The simple application technique used in the present study and the high reproducibility of positive responses suggest that the potential applications of Radiesse could expand beyond that of facial soft tissue augmentation to include correction of flaccid skin in the abdomen, thigh, and brachial zones. Such a procedure may be desirable to counteract the structural changes in the skin that are associated with aging and with substantial weight loss.1,3
In addition to an immediate volumetric effect, Radiesse has the ability to stimulate long-term physiological remodeling of the extracellular matrix.7,8 Specifically, Radiesse has demonstrated an effect on collagen, elastin, and fibroblasts,7–9 which may give Radiesse the potential to have long-lasting esthetic effects in skin rejuvenation. On the basis of the positive results in the present study, it is envisaged that this body vectoring technique could be used as a preventative measure; treatments would probably not be needed more than once per year, due to the potential of Radiesse to have sustained efficacy.11
In this study, Radiesse was reconstituted with lidocaine, which has previously not demonstrated any notable effect on the physical properties of the filler; however, any impact on subject comfort, durability, and efficacy remains to be determined.16 The use of lidocaine injection followed by a massaging technique has been found to facilitate the dispersion of Radiesse.17
The long-term maintenance of the esthetic effect was not explored in this preliminary study, but long-term durability of the effect of Radiesse in facial augmentation has previously been demonstrated.11,18 Future studies should monitor subjects in the longer term to explore the sustained efficacy of the treatment. Another limitation of the present study was the small number of subjects who participated. We recorded improvements in self-evaluation scores following treatment, indicative of a good level of patient satisfaction. It is important to note the subjective nature of these data, and the impact that patient expectations of the treatment might have had on their assessment of the results.
Conclusion
This novel body vectoring technique with Radiesse had notable positive results on skin flaccidity in the thigh, abdominal, and brachial zones, was well tolerated, and had good subject satisfaction.
Acknowledgments
Editorial assistance was provided by SCI Scientific Communications and Information, Oxford, UK, and was funded by Merz Pharmaceuticals GmbH, Frankfurt, Germany.
Disclosure
The author is employed by Merz Pharmaceuticals GmbH in a part-time role to deliver clinical education and training.
References
Montagna W, Carlisle K. Structural changes in ageing skin. Br J Dermatol. 1990;122 Suppl 35:61–70. | |
Baumann L. Skin ageing and its treatment. J Pathol. 2007;211(2):241–251. | |
Choo S, Marti G, Nastai M, Mallalieu J, Shermak MA. Biomechanical properties of skin in massive weight loss patients. Obes Surg. 2010; 20(10):1422–1428. | |
Carruthers J, Cohen SR, Joseph JH, Narins RS, Rubin M. The science and art of dermal fillers for soft-tissue augmentation. J Drugs Dermatol. 2009;8(4):335–350. | |
Emer J, Sundaram H. Aesthetic applications of calcium hydroxylapatite volumizing filler: an evidence-based review and discussion of current concepts: (part 1 of 2). J Drugs Dermatol. 2013;12(12):1345–1354. | |
The American Society for Aesthetic Plastic Surgery. Statistics 2012. Available from: http://www.surgery.org/media/statistics. Accessed February 10, 2014. | |
Marmur ES, Phelps R, Goldberg DJ. Clinical, histologic and electron microscopic findings after injection of a calcium hydroxylapatite filler. J Cosmet Laser Ther. 2004;6(4):223–226. | |
Berlin AL, Hussain M, Goldberg DJ. Calcium hydroxylapatite filler for facial rejuvenation: a histologic and immunohistochemical analysis. Dermatol Surg. 2008;34 Suppl 1:S64–S67. | |
Yutskovskaya Y, Kogan E, Leshunov E. A Randomized, split-face, histomorphologic study comparing a volumetric calcium hydroxylapatite and a hyaluronic acid-based dermal filler. J Drugs Dermatol. 2014;13(9):1047–1052. | |
Tzikas TL. Evaluation of the Radiance FN soft tissue filler for facial soft tissue augmentation. Arch Facial Plast Surg. 2004;6(4):234–239. | |
Tzikas TL. A 52-month summary of results using calcium hydroxylapatite for facial soft tissue augmentation. Dermatol Surg. 2008;34 Suppl 1:S9–S15. | |
Smith S, Busso M, McClaren M, Bass LS. A randomized, bilateral, prospective comparison of calcium hydroxylapatite microspheres versus human-based collagen for the correction of nasolabial folds. Dermatol Surg. 2007;33 Suppl 2:S112–S121. | |
Silvers SL, Eviatar JA, Echavez MI, Pappas AL. Prospective, open-label, 18-month trial of calcium hydroxylapatite (Radiesse) for facial soft-tissue augmentation in patients with human immunodeficiency virus-associated lipoatrophy: one-year durability. Plast Reconstr Surg. 2006;118(3 Suppl):34S–45S. | |
Pavicic T. Calcium hydroxylapatite filler: an overview of safety and tolerability. J Drugs Dermatol. 2013;12(9):996–1002. | |
Busso M. Vectoring approach to midface recontouring using calcium hydroxyaoatine and hyaluronic acid. Cosmet Dermatol. 2009;22(10):522–528. | |
Busso M, Voigts R. An investigation of changes in physical properties of injectable calcium hydroxylapatite in a carrier gel when mixed with lidocaine and with lidocaine/epinephrine. Dermatol Surg. 2008;34(S1):S16–S24. | |
Voigt R, Devore DP, Grazer JM. Dispersion of calcium hydroxylapatite accumulations in the skin: animal studies and clinical practices. Dermatol Surg. 2010;36(S1):798–803. | |
Bass LS, Smith S, Busso M, McClaren M. Calcium hydroxylapatite (Radiesse) for treatment of nasolabial folds: long-term safety and efficacy results. Aesthet Surg J. 2010;30(2):235–238. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


