Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Blood pressure response to exposure to moderate altitude in patients with COPD
Authors Schwarz EI , Latshang TD, Furian M , Flück D, Segitz S, Müller-Mottet S, Ulrich S , Bloch KE , Kohler M
Received 13 November 2018
Accepted for publication 31 January 2019
Published 14 March 2019 Volume 2019:14 Pages 659—666
DOI https://doi.org/10.2147/COPD.S194426
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Esther I Schwarz,1 Tsogyal D Latshang,1 Michael Furian,1 Deborah Flück,1 Sebastian Segitz,1 Severine Müller-Mottet,1 Silvia Ulrich,1 Konrad E Bloch,1,2 Malcolm Kohler1,2
1Department of Pulmonology and Sleep Disorders Centre, University Hospital of Zurich, Zurich, Switzerland; 2Centre for Integrative Human Physiology, University of Zurich, Zurich, Switzerland
Purpose: Patients with COPD might be particularly susceptible to hypoxia-induced autonomic dysregulation. Decreased baroreflex sensitivity (BRS) and increased blood pressure (BP) variability (BPV) are markers of impaired cardiovascular autonomic regulation and there is evidence for an association between decreased BRS/increased BPV and high cardiovascular risk. The aim of this study was to evaluate the effect of short-term exposure to moderate altitude on BP and measures of cardiovascular autonomic regulation in COPD patients.
Materials and methods: Continuous morning beat-to-beat BP was noninvasively measured with a Finometer® device for 10 minutes at low altitude (490 m, Zurich, Switzerland) and for 2 days at moderate altitude (2,590 m, Davos Jakobshorn, Switzerland) – the order of altitude exposure was randomized. Outcomes of interest were mean SBP and DBP, BPV expressed as the coefficient of variation (CV), and spontaneous BRS. Changes between low altitude and day 1 and day 2 at moderate altitude were assessed by ANOVA for repeated measurements with Fisher’s exact test analysis.
Results: Thirty-seven patients with moderate to severe COPD (mean±SD age 64±6 years, FEV1 60%±17%) were included. Morning SBP increased by +10.8 mmHg (95% CI: 4.7–17.0, P=0.001) and morning DBP by +5.0 mmHg (95% CI: 0.8–9.3, P=0.02) in response to altitude exposure. BRS significantly decreased (P=0.03), whereas BPV significantly and progressively increased (P<0.001) upon exposure to altitude.
Conclusion: Exposure of COPD patients to moderate altitude is associated with a clinically relevant increase in BP, which seems to be related to autonomic dysregulation.
Clinical trial registration: ClinicalTrials.gov (NCT01875133).
Keywords: COPD, hypobaric hypoxia, baroreflex sensitivity, blood pressure variability
Plain language summary
Why was the study done? Hypoxia is a potent stressor for the cardiovascular system and patients with COPD might be particularly susceptible to hypoxia-induced autonomic dysregulation.
What did the researchers do and find? When patients with moderate to severe COPD were exposed to hypobaric hypoxia at moderate altitude, they reacted with a clinically relevant increase of both blood pressure and heart rate accompanied by an increased blood pressure variability and a decreased baroreflex sensitivity.
What do these results mean? Disturbed autonomic cardiovascular regulation and increase in blood pressure and heart rate in response to hypoxia have important clinical implications for patients with COPD for altitude and air traveling and could explain part of the underlying pathophysiology of the observed risk of cardiovascular events during an acute exacerbation of COPD.
Introduction
COPD is characterized by progressive airflow limitation resulting from diffuse small airway disease and destruction of the lung parenchyma, leading to hypoxia and lung hyperinflation as well as worsening dyspnea on exertion, wheezing, and cough.1 Many patients with COPD suffer from comorbidities such as cardiovascular disease and COPD is one of the leading causes of mortality worldwide.2
Barometric pressure and partial pressure of inspired and arterial oxygen fall with increasing altitude. Physiologic studies exposing healthy subjects to simulated altitude in the hypobaric chamber have shown an increase in sympathetic activity and autonomic dysregulation.3–5
Arterial oxygen partial pressure and saturation in patients with moderate to severe COPD are usually already decreased at rest at lowlands when compared with healthy subjects. Patients with COPD might develop severe hypoxemia at altitude that is further aggravated during physical activity.6 Hypoxia-induced changes affect the cardiovascular system in particular. In addition, COPD is thought to be associated with impaired autonomic regulation,7,8 which has been shown to be improved by supplemental oxygen.9 Thus, COPD patients might be particularly susceptible to hypoxia-induced changes of cardiovascular regulation at altitude.10 In addition, other conditions of marked hypoxemia, such as acute exacerbations of COPD, are associated with an increased risk for cardiovascular events in COPD.11
BP was shown to increase in healthy subjects at ascent to altitude.10,12 There is only very limited data from simulated altitude13,14 and none from field studies on the effects of altitude exposure on BP or autonomic cardiovascular regulation in COPD. However, considering the high prevalence of COPD, the number of patients with COPD traveling to altitude for recreational activities or pulmonary rehabilitation in clinics located at moderate altitude is expected to be high. This implies a clinical importance of studying the effects of altitude exposure on pathophysiologic changes of the cardiovascular system in patients with COPD, who often suffer from comorbid cardiovascular disease.
Decreased BRS15,16 and increased BPV17–22 are established markers of impaired cardiovascular autonomic regulation. BPV underlies several cardiovascular control mechanisms and its increase is observed in conditions of impaired autonomic regulation. Baroreceptors as part of the autonomic nervous system operate within seconds and modulate moment-by-moment variations in BP. The baroreflex works via a negative feedback loop: an increase in BP results in stretching the baroreceptors in the aortic arch and the carotid sinus that leads to lowering of the heart rate via the medulla oblongata and results in BP lowering by reducing cardiac output. Arterial baroreceptors (stretch receptors in the aortic arch and the carotid sinus) can be reset during, for example, exercise or sleep.23 Spontaneous BRS is assessed by analyzing the relation of BP and heart rate variations in the time or frequency domain.
Based on the knowledge of physiologic studies on the effects of hypobaric hypoxia in healthy subjects, we hypothesized that acute altitude exposure of COPD patients will result in a relevant increase in BP by disturbed autonomic regulation of the cardiovascular system. To test this hypothesis, we performed a randomized altitude trial investigating the effect of a short-term stay at moderate altitude on BP and measures of cardiovascular autonomic regulation in COPD patients living at low altitude.
Material and methods
Trial design and intervention
This study was performed as a substudy of a randomized trial on the effects of altitude exposure in COPD on exercise capacity that took place in Switzerland between May 2013 (first recruitment) and December 2013 (last patient visit). Participants were examined at low altitude at 490 m a.s.l. (Zurich, barometric pressure [PB] 959 hPa) and at moderate altitude at 2,590 m a.s.l. (Davos Jakobshorn, PB 749 hPa) in random order (interval between assessments at different altitude levels ≥2 weeks) under a standardized environmental condition (temperature, position, time of the day). Block randomization was performed by choosing a time schedule for the assessments being blinded to the order of altitudes.24 The data analyzer was blinded to the group allocation. Transport between study locations was by train and cable car. Outcome assessments were performed on 2 consecutive days to study a potential acclimatization effect by physiologic adaptation. The study protocol was approved by the local ethics committee (EK-2013–0088) and registered at ClinicalTrials.gov (NCT01875133). Written informed consent was obtained from each participant before inclusion. The study was conducted according to the Declaration of Helsinki.
Participants
Patients aged 18–75 years who were diagnosed with moderate to severe COPD (COPD Gold grade 2–3) and were living at low altitude (<800 m a.s.l.) were included. Patients with mild (FEV1 >80% predicted) or very severe COPD (FEV1 <30% predicted, paO2 at 490 m <7.3 kPa [55 mmHg], paCO2 at 490 m >6.7 kPa [50 mmHg]), requiring supplemental oxygen at low altitude, currently suffering from a COPD exacerbation, currently smoking >20 cigarettes/day, with poorly controlled cardiovascular disease or with known altitude intolerance were excluded. Low and moderate altitude settings were the Sleep Unit of the University Hospital of Zurich and a cable car station in Davos Jakobshorn, respectively. The moderate altitude study location of 2,590 m (PB 749 hPa) corresponds to many mountain huts as well as to cabin pressure during air travel.
Sample size estimation based on expected minimally important differences in the primary outcome of the main trial (six-minute walk test)25,26 and data from previous altitude trials.27
Outcomes
The main outcome of interest was the change in both morning SBP and morning DBP. Other outcomes of interest were spontaneous BRS and BPV, both as measures of sympathovagal function. Heart rate was an additional outcome.
Continuous morning beat-to-beat BP was noninvasively measured by a physician with the Finometer® MIDI device (Finapres Medical Systems B.V., Amsterdam, the Netherlands) for 10 minutes in the early morning with the patient in supine position after a period of rest of >10 minutes in a quiet room with stable temperature and lightening. Application of the Finapres (FINger Arterial PRESsure) technique28 allows noninvasive continuous measurement of finger arterial pressure by a servo-plethysmo-manometer with infra-red transmission whereby volume fluctuations serve as surrogate of intra-arterial BP. It provides accurate assessments of short-term changes of BP as previously described and validated.29 BPV was expressed as the CV (ratio of the SD to the mean) of continuous beat-to-beat BP. BRS was automatically quantified with dedicated software (BeatScope Easy v1.1a, ADInstruments, Sydney, Australia).30
Statistical methods
Normality of distribution was tested by Kolmogorov-Smirnov tests. Normally distributed data are described as mean (SD) and nonnormally distributed data as median (IQR). Changes between low altitude and day 1 and day 2 at moderate altitude were assessed by ANOVA for repeated measurements with Fisher post hoc analysis. The average of the two measurements at low altitude (day 1 and day 2) was compared with the measurements at day 1 and day 2 at moderate altitude. Statistical significance level was set at alpha <0.05. In addition to univariate regression, multivariate regression analysis was used to study the relationship between changes in BP, BPV and BRS and clinical characteristics (airflow limitation at baseline, sleep apnea severity at altitude, hypoxemia, and exercise desaturation) correcting for potential confounders (age and baseline value of the actual variable, eg, BP or BRS). Statistical analysis was performed with Statistica version 6 software (StatSoft, Inc., Tulsa, OK, USA).
Ethics statement
The study was approved by the Ethics Committee of the Canton of Zurich, Switzerland (Kantonale Ethikkommission Zürich); registration reference EK-2013 – 0088.
Results
Participants
Forty patients with moderate to severe COPD were randomly assigned to the order of altitude exposure. Three participants refused to take part in the assessments on the other altitude level and withdrew consent. Finometer measurements at both altitudes were available for 37 patients. The study flowchart is shown in Figure 1. Patient characteristics are shown in Table 1.
  | Figure 1 Study flowchart. |
Main outcome of interest
Morning SBP increased by +10.8 mmHg (95% CI: 4.7–17.0, P=0.001) and morning DBP by +5.0 mmHg (95% CI: 0.8–9.3, P=0.02) at the first day of altitude exposure (Table 2). On the second day of the altitude sojourn, BP slightly decreased, but SBP was still statistically significantly elevated when compared to baseline (Table 2, Figure 2).
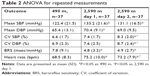  | Table 2 ANOVA for repeated measurements |
Other outcomes of interest
BRS significantly decreased (−3.0 msec/mmHg, 95% CI: −5.7 to −0.3, P=0.03) at moderate altitude, whereas BPV significantly and progressively increased (+1.8%, 95% CI: 0.9–2.6, P<0.001) upon ascent to moderate altitude (Table 2, Figure 2). Heart rate increased by +6.7 bpm (95% CI: 4.3–9.1, P<0.001).
Predictors of BP and autonomous response to altitude
In multivariate regression modelling including age, severity of airflow limitation (FEV1 percent predicted), oxygenation (paO2), oxygen desaturation during exercise at altitude, and severity of sleep-disordered breathing at altitude (oxygen desaturation index), only severity of airflow limitation and desaturation during exercise were independently associated with the response of blood pressure and its variability to altitude exposure. Within the multivariate model, no independent association of these parameters with BRS were identified. Changes in BRS were significantly correlated with intermittent nocturnal hypoxia (oxygen desaturation index, r=−0.43, P=0.025) but not with daytime oxygen saturation at altitude in univariate regression.
Discussion
This trial has shown that acute exposure of patients with moderate to severe COPD to moderate altitude results in a clinically relevant increase in BP. This was accompanied by a significant increase in BPV and a decrease in BRS, indicating autonomic dysregulation as the cause of the BP increase. Although an increase in cardiac output and in BP are a physiologic response to hypoxia, the increase in heart rate and BP seen in COPD patients was more extensive than in healthy young subjects exposed to the same level of hypobaric hypoxia.31 The significantly more pronounced increase in SBP and heart rate in this COPD cohort compared to healthy controls31 upon exposure to moderate altitude (same location, same environmental conditions) point towards a higher susceptibility of COPD patients to respond to hypobaric hypoxia with an increase in sympathetic activity.
There were moderate linear relationships between measures of exercise hypoxemia at altitude and changes in BP and its variability in response to altitude exposure. This finding leads to the conclusion that an increase in both BP and BPV may also be expected in other conditions of marked hypoxia in COPD patients, such as during a COPD exacerbation. Whereas the change in BRS was not associated with daytime hypoxemia at altitude, BRS negatively correlated with intermittent nocturnal hypoxia, meaning that concomitant sleep apnea might impair BRS.
BRS- and BPV-values in our patients were within the normal limit and BP was in the normotensive range at low altitude. BRS significantly declined to pathologic values upon altitude exposure. Mean BRS in healthy subjects aged 50–60 years was 6.8 ms/mmHg (95% CI: 6.2–7.3 ms/mmHg) in a large population-based study and was shown to decline with age.30 Normal values of short-term BPV are expected to be ~6%–8%.32
There is evidence of an association between increased BPV – visit-to-visit variability20,21 or variability during 24 h BP monitoring22 – and high cardiovascular risk. Less is known on beat-to-beat BPV. The baroreceptor reflex is responsible for fast, short-term, neuronal regulation of BP. However, beat-to-beat BPV was shown to be associated with visit-to-visit BPV despite different and complex underlying mechanisms.33,34
Autonomic regulation is influenced by hypoxemia and hypocapnia. In our patients, both paO2 and paCO2 from arterial blood gas analysis significantly decreased upon altitude exposure (paO2 from 8.2±1.9 to 6.0±1.1 kPa, and paCO2 from 5.6±0.9 to 4.7±0.5 kPa).35 In the same study population, exposure to moderate altitude was shown to be associated with a reduced oxygen uptake and reduced cerebral tissue oxygenation when compared to low altitude.35 Echocardiography in this population revealed a reduction of parameters assessing right ventricular function and an increase in calculated mean pulmonary artery pressure in response to moderate altitude that were mainly interpreted as a consequence of hypoxic pulmonary vasoconstriction.36 Of possible pathophysiologic importance to the BP increase at moderate altitude is the development of moderate to severe central sleep apnea at 2,590 m, additionally resulting in increased sympathetic activity due to the intermittent hypoxia and disturbed sleep.24 However, sleep apnea severity expressed as the apnea–hypopnea index or oxygen desaturation index was not independently associated with the BP response to altitude exposure. Nevertheless, known breathing disturbances during sleep at altitude due to frequent central events may play a role in the observed autonomic changes.
In accordance with our finding, Kourtidou-Papadeli et al14 have shown a statistically significant increase in SBP in patients with COPD and a less pronounced increase in SBP in healthy subjects exposed to hypobaric hypoxia by simulated altitude. Our findings of a slight decrease in BP over days of the altitude sojourn is in line with the finding of Kanstrup et al,37 which showed a BP increase upon ascent followed by a slight decrease on day 2 at altitude.
Patakas et al7 found a lower BRS after injection of phenylephrine in COPD patients than in healthy controls. In their study, pulmonary artery pressure during right heart catheterization showed the strongest association with BRS. They concluded that blunted baroreceptor response to pharmacologically induced BP changes in COPD is partially related to pulmonary hypertension and partly to the central effects of hypoxia and hypercapnia.7 Mazzeo et al38 found an increase in urinary and arterial epinephrine at initial exposure to altitude (4,300 m) in healthy men and observed a decrease after 3 weeks of acclimatization.
Sympathetic hyperactivation along with compromise in parasympathetic activity was found in healthy subjects exposed to high altitude at 6 and 18 months compared to baseline at low altitude. However, a reduction of sympathetic activity and an increased parasympathetic response was observed over time at altitude suggesting a partial acclimatization.39
Our findings are the first field study data on the effect of altitude on measures of autonomic cardiovascular regulation in COPD patients. This study provides an explanation for the observed increase in BP by showing autonomic dysregulation in COPD patients exposed to altitude.
A strength of the study is the randomization of altitude exposure, therefore avoiding influence of adaption to the measurements taken. Knowledge on the cardiovascular effects of exposing COPD to the altitude of 2,590 m is of clinical importance since barometer pressure at this altitude is comparable to that of many mountain huts and to the maximal altitude equivalent for commercial air flight. The findings on the effect of hypoxia on cardiovascular autonomic regulation have important implications for other conditions of acutely aggravated hypoxia, eg, acute exacerbations of COPD.
Limitations
A limitation for the interpretation of secondary outcomes is the fact that the sample size was not powered to analyze independent associations between the main outcomes and clinical predictors and some associations may not have been identified – these analyses are purely explorative. In addition, the sample size limits subgroup analyses on the role of antihypertensive drugs and some of the statistical approaches (eg, multivariable regression). Another limitation of the study is a potential influence of the results by requirement of supplemental oxygen at moderate altitude in five patients. However, oxygen was not supplemented during the outcome assessments. The potential influence of anticholinergic and beta-adrenergic agents on autonomic regulation was not studied in this trial. However, daily tiotropium for 3 months did not affect heart rate variability in 70 COPD patients.40 Although the findings in this COPD cohort can be compared to healthy controls in a previous study,31 the control subjects were not matched in terms of age or the presence of hypertension, which limits the interpretation of such a comparison.
Future altitude trials are warranted to assess the role of supplemental oxygen and therefore reduction of hypoxemia at altitude on BP and measures of autonomic cardiovascular regulation.
Conclusion
Short-term exposure of patients with COPD to moderate altitude is associated with a clinically relevant increase in BP, which is related to autonomic dysregulation. This finding should be considered when COPD patients with cardiovascular comorbidities are counselled on vacation or rehabilitation at altitude or for air traveling and in other conditions of hypoxemia such as acute exacerbations of COPD.
Acknowledgments
This work was supported by a Swiss National Science Foundation grant (32003B_143875) and the Clinical Research Priority Program (CRPP) Sleep and Health of the University of Zurich. The sponsors had no role in the design or conduct of the study, analysis and interpretation of the data, or writing the manuscript. The abstract of this paper was presented at the Annual Conference of the Schweizerische Gesellschaft für Pneumologie in Lugano, Switzerland (16–17 April 2015) as a conference talk and at the Annual Congress of the European Respiratory Society in Amsterdam, the Netherlands (26–30 September 2015) as a poster. The poster’s abstract was published in a supplement of the European Respiratory Journal, 1 September 2015, volume 46, issue suppl 59. DOI 10.1183/13993003.congress-2015.PA2309.
Author contributions
Drafting the article: EIS. All authors contributed to data analysis, revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest in this work.
References
Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(4):347–365. | ||
Chen W, Thomas J, Sadatsafavi M, Fitzgerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–639. | ||
Sevre K, Bendz B, Hanko E, et al. Reduced autonomic activity during stepwise exposure to high altitude. Acta Physiol Scand. 2001;173(4):409–417. | ||
Sevre K, Bendz B, Rostrup M. Reduced baroreceptor reflex sensitivity and increased blood pressure variability at 2400 M simulated cabin altitude. Aviat Space Environ Med. 2002;73(7):632–634. | ||
Heindl S, Lehnert M, Criée CP, Hasenfuss G, Andreas S. Marked sympathetic activation in patients with chronic respiratory failure. Am J Respir Crit Care Med. 2001;164(4):597–601. | ||
Graham WG, Houston CS. Short-term adaptation to moderate altitude. Patients with chronic obstructive pulmonary disease. JAMA. 1978;240(14):1491–1494. | ||
Patakas D, Louridas G, Kakavelas E. Reduced baroreceptor sensitivity in patients with chronic obstructive pulmonary disease. Thorax. 1982;37(4):292–295. | ||
van Gestel AJR, Kohler M, Clarenbach CF. Sympathetic overactivity and cardiovascular disease in patients with chronic obstructive pulmonary disease (COPD). Discov Med. 2012;14(79):359–368. | ||
Bartels MN, Gonzalez JM, Kim W, de Meersman RE. Oxygen supplementation and cardiac-autonomic modulation in COPD. Chest. 2000;118(3):691–696. | ||
Rhodes HL, Chesterman K, Chan CW, et al. Systemic blood pressure, arterial stiffness and pulse waveform analysis at altitude. J R Army Med Corps. 2011;157(1):110–113. | ||
Kunisaki KM, Dransfield MT, Anderson JA, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events: a cohort analysis. Am J Respir Crit Care Med. 2018;198(1):51–57. | ||
Veglio M, Maule S, Cametti G, et al. The effects of exposure to moderate altitude on cardiovascular autonomic function in normal subjects. Clin Auton Res. 1999;9(3):123–127. | ||
Berg BW, Dillard TA, Derderian SS, Rajagopal KR. Hemodynamic effects of altitude exposure and oxygen administration in chronic obstructive pulmonary disease. Am J Med. 1993;94(4):407–412. | ||
Kourtidou-Papadeli C, Papadelis C, Koutsonikolas D, Boutzioukas S, Styliadis C, Guiba-Tziampiri O. High altitude cognitive performance and COPD interaction. Hippokratia. 2008;12(Suppl 1):84–90. | ||
La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351(9101):478–484. | ||
Ormezzano O, Cracowski JL, Quesada JL, Pierre H, Mallion JM, Baguet JP. Evaluation of the prognostic value of baroreflex sensitivity in hypertensive patients: the EVABAR study. J Hypertens. 2008;26(7):1373–1378. | ||
Parati G, Ulian L, Santucciu C, Omboni S, Mancia G. Blood pressure variability, cardiovascular risk and antihypertensive treatment. J Hypertens Suppl. 1995;13(4):S27–S34. | ||
Bombelli M, Fodri D, Toso E, et al. Relationship among morning blood pressure surge, 24-hour blood pressure variability, and cardiovascular outcomes in a white population. Hypertension. 2014;64(5):943–950. | ||
Parati G, Ochoa JE, Bilo G. Blood pressure variability, cardiovascular risk, and risk for renal disease progression. Curr Hypertens Rep. 2012;14(5):421–431. | ||
Suchy-Dicey AM, Wallace ER, Mitchell SV, et al. Blood pressure variability and the risk of all-cause mortality, incident myocardial infarction, and incident stroke in the cardiovascular health study. Am J Hypertens. 2013;26(10):1210–1217. | ||
Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895–905. | ||
Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens. 1987;5(1):93–98. | ||
DiCarlo SE, Bishop VS. Central baroreflex resetting as a means of increasing and decreasing sympathetic outflow and arterial pressure. Ann N Y Acad Sci. 2001;940(1):324–337. | ||
Latshang TD, Tardent RPM, Furian M, et al. Sleep and breathing disturbances in patients with chronic obstructive pulmonary disease traveling to altitude: a randomized trial. Sleep. 2019;42(1). | ||
Latshang TD, Furian M, Flück D, et al. Effect of travelling to 2590 m on 6-minute walk distance, blood gases and symptoms of acute mountain sickness in COPD patients. Eur Respir J. 2014;44:P3630. | ||
Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schunemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32(3):637–643. | ||
Latshang TD, Lo Cascio CM, Stöwhas AC, et al. Are nocturnal breathing, sleep, and cognitive performance impaired at moderate altitude (1,630–2,590 M)? Sleep. 2013;36(12):1969–1976. | ||
Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. | ||
Imholz BP, Wieling W, van Montfrans GA, Wesseling KH. Fifteen years experience with finger arterial pressure monitoring: assessment of the technology. Cardiovasc Res. 1998;38(3):605–616. | ||
Kardos A, Watterich G, de Menezes R, Csanády M, Casadei B, Rudas L. Determinants of spontaneous baroreflex sensitivity in a healthy working population. Hypertension. 2001;37(3):911–916. | ||
Stöwhas AC, Latshang TD, Lo Cascio CM, et al. Effects of acute exposure to moderate altitude on vascular function, metabolism and systemic inflammation. PLoS One. 2013;8(8):e70081. | ||
Mancia G, Ferrari A, Gregorini L, et al. Blood pressure variability in man: its relation to high blood pressure, age and baroreflex sensitivity. Clin Sci. 1980;59(s6):401s–404s. | ||
Johansson JK, Puukka PJ, Virtanen R, Jula AM. Beat-to-beat, ambulatory hour-to-hour, and home day-to-day variabilities in blood pressure, pulse pressure, and heart rate in comparison with each other and with target-organ damage. Blood Press Monit. 2015;20(3):113–120. | ||
Webb AJS, Rothwell PM. Physiological correlates of beat-to-beat, ambulatory, and day-to-day home blood pressure variability after transient ischemic attack or minor stroke. Stroke. 2014;45(2):533–538. | ||
Furian M, Hartmann SE, Latshang TD, et al. Exercise performance of Lowlanders with COPD at 2,590 m: data from a randomized trial. Respiration. 2018;95(6):422–432. | ||
Lichtblau M, Latshang TD, Furian M, et al. Right and left heart function in lowlanders with COPD at altitude: data from a randomized study. Respiration. 2019;97(2):125–134. | ||
Kanstrup IL, Poulsen TD, Hansen JM, et al. Blood pressure and plasma catecholamines in acute and prolonged hypoxia: effects of local hypothermia. J Appl Physiol (1985). 1999;87(6):2053–2058. | ||
Mazzeo RS, Wolfel EE, Butterfield GE, Reeves JT. Sympathetic response during 21 days at high altitude (4,300 M) as determined by urinary and arterial catecholamines. Metabolism. 1994;43(10):1226–1232. | ||
Dhar P, Sharma VK, Hota KB, et al. Autonomic cardiovascular responses in acclimatized lowlanders on prolonged stay at high altitude: a longitudinal follow up study. PLoS One. 2014;9(1):e84274. | ||
Wu YK, Huang CY, Yang MC, Huang GL, Chen SY, Lan CC. Effect of tiotropium on heart rate variability in stable chronic obstructive pulmonary disease patients. J Aerosol Med Pulm Drug Deliv. 2015;28(2):100–105. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


