Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Blood Neutrophils In COPD But Not Asthma Exhibit A Primed Phenotype With Downregulated CD62L Expression
Authors Lokwani R , Wark PAB , Baines KJ , Fricker M , Barker D , Simpson JL
Received 2 August 2019
Accepted for publication 23 September 2019
Published 15 November 2019 Volume 2019:14 Pages 2517—2525
DOI https://doi.org/10.2147/COPD.S222486
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Ravi Lokwani,1–3 Peter AB Wark,1–3 Katherine J Baines,1,3 Michael Fricker,1,3 Daniel Barker,3 Jodie L Simpson1–3
1Priority Research Centre for Healthy Lungs, Hunter Medical Research Institute, University of Newcastle, Callaghan, NSW 2308, Australia; 2Department of Respiratory and Sleep Medicine, John Hunter Hospital, New Lambton Heights, NSW 2305, Australia; 3School of Medicine and Public Health, Faculty of Health and Medicine, University of Newcastle, Callaghan, NSW 2308, Australia
Correspondence: Jodie L Simpson
Level 2, East Wing, Hunter Medical Research Institute, Locked Bag, New Lambton 1000, NSW 2305, Australia
Tel +61 422220992
Email [email protected]
Purpose: To characterize neutrophils in obstructive airway disease by measuring their surface adhesion molecules and oxidative burst along with characterizing them into different subsets as per their adhesion molecule expression.
Patients and methods: Peripheral blood from adults with COPD (n=17), asthma (n=20), and healthy participants (n=19) was examined for expression of CD16, CD62L, CD11b, CD11c, and CD54, and analyzed by flow cytometry. For oxidative burst and CD62L shedding analysis, CD16 and CD62L stained leukocytes were loaded with Dihydrorhodamine-123 (DHR-123) and stimulated with N-Formylmethionine-leucyl-phenylalanine (fMLF). Neutrophil subsets were characterized based on CD16 and CD62L expression. Marker surface expression was recorded on CD16+ neutrophils as median fluorescence intensity (MFI).
Results: Neutrophil surface expression of CD62L was significantly reduced in COPD (median (IQR) MFI: 1156 (904, 1365)) compared with asthma (1865 (1157, 2408)) and healthy controls (2079 (1054, 2960)); p=0.028. COPD neutrophils also demonstrated a significant reduction in CD62L expression with and without fMLF stimulation. Asthma participants had a significantly increased proportion and number of CD62Lbright/CD16dim neutrophils (median: 5.4% and 0.14 × 109/L, respectively), in comparison with healthy (3.54% and 0.12 × 109/L, respectively); p<0.017.
Conclusion: Reduced CD62L expression suggests blood neutrophils have undergone priming in COPD but not in asthma, which may be the result of systemic inflammation. The increased shedding of CD62L receptor by COPD blood neutrophils suggests a high sensitivity for activation.
Keywords: COPD, asthma, neutrophils, adhesion molecules, neutrophils phenotype
Introduction
Obstructive airway diseases such as asthma and COPD are common respiratory diseases with a burden that is expected to continue to increase.1 The presence of airway inflammation is a common feature of both diseases2 contributing to exacerbations and airway remodeling where neutrophils are common and associated with airflow obstruction and lung function decline.2–4
Neutrophils are the most abundant leukocyte cells in circulation5 and provide a regulated immune response at the site of injury or infection.6 In a healthy state, the migration of neutrophils into the airways is tightly regulated.7–9 Infection or inflammation in the airways can trigger the release of inflammatory chemokines and cytokines, which stimulate endothelial cell expression of adhesion molecules. The adhesion molecules present on the surface of neutrophils, such as CD62L (L-selectin), initiate the process of engagement by anchoring with P and E-selectins of endothelial cells, resulting in capture and rolling of neutrophils6 followed by firm adhesion,10 mediated by integrins such as CD11b (Integrin α M) and CD11c (Integrin α X) which bind with intercellular adhesion molecules of endothelial cells.11,12 After firm adhesion, neutrophils aggregate and clump at endothelial junctions via intercellular adhesion molecules such as CD54 (ICAM-1)13 and finally transmigrate through the endothelial junctions, cleaving endothelial cell basement membrane proteins by using their proteolytic enzyme neutrophil elastase (NE) to enter the inflamed and/or infected tissue.6,14
Persistent inflammation in the airways in asthma and COPD can result in increased production of cytokines such as CXCL-8, and TNF-α, which upon prolonged release can prime the endothelial cells of blood vessels for capturing the neutrophils in circulation, resulting in premature activation or priming of circulatory neutrophils.6,15,16 The priming of neutrophils can trigger production of intracellular reactive oxygen species (ROS), along with changes in regulation of surface adhesion molecules,17 such as shedding of the CD62L receptor,18 before homing into airways. Thus, the analysis of surface adhesion molecules and ROS production of blood neutrophils of obstructive airway disease can help in understanding the influence of airway inflammation on systemic circulation.19
In this study, we analyzed the priming associated signatures of blood neutrophils from participants with COPD, asthma, and healthy controls by measuring the surface expression of neutrophil adhesion molecules (CD62L, CD11b, CD11c, and CD54) and neutrophils oxidative burst capacity. As we previously identified neutrophil subsets based on nuclear morphology,20 in this study, we have characterized them as per their surface expression of CD16 and CD62L.21–24 We hypothesized that participants with obstructive airway disease would have downregulated CD62L expression, upregulated CD11b, CD11c, CD54 expression, elevated oxidative burst, and elevated proportion of CD62Ldim neutrophils in comparison with healthy controls.
Materials And Methods
Participants
Adults (>18 years) were recruited from the respiratory ambulatory care clinic service of John Hunter Hospital (Newcastle, Australia), the clinical research databases of the Priority Research Centre for Healthy Lungs at The University of Newcastle, Australia, the Hunter Medical Research Institute (Newcastle Australia) volunteer register, and through community advertisement. All participants provided written informed consent, and the ethics approval was obtained from the Hunter New England Human Research Ethics Committee.
All participants had no history of a clinical respiratory tract infection in the previous 4 weeks, no current lung cancer or other blood, lymphatic, or solid organ malignancy. Those with asthma (n=20) had a physician’s diagnosis with objective evidence of variable airway obstruction or bronchial hyper-responsiveness. Those with COPD (n=17) had a physician’s diagnosis in combination with a post-bronchodilator FEV1 of less than 80% of the predicted value and/or a post-bronchodilator FEV1/FVC less than 70%. Healthy non-smokers (n=19) had normal lung function assessed by spirometry and had no previous history of respiratory disease.
As previous studies have shown that smoking can influence the adhesion molecule expression on neutrophils,19,25 current smokers or those who had ceased smoking within the last six months were excluded. Any participant with a primary diagnosis of bronchiectasis or those receiving monoclonal antibodies for the treatment of asthma or COPD was also excluded from the study.
Study Design
A cross-sectional study was conducted in which peripheral blood samples were collected in EDTA tubes (BD Vacutainer, BD Worldwide, NSW, Australia) and spirometry was performed as described previously,26 after an assessment of clinical history including respiratory symptoms, smoking status, and medication.
Blood Inflammatory Cell Count
A full blood count was performed by using CELL-DYN Ruby hematology analyzer (Abbott, Lake Bluff, IL, USA). Blood samples were not available for full blood count for two participants.
Antibodies Utilized For Flow Cytometry
Antibodies were prepared by diluting the titrated volumes (calculated based on the ratio of MFI of isotype and antibody) of CD16-PE-Cy7, CD62L-BV421, CD54-BV650, CD11b-PE (BD Bioscience, Franklin Lakes, NJ, USA), and CD11c-APC (Bio Legend, San Diego, CA, USA) in 50 µL of BD stain buffer (BD Bioscience, Franklin Lakes, NJ, USA). The antibody cocktail was then stored at 4°C until further use.
Whole Blood Staining
Whole blood was incubated with antibody cocktail for 30 mins at 4°C followed by lysis of red blood cells by erythrocyte lysis buffer (Qiagen, Hilden, Germany). The cells were suspended in staining buffer (1% bovine serum albumin (Roche, Mannheim, Germany) in Dulbecco’s phosphate-buffered saline (PBS) (Gibco, Grand Island, NY, USA)) and treated with propidium iodide (PI) (BD Bioscience, Franklin Lakes, NJ, USA). The cell suspensions were then analyzed on a flow cytometer (Fortessa X-20) (BD Bioscience, Franklin Lakes, NJ, USA) and marker surface expression was recorded on CD16+ granulocytes as median fluorescence intensity (MFI). Data were analyzed using FlowJo version 10.5.3 (BD Bioscience, Franklin Lakes, NJ, USA).
Gating Strategy For Neutrophil Subset Characterization
The neutrophil subsets were gated and characterized based on dim and bright expression of CD16 and CD62L as previously described.21 Gate assignment was undertaken blinded to sample ID.
Oxidative Burst Measurement
Oxidative burst measurement was performed as previously described.21 Briefly, cells were stained with CD16 and CD62L followed by red blood cell lysis. Cells were suspended in Hank’s balanced salt solution (Hyclone, Logan, Utah, USA) and treated with 1µM Dihydrorhodamine-123 (DHR-123, Sigma-Aldrich, St. Louis, MO, USA). Cell suspensions were then treated with N-Formylmethionine-leucyl-phenylalanine (fMLF) (Sigma-Aldrich, St. Louis, MO, USA). The response of the ROS indicator dye DHR-123 in CD16+ granulocytes was recorded as MFI.21,27 In addition to DHR-123 MFI, the experiment also utilized the MFI of CD62L to gauge the level of activation of neutrophils by observing the CD62L shedding (drop in CD62L MFI) with subsequent stimulation with fMLF.
Statistical Analysis
Data were analyzed using Stata version 15.1 (StataCorp, College Station, TX, USA). Results are reported as mean (SD) or median (interquartile range), unless otherwise stated. Continuous measures were analyzed using the two-sample Wilcoxon’s rank-sum test or t-test (two sample t test with equal variances) and Kruskal–Wallis test or one-way analysis of variance (ANOVA) as appropriate. Matched samples were analyzed by paired t-test or Wilcoxon matched-pairs signed-rank test as appropriate. Categorical data were analyzed using Fisher’s exact test. Number of participants analyzed for each measurement is indicated in tables and figure legends.
Results
Clinical Characteristics
The clinical and demographic details of the participants are provided in Table 1, and the blood inflammatory cell counts are provided in Table 2. Participants with COPD (n=17) were older with a greater smoking history (Table 1). Participants with asthma and COPD were prescribed inhaled corticosteroids and had significantly reduced lung function in comparison with healthy controls (Table 1). The total number of white blood cells and neutrophils were significantly elevated in both asthma and COPD in comparison with healthy controls. The number of blood eosinophils were significantly higher in asthma in comparison with healthy (Table 2). The asthma participants also had elevated proportion of blood neutrophils in comparison with healthy controls (Table 2).
 |
Table 1 Clinical Characteristics Of Participants With COPD, Asthma, And Healthy Controls |
 |
Table 2 Blood Inflammatory Cell Count From Participants With COPD, Asthma, And Healthy Controls |
Adhesion Molecule Surface Expression Of Blood Neutrophils
CD62L expression on blood neutrophils was significantly reduced in COPD in comparison with asthma and healthy controls (Figure 1). There was no difference observed in the expression of CD11c, CD11b, and CD54 between the groups. There was no correlation between any of the adhesion molecule surface expressions and clinical characteristics such as age, sex, smoking history, lung function, and ICS dose of participants (data not shown).
To further characterize the CD62L expression on COPD blood neutrophils, we also analyzed the percentage of CD62L+ neutrophils in all three groups. The COPD participants had a significantly reduced proportion of CD62L+ neutrophils in comparison with asthma and healthy (CD62L+ neutrophils percentage, median (IQR), asthma: 99.6 (99.2, 99.8), COPD: 97.9 (96.3, 99.5), and healthy: 99.7 (98.9, 99.9); p=0.007).
Surface Expression Of Blood Eosinophils
There was no significant difference in surface CD16 and adhesion molecule (CD62L, CD11b, CD11c, and CD54) expression on eosinophils (CD16 negative granulocytes) between groups (Table 3). We also did not observe any significant difference in the proportion of CD16 negative cells between groups (CD16− cells percentage, median IQR asthma: 6.4 (3.35, 8.30), COPD: 4.30 (3.30, 6.20), and healthy: 3.5 (2.40, 5.20); p=0.084).
 |
Table 3 Surface Expression Of Blood Eosinophils |
While not reaching statistical significance, CD62L expression on eosinophils tended to be lowest in COPD followed by asthma and then healthy control [CD62L MFI median (IQR), asthma: 826 (582, 1091), COPD: 689 (507, 853), and healthy: 1018 (679, 1268); p=0.052] (Table 3).
ROS Production
Spontaneous ROS production (basal oxidative burst) of blood neutrophils was similar in all three groups (Figure 2). The production of ROS in blood neutrophils was significantly increased from baseline following stimulation with fMLF in a dose-dependent manner in all participants (n=55) [DHR-123 MFI median (IQR) at baseline: 450 (357, 591), 10nM fMLF: 638 (474, 967), and 100nM fMLF: 871 (554, 1525); p<0.05 for both stimulations in comparison with baseline and for comparison between 10nM and 100nM stimulation]. However, on comparison between groups, the level of oxidative burst was similar at both stimulations (10nM and 100nM fMLF) regardless of disease status (Figure 2).
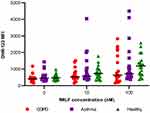 |
Figure 2 ROS production of unstimulated (0nM fMLF) and stimulated blood neutrophils of COPD (n=17), asthma (n=19), and healthy participants (n=19); line between dots represents the median value. |
CD62L Shedding
Spontaneous shedding of CD62L receptors was significantly higher in the COPD group in comparison with both asthma and healthy [CD62L MFI median (IQR), COPD: 1147 (730, 1402), asthma: 1750 (1074, 2528), and healthy: 2185 (1103, 3042); p=0.011] (Figure 3). Blood neutrophils from all participants (n=55) demonstrated significant shedding of CD62L from baseline after stimulation with fMLF [CD62L MFI median (IQR) at baseline: 1530 (986, 2499), 10nM fMLF: 1067 (720, 1903) and 100nM fMLF: 1008 (687, 1849); p<0.05 for both stimulations in comparison with baseline and for comparison between 10nM and 100nM stimulation]. Neutrophils from COPD participants demonstrated significantly increased CD62L shedding after stimulation in comparison with both asthma and healthy controls, ie, at 10nM fMLF stimulation [CD62L MFI median (IQR), COPD: 732 (537,1032), asthma: 1183 (891,1997), and healthy: 1489 (639, 2129); p=0.014] and at 100nM fMLF stimulation [COPD: 737 (569, 777), asthma: 1181 (815, 1878), and healthy: 1356 (641, 2116); p=0.012] (Figure 3).
CD16/CD62L Neutrophil Subsets
Neutrophils were characterized according to CD16 and CD62L expression into subsets as shown in Figure 4A. No significant difference was observed for CD62Ldim/CD16bright neutrophil (a surrogate for hypersegmented neutrophils) proportion and number between groups (Figure 4B and C, respectively.) The proportion and number of CD62Lbright/CD16dim neutrophils (a surrogate of banded neutrophils) were significantly elevated in asthma participants in comparison with healthy participants as shown in Figure 4D and E, respectively. Though no differences were observed for proportion of classical neutrophils (CD62Lbright/CD16bright, shown as ungated CD16 positive cells population between two neutrophils subset gates in Figure 4A) between groups (Figure 4F), their numbers were significantly high in both asthma and COPD in comparison with healthy (Figure 4G).
Discussion
The study has demonstrated downregulated CD62L surface expression on blood neutrophils from COPD participants in comparison with asthma and healthy controls. Blood neutrophils from those with COPD also demonstrated increased shedding of CD62L spontaneously and after stimulation with fMLF in comparison with asthma and healthy controls. While others have studied adhesion molecules on the surface of blood neutrophils in either asthma28 or COPD,19 this study compared the expression of adhesion molecules, oxidative burst of neutrophils, and also examined blood neutrophil subsets in asthma and COPD along with healthy controls.
Downregulation of CD62L is reported to coincide with upregulation of CD11b and is considered a common signature of neutrophil priming in response to microbial products, chemoattractant, and inflammatory cytokines.29,30 We observed a significant downregulation of CD62L expression on COPD neutrophils both in terms of MFI and % positive cells while the expression of CD11b was not significantly altered, suggesting reduced CD62L receptor density and that neutrophils might be going through an early stage of priming. Alternatively, this specific downregulation of CD62L in COPD blood neutrophils may be the result of an exposure to a specific stimulant, which may have not affected regulation of other adhesion molecules. Previous studies have demonstrated that regulation of adhesion molecules can vary according to the stimulant. For example, a challenge with lipopolysaccharide can cause downregulation of CD62L on neutrophils without causing any major changes to CD11b. Conversely, a challenge with platelet-activating factor can cause upregulation of CD11b without affecting CD62L.31 In agreement with Mann et al, we did not observe any alteration in CD62L expression at basal level between asthma and healthy participants.28 An alternative possibility may be that the downregulation of CD62L we have reported in COPD may be due to the existence of low-intensity systemic inflammation which is common in stable COPD.32
The production of ROS by neutrophils plays an important role in their anti-microbial activity against wide range of pathogens. We utilized a pre-established method to measure ROS21 and found no significant differences between groups for ROS production levels of blood neutrophils at both baseline and following stimulation with fMLF, suggesting no altered capacity of blood neutrophils to generate ROS in asthma or COPD. This is in agreement with another study in COPD where a difference was only observed in sputum but not in blood neutrophils,33 suggesting any alteration in ROS production may occur after neutrophils reach the site inflammation or infection.
Shedding of CD62L is also a signature corresponding to neutrophil activation which can occur in response to stimuli such as CXCL-8 and fMLF.18 We observed a greater reduction in CD62L expression on blood neutrophils from COPD participants at both baseline and following fMLF stimulation in comparison with blood neutrophils from other participant groups, suggesting that COPD blood neutrophils might have already gone through some activation while in the circulation. This could result in an enhanced sensitivity for further activation with subsequent exposures.6 The same was not observed in the blood neutrophils of asthma participants, which is an interesting observation and may be due to the presence of low-grade systemic inflammation, which is more common in COPD than asthma.32,34 Further research is required to fully elucidate these differences.
In this study, we have also characterized neutrophil subsets based on CD16 and CD62L expression as previously reported in a model of acute systemic inflammation.21 These subsets were present in each of our participant groups, with a significantly increased proportion of CD16dim/CD62Lbright neutrophils (a surrogate for banded or immature neutrophils) observed in asthma in comparison with healthy controls. In the context of acute systemic inflammation, Pillay et al reported significant increases in both CD16dim/CD62Lbright and CD16bright/CD62Ldim neutrophil subsets after administering low dose LPS.21 A recent study from the same group has shown that CD16dim/CD62Lbright neutrophils isolated from blood after inducing acute inflammation were better in the containment of bacterial pathogens (retaining bacteria in phagosome for longer period of time) in comparison with their counterparts.35 This suggests that increased presence of CD16dim/CD62Lbright neutrophils in the blood of asthma participants may be a physiological response against presence of infections in asthma. However, lack of microbial data is a limitation in establishing this link in our study and is an important area of further research in order to understand the causes of increase in CD16dim/CD62Lbright in asthma participants.
Our recent study of airway neutrophil subsets defined using nuclear morphology found an increased proportion of banded neutrophils in the airways of asthma in comparison with COPD but not compared with healthy controls. Conversely, we reported the increased presence of hypersegmented airway neutrophils (surrogate for CD16bright/CD62Ldim neutrophils) in bronchial lavage of obstructive airway disease participants and the hypersegmented neutrophils were also associated with reduced lung function in obstructive airway disease.20 CD16bright/CD62Ldim neutrophils can be produced from normal neutrophils (CD16bright/CD62Lbright) following stimulation with LPS in vitro and have been shown to be important in the development of airway hyperactivity in isolated small airway epithelial cells in co-culture. This suggests that normal neutrophils might be able to express a different phenotype following exposure to bacterial products such as LPS.36
This study extends our understanding of the neutrophil in airway disease. A better understanding of neutrophil function and the factors that influence neutrophil migration and activation are needed in order to develop better targeted anti-inflammatory therapies for asthma and COPD. Our data suggest there are systemic differences in the expression of key receptors on blood neutrophils in COPD but not in asthma. The small sample size of this study is a limitation in categorizing the disease groups into different subgroups according to their severity or inflammatory phenotypes. The cross-sectional nature of the study is also a limitation in understanding the effect of CD62L downregulation on future clinical outcomes of disease, and longitudinal studies of blood and airway neutrophils are needed.
Conclusion
COPD blood neutrophils feature reduced expression of CD62L and increased shedding of CD62L both spontaneously and after stimulation, in comparison with asthma and healthy participants, suggesting blood neutrophils in COPD have undergone partial priming in the circulation.
Ethics Statement
This study was approved by Hunter New England Human Research Ethics Committee (Reference No’s: 15/03/18/3.04, 05/08/10/3.09, and 17/06/21/3.04), and all participants provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Data Availability
Raw data can be obtained by contacting the corresponding author.
Acknowledgments
We acknowledge technical support from Bridgette Donati, Lakshitha Gunawardhana, Natalie Niessen and Andrew Reid, and clinical support from Penelope Chan, Netsanet Negewo, Emilly Amico, Joanne Howes, Stephany Sanchez Ovando and Rebecca McKerrow of The Priority Research Centre for Healthy Lungs.
Disclosure
Dr Michael Fricker reports grants from NHMRC, grants from Thoracic Society of Australia and New Zealand, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Siddiqui S, Brightling CE. Airways disease: phenotyping heterogeneity using measures of airway inflammation. Allergy Asthma Clin Immunol. 2007;3(2):60–69. doi:10.1186/1710-1492-3-2-60
2. Simpson JL, Phipps S, Gibson PG. Inflammatory mechanisms and treatment of obstructive airway diseases with neutrophilic bronchitis. Pharmacol Ther. 2009;124(1):86–95. doi:10.1016/j.pharmthera.2009.06.004
3. Demkow U, van Overveld FJ. Role of elastases in the pathogenesis of chronic obstructive pulmonary disease: implications for treatment. Eur J Med Res. 2010;15(Suppl 2):27–35. doi:10.1186/2047-783x-15-s2-27
4. Shaw DE, Berry MA, Hargadon B, et al. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest. 2007;132(6):1871–1875. doi:10.1378/chest.07-1047
5. Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol. 2018;9:113. doi:10.3389/fphys.2018.00113
6. Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi:10.1146/annurev-immunol-020711-074942
7. Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol. 2008;48:171–197. doi:10.1146/annurev.pharmtox.48.121806.154841
8. Vaguliene N, Zemaitis M, Lavinskiene S, Miliauskas S, Sakalauskas R. Local and systemic neutrophilic inflammation in patients with lung cancer and chronic obstructive pulmonary disease. BMC Immunol. 2013;14:36. doi:10.1186/1471-2172-14-36
9. Fortunati E, Kazemier KM, Grutters JC, Koenderman L, Van Den Bosch VJMM. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin Exp Immunol. 2009;155(3):559–566. doi:10.1111/j.1365-2249.2008.03791.x
10. Mallia P, Message SD, Contoli M, et al. Neutrophil adhesion molecules in experimental rhinovirus infection in COPD. Respir Res. 2013;14(1):72. doi:10.1186/1465-9921-14-72
11. Hyun Y-M, Lefort CT, Kim M. Leukocyte integrins and their ligand interactions. Immunol Res. 2009;45(2–3):195–208. doi:10.1007/s12026-009-8101-1
12. Sadhu C, Ting HJ, Lipsky B, et al. CD11c/CD18: novel ligands and a role in delayed-type hypersensitivity. J Leukoc Biol. 2007;81(6):1395–1403. doi:10.1189/jlb.1106680
13. Wang JH, Sexton DM, Redmond HP, Watson RW, Croke DT, Bouchier-Hayes D. Intercellular adhesion molecule-1 (ICAM-1) is expressed on human neutrophils and is essential for neutrophil adherence and aggregation. Shock. 1997;8(5):357–361. doi:10.1097/00024382-199711000-00007
14. Wang S, Dangerfield JP, Young RE, Nourshargh S. PECAM-1, alpha6 integrins and neutrophil elastase cooperate in mediating neutrophil transmigration. J Cell Sci. 2005;118(Pt 9):2067–2076. doi:10.1242/jcs.02340
15. Oudijk EJ, Lammers JW, Koenderman L. Systemic inflammation in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2003;46:5s–13s.
16. Sokol CL, Luster AD. The chemokine system in innate immunity. Cold Spring Harb Perspect Biol. 2015; 7(5):a016303. doi:10.1101/cshperspect.a016303
17. Yao Y, Matsushima H, Ohtola JA, Geng S, Lu R, Takashima A. Neutrophil priming occurs in a sequential manner and can be visualized in living animals by monitoring IL-1β promoter activation. J Immunol. 2015;194(3):1211–1224. doi:10.4049/jimmunol.1402018
18. Hafezi-Moghadam A, Thomas KL, Prorock AJ, Huo Y, Ley K. L-selectin shedding regulates leukocyte recruitment. J Exp Med. 2001;193(7):863–872. doi:10.1084/jem.193.7.863
19. Blidberg K, Palmberg L, James A, et al. Adhesion molecules in subjects with COPD and healthy non-smokers: a cross sectional parallel group study. Respir Res. 2013;14:47. doi:10.1186/1465-9921-14-19
20. Lokwani R, Wark PAB, Baines KJ, Barker D, Simpson JL. Hypersegmented airway neutrophils and its association with reduced lung function in adults with obstructive airway disease: an exploratory study. BMJ Open. 2019;9(1):e024330. doi:10.1136/bmjopen-2018-024330
21. Pillay J, Kamp VM, van Hoffen E, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122(1):327–336. doi:10.1172/JCI57990
22. Tak T, Wijten P, Heeres M, et al. Human CD62L(dim) neutrophils identified as a separate subset by proteome profiling and in vivo pulse-chase labeling. Blood. 2017;129(26):3476–3485. doi:10.1182/blood-2016-07-727669
23. Hao S, Andersen M, Yu H. Detection of immune suppressive neutrophils in peripheral blood samples of cancer patients. Am J Blood Res. 2013;3(3):239–245.
24. Millrud CR, Kågedal Å, Kumlien Georén S, et al. NET-producing CD16high CD62Ldim neutrophils migrate to tumor sites and predict improved survival in patients with HNSCC. Int J Cancer. 2017;140(11):2557–2567. doi:10.1002/ijc.30671
25. Pitzer JE, Del Zoppo GJ, Schmid-Schonbein GW. Neutrophil activation in smokers. Biorheology. 1996;33(1):45–58.
26. Gibson PG, Wlodarczyk JW, Hensley MJ, et al. Epidemiological association of airway inflammation with asthma symptoms and airway hyperresponsiveness in childhood. Am J Respir Crit Care Med. 1998;158(1):36–41. doi:10.1164/ajrccm.158.1.9705031
27. Chen Y, Junger WG. Measurement of oxidative burst in neutrophils. Methods Mol Biol. 2012;844:115–124.
28. Mann BS, Chung KF. Blood neutrophil activation markers in severe asthma: lack of inhibition by prednisolone therapy. Respir Res. 2006;7:59. doi:10.1186/1465-9921-7-59
29. Wittmann S, Rothe G, Schmitz G, Frohlich D. Cytokine upregulation of surface antigens correlates to the priming of the neutrophil oxidative burst response. Cytometry Part A. 2004;57(1):53–62. doi:10.1002/cyto.a.10108
30. Miralda I, Uriarte SM, McLeish KR. Multiple phenotypic changes define neutrophil priming. Front Cell Infect Microbiol. 2017;7:217. doi:10.3389/fcimb.2017.00217
31. Condliffe AM, Chilvers ER, Haslett C, Dransfield I. Priming differentially regulates neutrophil adhesion molecule expression/function. Immunology. 1996;89(1):105–111. doi:10.1046/j.1365-2567.1996.d01-711.x
32. Tkacova R. Systemic inflammation in chronic obstructive pulmonary disease: may adipose tissue play a role? Review of the literature and future perspectives. Mediators Inflamm. 2010;2010:585989. doi:10.1155/2010/585989
33. Vaitkus M, Lavinskiene S, Barkauskiene D, Bieksiene K, Jeroch J, Sakalauskas R. Reactive oxygen species in peripheral blood and sputum neutrophils during bacterial and nonbacterial acute exacerbation of chronic obstructive pulmonary disease. Inflammation. 2013;36(6):1485–1493. doi:10.1007/s10753-013-9690-3
34. Wouters EF, Reynaert NL, Dentener MA, Vernooy JH. Systemic and local inflammation in asthma and chronic obstructive pulmonary disease: is there a connection? Proc Am Thorac Soc. 2009;6(8):638–647. doi:10.1513/pats.200907-073DP
35. Leliefeld PHC, Pillay J, Vrisekoop N, et al. Differential antibacterial control by neutrophil subsets. Blood Adv. 2018;2(11):1344–1355. doi:10.1182/bloodadvances.2017015578
36. Ekstedt S, Safholm J, Georen SK, Cardell LO. Dividing neutrophils in subsets reveals a significant role for activated neutrophils in the development of airway hyperreactivity. Clin Exp Allergy. 2019;49(3):285–291. doi:10.1111/cea.13311
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



