Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Blood Eosinophil and Risk of Exacerbation in Chronic Obstructive Pulmonary Disease Patients: A Retrospective Cohort Analysis
Authors Chan MC , Yeung YC , Yu ELM , Yu WC
Received 17 June 2020
Accepted for publication 31 August 2020
Published 10 November 2020 Volume 2020:15 Pages 2869—2877
DOI https://doi.org/10.2147/COPD.S268018
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Ming Chiu Chan, 1 Yiu Cheong Yeung, 1 Ellen Lok Man Yu, 2 Wai Cho Yu 1
1Department of Medicine and Geriatrics, Princess Margaret Hospital, Hong Kong, China; 2Clinical Research Centre, Princess Margaret Hospital, Hong Kong, China
Correspondence: Ming Chiu Chan
Department of Medicine and Geriatrics, Princess Margaret Hospital, Hong Kong, China
Tel +852 2990 3771
Correspondence Email [email protected]
Purpose: Blood eosinophil is a readily available biomarker to reflect the eosinophilic inflammation in chronic obstructive pulmonary disease (COPD) patients, yet its association with exacerbation is inconclusive. It is uncertain which measurement, eosinophil percentage or absolute eosinophil count, should be used and what is the optimal cutoff for exacerbation prediction.
Patients and Methods: A total of 247 COPD patients were included in this retrospective cohort study. Blood eosinophil during stable disease state, baseline demographics, and clinical characteristics in 12 months after the index complete blood count (CBC) were recorded. Exacerbation frequencies were compared between patients with high and low blood eosinophil percentage using 2% as cut-off. Logistic regression and receiver operating characteristics (ROC) curve analyses were conducted.
Results: Patients with blood eosinophil ≥ 2% were associated with more frequent exacerbations than patients with eosinophil < 2% in the 12 months after the index CBC (mean exacerbation 1.07 vs 0.34, p < 0.001). Higher blood eosinophil percentage conferred a higher risk of exacerbation. Adjusted odds ratio for exacerbation in 12 months after the index CBC for blood eosinophil ≥ 2% was 2.98 (95% confidence interval = 1.42– 6.25). The area under the ROC curve of eosinophil percentage was significantly higher than that of absolute eosinophil count (0.678 vs 0.640, p = 0.010). The optimal cutoff of blood eosinophil percentage for exacerbation prediction was 2.8%.
Conclusion: Blood eosinophilia was associated with higher exacerbation risk in COPD patients. Further studies are required to elucidate the mechanism of eosinophilic inflammation in COPD and determine the optimal treatment strategy to reduce exacerbations.
Keywords: COPD, eosinophil, exacerbation, receiver operating characteristics curve
Introduction
Chronic obstructive pulmonary disease (COPD) is commonly associated with neutrophilic airway inflammation, but it is important to note that 20% to 40% of COPD patients have eosinophilic airway inflammation, as evidenced by sputum eosinophilia.1 Eosinophilic airway inflammation has been observed in acute exacerbations of COPD,2 which contributes to disease progression, morbidity and mortality in COPD patients.3,4 Blood eosinophil was found to be a readily available biomarker to reflect underlying eosinophilic airway inflammation2,5,6 and predicts inhaled corticosteroid (ICS) efficacy in exacerbation reduction.7–10 Blood eosinophilia, during stable disease state and during exacerbation, was associated with increased risk of COPD exacerbations and readmissions after hospitalization.11–13
However, several issues regarding blood eosinophil in COPD patients warrant further investigation. Some studies yielded conflicting results regarding the relationship between blood eosinophilia and exacerbation risk.14–17 The optimal cutoff level of eosinophil count to differentiate the exacerbation risk is unclear as well.3,18 Although the cutoff level of eosinophil count greater than or equal to 2% of peripheral white cell count was previously reported to have high sensitivity for predicting sputum eosinophilia, predict ICS efficacy, and was commonly adopted in published studies,2,8–10 other studies used a higher cutoff percentage or absolute eosinophil count.7,11,18
The primary objective of this study is to compare the COPD exacerbation frequencies between the group with high blood eosinophil (defined as patients with baseline eosinophil greater than or equal to 2% of total white cell count) and that of the group with low eosinophil (patients with baseline eosinophil less than 2%) in 12 months after the index blood count. Secondary objectives included comparing the clinical characteristics between the high and low eosinophil groups, investigating the exacerbation frequencies in relation to different cutoffs for blood eosinophil (2%, 4% and 6%), and determining the optimal cutoff level of blood eosinophil percentage or absolute count to differentiate risk of exacerbation.
Patients and Methods
This was a retrospective cohort study and was approved by the Kowloon West Cluster Research Ethics Committee (KWC-REC Reference: KW/EX-17-155 (118–03)). Patients with the diagnosis of COPD followed up in Princess Margaret Hospital department of Medicine and Geriatrics were identified by the Clinical Data Analyzing and Reporting System (CDARS) and a case notes review was performed. As this was a retrospective study with no active intervention, patient consent was not required by our Research Ethics Committees. The patient data was anonymized during the study and the study was conducted in accordance with the principles of the Declaration of Helsinki.
Patients who fulfilled the standard definition of COPD according to the Global Initiative for Chronic Obstructive Lung Disease (includes evaluation of symptoms, chest radiograph, and pulmonary function tests with forced expiratory volume in 1 second/forced vital capacity ratio < 0.7) and with previous complete blood count (CBC) taken during stable state within 5 years were recruited into the study. Exclusion criteria included COPD patients with 1) CBC taken only during or within 4 weeks of an exacerbation; 2) other concomitant chronic respiratory disease (such as bronchiectasis, thoracic cage deformity, interstitial lung disease, history of lung resection); and 3) other known causes of blood eosinophilia including active parasitic or fungal infection, hematological or solid organ malignancies, connective tissue diseases and adrenal insufficiency.
Taking reference from Couillard et al for the incidence of COPD exacerbation in the high and low eosinophil groups of 47.3% and 25% respectively and an overall incidence of exacerbation of 32.3%,13 the sample size needed for incidence comparison was 169 with 80% power and 95% confidence level. Using rule of thumb for estimating sample size for logistic regression, 80 subjects in the smaller outcome group (with exacerbation) was required for a regression model with 8 variables, thus the required study sample size was estimated to be 247.
Baseline demographics, clinical characteristics, lung function test results, physician diagnosis of asthma, and physician diagnosis of allergic rhinitis or eczema were recorded. Blood eosinophil percentage and absolute count were noted. If multiple CBCs were taken during stable state, then the most recent one, but at least taken 12 months ago, would be recorded. The number of exacerbations (defined as either emergency department visits or hospitalizations, or both) in the 12 months preceding the date of the chosen CBC, and the number of exacerbations in the subsequent 12 months after the CBC date were collected.
The baseline demographics and clinical characteristics of high and low eosinophil groups (blood eosinophil ≥2% and <2%) were compared by Pearson’s chi-square test or Fisher’s exact test for categorical variables and Student’s t-test or Mann–Whitney U-test for continuous variables. The exacerbation frequencies in 12 months after the index CBC across eosinophil percentage groups with cutoff 2%, 4% and 6% were compared using Jonckheere’s trend test. Association between eosinophil and COPD exacerbation in 12 months following the index CBC was assessed by multiple binary logistic regression analysis adjusted for baseline characteristics with p < 0.1 in simple regression analyses. The area under the receiver operating characteristics (ROC) curves (AUCs) of blood eosinophil percentage and absolute count to predict exacerbation in 12 months following the index CBC were calculated and compared using the DeLong test. The optimal cutoff level of eosinophil for exacerbation risk prediction was determined by using Youden index for maximal sensitivity and specificity.19 Accuracy, sensitivity, specificity, positive and negative predictive values with 95% confidence interval were calculated. Statistical analyses were performed using R version 3.6.1. Statistical significance was set at p < 0.05.
Results
A total of 350 patients with the diagnosis of chronic obstructive pulmonary disease were screened, in which 247 patients were included into the study (Figure 1). The mean forced expiratory volume in 1 second (FEV1) of the study population was 49.5% predicted or 1.06 L (Table 1). The percentage of subjects with very severe (FEV1 < 30%), severe (FEV1 30–49%) and moderate airflow limitation (50–79%) were 10.9%, 44.5% and 38.5% respectively. The median eosinophil percentage of total white cell count was 3.1% (interquartile range [IQR] 1.8–5.2%), and the median absolute eosinophil count was 0.2 x 109/L (IQR 0.1 x 109/L - 0.4 x 109/L). A total of 65 patients had blood eosinophil <2% and 182 patients had blood eosinophil ≥2%. The baseline characteristics did not differ significantly between the patients with blood eosinophil ≥2% and those with eosinophil <2%, except for the inhaler use. The elevated eosinophil group had higher percentage of patients on any form of long acting beta agonist (LABA) and long acting muscarinic agonist (LAMA) (Table 1). There were 12 (18.5%), 3 (4.6%) and 21 (32.3%) patients in the low blood eosinophil group and 32 (17.6%), 18 (9.9%) and 91 (50%) patients in the high eosinophil group receiving LABA/ICS, LABA/LAMA and LABA/LAMA/ICS, respectively.
 |
Table 1 Demographics and Clinical Characteristics |
 |
Figure 1 Recruitment flow chart. |
Patients with blood eosinophil ≥2% had significantly higher mean exacerbation frequencies in 12 months after the index CBC (1.07 vs 0.34, p < 0.001; Table 2). For the past exacerbation history, patients with blood eosinophil ≥2% also had a higher mean exacerbation frequency in 12 months before the index CBC (1.12 vs 0.68, p = 0.026). The cohort was further divided into four mutually exclusive groups according to blood eosinophil percentage (Figure 2) and there was significant increasing trend in the exacerbation frequency with higher blood eosinophil percentage (p < 0.001). The mean exacerbation frequencies were 0.34, 0.82, 0.93 and 1.69 in patients with eosinophil <2%, 2–3.9%, 4–5.9% and ≥6%, respectively (Figure 2).
 |
Table 2 Exacerbation Frequencies in 12 Months Before and 12 Months After the Index Complete Blood Count |
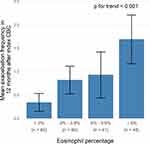 |
Figure 2 Mean exacerbation frequency in 12 months after the index complete blood count (CBC) by blood eosinophil percentage. |
The exacerbation frequencies in 12 months after the index CBC were also studied according to the use of ICS (Table 3) and there were no significant differences in the mean exacerbation in both the high and low eosinophil groups.
 |
Table 3 Exacerbation Frequencies According to the Use of Inhaled Corticosteroid |
Table 4 shows the results of logistic regression analysis. The unadjusted odds ratio (OR) for exacerbation for blood eosinophil ≥2% was 3.12. After adjusting for potential confounders including past 12-month exacerbation, age, spirometry, history of allergic rhinitis or eczema and inhaler use, the adjusted OR for exacerbation for blood eosinophil ≥2% was 2.98 (95% confidence interval [CI] 1.42–6.25, p = 0.004).
 |
Table 4 Factors Associated with Exacerbation in 12 Months After the Index Complete Blood Count – Regression Analysis |
The ROC curves of blood eosinophil percentage and absolute eosinophil count to predict exacerbation in 12 months following the index CBC are presented in Figure 3. The AUC for eosinophil percentage is 0.678, which was significantly higher than that of the absolute eosinophil count (AUC 0.640, p = 0.010). The optimal cutoff of blood eosinophil percentage was found to be 2.8%, and the test performance is shown in Table 5. The sensitivity and specificity of the blood eosinophil percentage threshold, 2.8% for exacerbation prediction, were 75.5% and 53.7% respectively, while the sensitivity and specificity of the blood eosinophil threshold 2% were 85.7% and 34.2% respectively. With blood eosinophil ≥2.8%, the adjusted OR for exacerbation in 12 months following the index CBC was 3.96 (95% CI 2.09–7.49, p < 0.001).
 |
Table 5 Test Performance of Blood Eosinophil for Exacerbation in 12 Months After the Index Complete Blood Count with 2.8% Threshold |
 |
Figure 3 Receiver operating characteristics curve of blood eosinophil for prediction of exacerbation in 12 months after the index complete blood count. |
Discussion
In our cohort of moderate to severe COPD patients, the median eosinophil percentage is 3.1%. It was higher than the median eosinophil percentage of other cohorts (2.61% in the Copenhagen General Population study and 1.9% in the French Initiatives cohort).11,15 Patients with concomitant diagnosis of asthma were not excluded in our cohort, while those corresponding patients were excluded in the Copenhagen study. However, the number of patients with concomitant asthma was small (7 patients only) and the median eosinophil percentage would be unaffected after excluding those patients. The proportion of patients with allergic rhinitis or eczema was also similar to the Copenhagen study. Therefore, the higher median eosinophil percentage could be related to the fact that a hospital-based cohort, as in our case, had more frequent exacerbations than a general population cohort, as reported by Erdal et al.20
There was no significant difference in demographics and patient characteristics between the high eosinophil group (≥2%) and the low eosinophil group (<2%) except for a more long-acting bronchodilator (LABA and LAMA) in the high eosinophil group. It would be reasonable to hypothesize that the more frequent exacerbation history in the high eosinophil group had led to more long acting bronchodilator in accordance with the current treatment guideline.3 The use of inhaled corticosteroid (ICS) did not differ between the high and low eosinophil group. ICS has been reported to cause only small change in the peripheral eosinophil count in ICS-naive patients (−0.03 x 109/L) in a small post-hoc analysis, and the change of eosinophil was not significantly different compared with the change in patients receiving placebo or long acting beta agonist.21
Eosinophil and Exacerbations
Our study has found that patients with blood eosinophilia (≥2%) were associated with a significantly higher exacerbation risk in the next 12 months, with an adjusted odds ratio (OR) of 2.98. Our finding was consistent with the result of the Copenhagen study which demonstrated an exacerbation rate ratio of 1.85 for clinical COPD cohort with blood eosinophil ≥2% and a history of exacerbation in the previous year.11 Higher eosinophil threshold conferred higher future exacerbation risk in our cohort, again supporting the association between the two. Similarly, progressive increase in exacerbation risk with increasing blood eosinophil was also observed in COPDGene study a retrospective study of over 7000 subjects done by Zeiger et al.12,22
On the contrary, in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) study,23 the largest observational study investigating sputum and blood eosinophils and clinical outcomes of COPD patients, there was no association between total exacerbation in the previous year and blood eosinophilia with cutoff of 0.2 x 109/L. However, 31% patients in the SPIROMICS cohort were GOLD stage 0, ie did not fulfill the diagnosis of COPD (at least 20 pack years of smoking history but preserved lung function). Not surprisingly, the SPIROMICS cohort had few exacerbations recorded, with 25% patients having one or more severe exacerbations in the previous year.24 Two recent large retrospective studies involving patients from primary care settings and a prospective cohort study in hospital care setting also did not show any association between COPD exacerbations and blood eosinophil levels.25–27 The lack of significant findings could be again due to the milder disease severity of the subjects, with about half of the study population not having any exacerbation histories in all three studies. Therefore, the differences in the study outcomes suggested that patient population, disease severity and past exacerbation history are all important in understanding the significance of eosinophil in COPD.28
There was a trend of more future exacerbations in patients who received ICS in both the high and low eosinophil groups, though not statistically significant. It could be due to the fact that patients with exacerbation histories were more likely to be put on ICS in this real-life study. For patients with blood eosinophil ≥ 2%, the mean exacerbation in 12 months before the index CBC was 1.27 in those who were on ICS, compared with 0.68 in those who were not on ICS (p=0.048).
Eosinophil: Percentage or Absolute Count
Another controversy regarding blood eosinophil in COPD patients is which measurement, eosinophil percentage or absolute eosinophil count, should be used when analyzing exacerbation data. Some postulated that eosinophil percentage may be more informative regarding the effect of eosinophil relative to other white cells, while absolute eosinophil count may better reflect the eosinophil burden,18 and therefore suggesting eosinophil percentage should be chosen for threshold in evaluation of exacerbation treatment and absolute eosinophil count for maintenance treatment. However, researches directly addressing this important topic were scarce. In our study, we used the area under the curve (AUC) of Receiver operating characteristics curve to compare the performance of eosinophil percentage and absolute eosinophil count. The AUC of eosinophil percentage for prediction of exacerbation in 12 months following the index CBC was significantly higher than that of the absolute eosinophil count (0.678 and 0.640 respectively). However, in the Copenhagen study, the result was the opposite and the AUC of absolute count was higher than that of the eosinophil percentage (0.63 and 0.59 respectively). The discrepancy in the result could be due to the different reporting accuracy of the absolute eosinophil count. It was reported up to 2 decimal places in the Copenhagen study while our laboratory only reported up to 1 decimal place. Therefore, in our local setting, eosinophil percentage would be a superior biomarker of exacerbation risk than eosinophil percentage.
In our study, the optimal eosinophil percentage threshold to identify exacerbation in 12 months following the index CBC was 2.8%. With this cutoff, blood eosinophil percentage had a good sensitivity (75.5%) and negative predictive value (77.1%), though the specificity (53.7%) and positive predictive value (51.8%) was fair. Therefore, in our population, patients with blood eosinophil lower than 2.8% can serve as an adjunctive measure to identify patients with low risk of exacerbation, especially in conjunction with past exacerbation history, to formulate personalized treatment strategies. Threshold of 2% was commonly adopted in earlier studies due to its correlation with sputum eosinophilia,2 but our result showed the 2% threshold lacked specificity (34.2% only), limiting its clinical use. More recent studies also found a threshold higher than 2% predicted exacerbation risk with better performance.11
Our study had the strength of being a real-life study and its result was more applicable to our daily practice than that of post hoc analysis of a drug trial. There was no published data regarding the risk of exacerbation for eosinophilic COPD patients in our local population and this study contributed to our local data. Our study also specifically excluded diseases that affect blood eosinophil signal, increasing the chance that eosinophilic inflammation was genuinely related to COPD. Blood eosinophil that was taken within 4 weeks of exacerbation would be excluded to avoid interference from exacerbation and systemic corticosteroid.
Our study had several limitations. It included patients with moderate to severe COPD followed up in a regional hospital with frequent exacerbations (39.7% patients had at least 1 exacerbation leading to emergency department visit or hospitalization, which was defined as severe exacerbation in most studies). The result of this study may not be generalized or directly applicable to the general population in Hong Kong especially patients with milder disease, though the Copenhagen General Population study previously discussed also yielded consistent result for the effect of eosinophil on COPD exacerbation. The majority of our patients were male and our cohort only contained 10% of females, but it reflected the COPD population commonly seen in daily practice.29
Our study was a retrospective one, of which missing data was a potential pitfall. In our study, 41 patients had to be excluded on screening because no CBC result was available during a stable state within the past 5 years. The lack of CBC could be due to the low awareness of blood eosinophil in COPD patients in the past, but a minority group of COPD patients had frequent exacerbations and readmissions, and they rarely attended outpatient follow-up and had blood taking at a stable disease state. These frequent exacerbators could not be captured in our study. Only exacerbations leading to emergency department visit or hospitalization were analyzed in this study, since no comprehensive or reliable data of mild or moderate exacerbation was available with our retrospective cohort.
Lastly, only one blood eosinophil count at stable disease state, which was the latest one taken at least 12 months ago, was analyzed. The effect of fluctuation of blood eosinophil count could not be evaluated in this study. The stability of blood eosinophil could be potentially affected by exacerbation or fluctuation of disease stability and treatment adaptation, alongside with seasonal and hormonal influence, but blood eosinophil was shown to be a reasonably stable marker, with 85% patients having stable blood eosinophil count over 6 months and 75% patients over 1 year.30
Conclusion
In this retrospective cohort of COPD patients, blood eosinophilia ≥2% at stable disease state was associated with more frequent exacerbations in 12 months after the index CBC, with an adjusted odds ratio of 2.98 (95% confidence interval 1.42–6.25, p = 0.004). Higher mean exacerbation frequency was observed with increasing blood eosinophil percentage. Using the ROC curve, the optimal cutoff of eosinophil for prediction of exacerbation in 12 months following the CBC was 2.8%. Further studies are required to elucidate the mechanism of eosinophilic inflammation in COPD and determine the optimal treatment strategy to reduce exacerbations.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(1):39–47. doi:10.2147/copd.2006.1.1.39
2. Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi:10.1164/rccm.201104-0597OC
3. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi:10.1183/13993003.00164-2019
4. Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi:10.1016/S0140-6736(07)61382-8
5. Schleich F, Corhay J, Louis R. Blood eosinophil count to predict bronchial eosinophilic inflammation in COPD. Eur Respir J. 2016;47(5):1562–1564. doi:10.1183/13993003.01659-2015
6. Wagener AH, de Nijs SB, Lutter R, et al. External validation of blood eosinophils, FE NO and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70(2):115–120. doi:10.1136/thoraxjnl-2014-205634
7. Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):523–525. doi:10.1164/rccm.201502-0235LE
8. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. doi:10.1016/s2213-2600(15)00106-x
9. Pavord ID, Lettis S, Locantore N, et al. Blood eosinophils and inhaled corticosteroid/long-acting β-2 agonist efficacy in COPD. Thorax. 2016;71(2):118–125. doi:10.1136/thoraxjnl-2015-207021
10. Barnes NC, Sharma R, Lettis S, Calverley PMA. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur Respir J. 2016;47(5):1374–1382. doi:10.1183/13993003.01370-2015
11. Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen general population study. Am J Respir Crit Care Med. 2016;193(9):965–974. doi:10.1164/rccm.201509-1869OC
12. Zeiger RS, Tran TN, Butler RK, et al. Relationship of blood eosinophil count to exacerbations in chronic obstructive pulmonary disease. J Allergy Clin Immunol Pract. 2018;6(3):944–954. doi:10.1016/j.jaip.2017.10.004
13. Couillard S, Larivée P, Courteau J, Vanasse A. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest. 2017;151(2):366–373. doi:10.1016/j.chest.2016.10.003
14. Bafadhel M, Greening NJ, Harvey-Dunstan TC, et al. Blood eosinophils and outcomes in severe hospitalized exacerbations of COPD. Chest. 2016;150(2):320–328. doi:10.1016/j.chest.2016.01.026
15. Zysman M, Deslee G, Caillaud D, et al. Relationship between blood eosinophils, clinical characteristics, and mortality in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1819–1824. doi:10.2147/COPD.S129787
16. Song JH, Lee C, Kim JW, et al. Clinical implications of blood eosinophil count in patients with non-asthma–COPD overlap syndrome COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2455–2464. doi:10.2147/COPD.S129321
17. Ho J, He W, Chan MTV, et al. Eosinophilia and clinical outcome of chronic obstructive pulmonary disease: a meta-analysis. Sci Rep. 2017;7(1):13451. doi:10.1038/s41598-017-13745-x
18. Bafadhel M, Pavord ID, Russell REK. Eosinophils in COPD: just another biomarker? Lancet Respir Med. 2017;5(9):747–759. doi:10.1016/S2213-2600(17)30217-5
19. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi:10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.CO;2-3
20. Erdal M, Johannessen A, Eagan TM, Bakke P, Gulsvik A, Grønseth R. Incidence of utilization- and symptom-defined COPD exacerbations in hospital- and population-recruited patients. Int J Chron Obstruct Pulmon Dis. 2016;11:2099–2108. doi:10.2147/COPD.S108720
21. Kreindler JL, Watkins ML, Lettis S, Tal-Singer R, Locantore N. Effect of inhaled corticosteroids on blood eosinophil count in steroid-naïve patients with COPD. BMJ Open Respir Res. 2016;3(1):e000151. doi:10.1136/bmjresp-2016-000151
22. Yun JH, Lamb A, Chase R, et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141(6):2037–2047. doi:10.1016/j.jaci.2018.04.010
23. Hastie AT, Martinez FJ, Curtis JL, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(12):956–967. doi:10.1016/S2213-2600(17)30432-0
24. Bafadhel M. Eosinophils in COPD: are we nearly there yet? Lancet Respir Med. 2017;5(12):913–914. doi:10.1016/S2213-2600(17)30445-9
25. Landis S, Suruki R, Maskell J, Bonar K, Hilton E, Compton C. Demographic and clinical characteristics of COPD patients at different blood eosinophil levels in the UK clinical practice research datalink. COPD. 2018;15(2):177–184. doi:10.1080/15412555.2018.1441275
26. Miravitlles M, Monteagudo M, Solntseva I, Alcazar B. Blood eosinophil counts and their variability and risk of exacerbations in COPD: A population-based study. Arch Bronconeumol. 2020;S0300-2896(19):30623–30624. doi:10.1016/j.arbres.2019.12.015
27. Turato G, Semenzat U, Bazzan E, et al. Blood eosinophilia neither reflects tissue eosinophils nor worsens clinical outcomes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(9):1216–1219. doi:10.1164/rccm.201708-1684LE
28. Brusselle G, Pavord ID, Landis S, et al. Blood eosinophil levels as a biomarker in COPD. Respir Med. 2018;138:21–31. doi:10.1016/j.rmed.2018.03.016
29. Yu WC, Tai EL, Fu SN, et al. Treatment of patients with chronic obstructive pulmonary disease as practised in a defined hong kong community: a cross-sectional pilot survey. Hong Kong Med J. 2011;17(4):306–314.
30. Oshagbemi OA, Burden AM, Braeken DCW, et al. Stability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline counts. Am J Respir Crit Care Med. 2017;195(10):1402–1404. doi:10.1164/rccm.201701-0009LE
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
