Back to Journals » Open Access Emergency Medicine » Volume 15
Bleeding Complications in Uremic Patients After Ultrasound-Guided Central Venous Catheter Placement
Authors Diaz C, Quintero JA , Zarama V, Bustamante-Cristancho LA
Received 28 July 2022
Accepted for publication 9 December 2022
Published 12 January 2023 Volume 2023:15 Pages 21—28
DOI https://doi.org/10.2147/OAEM.S384081
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hans-Christoph Pape
Carime Diaz,1 Jaime A Quintero,1,2 Virginia Zarama,1 Luis Alfonso Bustamante-Cristancho1
1Critical Medicine, Emergency Department, Fundación Valle del Lili, Cali, 760032, Colombia; 2Centro de Investigaciones Clínicas (CIC), Fundación Valle del Lili, Cali, 760032, Colombia
Correspondence: Jaime A Quintero, Critical Medicine, Emergency Department, Centro de Investigaciones Clínicas, Fundación Valle del Lili, Carrera 98 No. 18-49, Cali, 760032, Colombia, Tel +57 3184257759, Email [email protected]
Introduction: Bleeding associated with elevated blood urea nitrogen (BUN) is a known complication. Patients with uremia require a central venous catheter insertion by dialysis. The relation between BUN and bleeding complications during central venous catheter insertion is not yet clear.
Objective: We described the frequency of complications associated with central venous catheter implantation in uremic patients and evaluated the statistical relationship between bleeding complications and catheter type, number of punctures, and catheter insertion site. Also, we determined if any value of BUN is associated with bleeding complications.
Methods: We included patients with a serum value of BUN > 70 mg/dl that required insertion of a central venous catheter. The quantitative variables were expressed through the measure of central tendency. A bivariate analysis and a ROC curve were performed.
Results: A total of 273 catheters were included in this study. Bleeding complications were detected in 69 cases (25.3%), and local bleeding was the most frequent complication in 51/69 cases. Statistically significant association was not established. We did not find a specific cut-off value directly related to BUN levels and the rate of complications.
Conclusion: Bleeding complications associated with the insertion of central venous catheter and the suspected disorder of hemostasis given by BUN levels > 70 mg/dl are common. It was not possible to determine a BUN cut-off value to predict complications. The association analysis was not conclusive. High BUN levels should not be considered a high-risk condition for central venous cannulation under ultrasound guidance performed by trained personnel.
Keywords: central catheterization, vascular catheter, ultrasound imaging, blood coagulation disorders, blood urea nitrogen
Background
Uremia is a clinical condition associated with worsening renal function secondary to acute or chronic renal failure, systemic infection, cancer, and other diseases. Uremic patients can develop bleeding complications due to platelet aggregation dysfunction, Von Willebrand factor dysfunction, loss of erythrocyte mass, and high levels of cAMP (cyclic adenosine monophosphate) or cGMP (cyclic guanosine monophosphate).1–9
High BUN levels are associated with gastrointestinal bleeding11,12 or spontaneous brain bleeding and increased bleeding episodes in trauma patients.10
Despite the potential risk of bleeding complications due to uremic coagulopathy,52–56 uremic patients frequently require the implantation of a central venous catheter to provide renal replacement therapy or invasive treatment.
In general, risk of major and minor bleeding associated with a central line placement in patients with coagulation abnormalities is highly variable, with bleeding rates ranging between 0% and 32%.13–15 However, the ultrasound guide for insertion of a central venous catheter reduces the number of punctures, provides a greater chance of success in the first attempt, reduces the risk of arterial puncture, and reduces the incidence of hematomas in non-compressible places.10,16–19
Bleeding complications after the insertion of a central venous catheter in uremic patients are relatively uncommon.14,20–23
The aim of this study was to describe the frequency of complications associated with the insertion of central venous catheters in patients with a suspected disorder of hemostasis given by levels of >70 mg/dl.19 Additionally, we performed a bivariate analysis to determine possible relationship between catheter type, number of puncture, catheter insertion site and bleeding complications. Finally, we evaluate the possible utility of a cut-off value for serum BUN associated with bleeding complications related to the procedure.
Materials and Methods
Data Source
This retrospective descriptive study was conducted in an emergency department of a highly complexity University Hospital (Fundación Valle del Lili) in Southwestern Colombia between January 2013 and July 2019. Patients with serum urea nitrogen levels >70 mg/dl were selected. We defined the value of serum BUN of the results obtained by Jamale19 for the initiation of renal replacement therapy defined.
The ethics committee in biomedical research at University Hospital (Comité de Ética en Investigación Biomédica de la Fundación Valle del Lili) approved this study (Register No. 210-2018, approved on August 01, 2018. Institutional act No. 15 of August 01, 2018, protocol number 1279). This study applying the declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. This study adhered to the standards of the STROBE guidelines.
This study did not present risk (according to resolution 8430 of 1993 of the Colombian Ministry of Health); since it is documentary in nature, and the researchers do not assign biological, psychological, or social exposures to patients. Therefore, the ethics committee omitted the use of informed consent. All patient data accessed complied with relevant data protection and privacy regulations.
Study Population
The selected medical records had to meet the following inclusion criteria: 1) age greater than or equal to 18 years, 2) blood samples taken 24 hours before or after insertion of the central venous catheter, 3) BUN> 70 mg/dL, 4) central venous catheter inserted with ultrasound guidance and 5) recording of the procedure data by the specialist who performed the procedure. The professionals who performed the catheter insertion are all emergency medicine specialists with certified training in this ultrasound-guided practice.
All patients who received platelet transfusion 24 hours before the procedure were excluded.
Statistical Analysis
A descriptive analysis was performed. The normality of the variables was determined through a Shapiro–Wilk test. According to their distribution, the quantitative variables were expressed through the measure of central tendency and dispersion. Categorical variables were described in proportions and compared with Chi-square test or Fisher’s test. Receiver operatoring characteristic (ROC) analysis was applied to determine the threshold value of BUN related to the bleeding complications after catheter insertion.
Bleeding complications were defined as the presence of oozing from the skin insertion site, hematoma that did not require intervention and resolved with manual compression (Grades 1–2), a decrease in hemoglobin level ≥1.5g/dL judged to be associated with the catheter insertion or arterial puncture. Coagulopathy factors were documented as a partial thromboplastin time, prothrombin time value in platelets.
Results
A total of 1398 medical records were reviewed. A total of 273 records of procedures performed (273 inserted catheters) were included and analyzed. (Figure 1). There was a predominance of females, with a median age of 61 years. The most commonly used type of central venous catheter was Mahurkar (n: 184–67.4%), followed by the triple lumen catheter (n: 49–17.9%) and double-lumen catheter (n: 34–12.5%). The internal jugular vein was the most common insertion site chosen by the specialist (n: 146–53.5%). The success rate in the first puncture by ultrasound guidance was 93%. Chronic kidney disease was the first cause of indication for inserting the catheter (46.2%). The second cause was Sepsis indicated for the management with vasopressors (21.6%). Median of platelet count was 176,000. Median PTT values were prolonged (Table 1).
 |
Table 1 Clinical Characteristics |
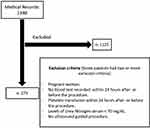 |
Figure 1 Study flowchart. |
Bleeding complications were detected in 69 cases (25.3%). Local bleeding after the intervention was the most frequent complication in 51/69 cases (73.9%). Post puncture hematoma was documented in 15/67 cases (21.7%) and Arterial puncture in cases 5/69 (7.2%) (Table 2).
 |
Table 2 Complications Related to Insertion of Central Venous Catheter |
The bivariate analysis showed a statistically significant association between the hematoma and catheter type (p:0.016) and, hematoma and catheter insertion site (p: 0.01) (Table 3).
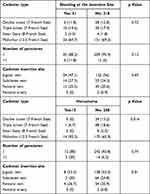 |
Table 3 Bivariate Analysis |
There was no cut-off value for BUN related to a greater probability of bleeding as a complication associated with the procedure (median within the cohort of 89.7 mg/dl and interquartile range between 80 and 111.9 mg/dl) (Figure 2).
 |
Figure 2 Area under the receiver operating characteristic (ROC) curve of blood urea nitrogen (BUN) for predicting bleeding complications after placement of central venous catheter. |
Discussion
In acutely ill patients with elevated BUN levels and the presence of bleeding complications after the ultrasound-guided central venous catheter placement was presented in 26.7%.
Complications after the insertion of a catheter in patients with uremia are common.24–29 There is a potential risk of complications suggested by pathophysiological mechanisms in patients with elevated BUN levels and the ultrasound-guided central venous catheter placement present.30–36 We identified patients with a high BUN value without bleeding complications reported. However, 10 patients (12 catheters implanted) presented a BUN value greater than 150 mg/dl and 2 patients reported any bleeding complication. The coagulopathy factors (Partial Thromboplastin Time and Prothrombin time) was altered in one patient.
Previous studies showed that threshold BUN value for distinguishing upper from lower gastrointestinal bleeding was 21.0 mg/dl,11 and BUN/Cr ratio over 35 mg/dL can predict a gastrointestinal bleeding.12 Lin et al10 described BUN levels between 150 and 200 mg/dl of urea nitrogen are associated with spontaneous brain bleeding and increased bleeding episodes in trauma patients. Perhaps, the low number of bleeding complications in our study was related to the fact that the distribution of patients in the cohort had a median BUN of 89.7 mg/dl (IQR 80–111.9 mg/dl). This value of BUN was not severely increased, even when it is among the uremia criteria according to the reference point we used for this analysis (Supplementary Table 1).
A total of 110/269 patients presented thrombocytopenia (platelets accounts <150 × 103/µL) of which 36/110 documented any bleeding complication related to cvc placement. Some authors considered an optimal platelet account >50 × 103/µL before central venous catheter could be safe.48 Previous studies have reported a low rate of bleeding complications in patients with thrombocytopenia undergoing central venous cannulation.14 Our results are according with the literature reported with rates ranging between 0% and 32% after cvc placement.49–51
Previous studies have described that the major complications occur about 3% in experimented operator.47 In our study, one patient (0.3%) presented a major bleeding complication in the first 48 hours after insertion of the catheter. Coagulopathy factors were normal range and value of BUN was 86 mg/dl. Due to profuse bleeding at the insertion site, he required a transfusion of two red blood cell units.
The use of ultrasound-guided central venous catheter placement decreases the number of complications (especially with a lower risk of arterial puncture) related to bleeding events.37–40 Randolph et al41 demonstrated a reduction in the risk of failed attempts compared to the anatomical technique (RR 0.66; 95% CI 0.45 to 0.79) and a reduction in complications (RR 0.22; 95% CI 0.10 to 0.45). Our population is not representative to conclude safety or risk for bleeding complications with ultrasound guidance. However, we considered the useful of ultrasound guidance as a safe procedure for catheter placement.42–44
A bivariate analysis was performed. There was a statistically significant association between the hematoma and two independent variables (the type of catheter and catheter insertion site). However, it is not possible to attribute this finding due to the reduced number of patients in the group that presented the event. It is necessary to conduct a study with more cases to determine that this finding is demonstrable. We did not identify a correlation with any cut-off point between high levels of urea nitrogen and any bleeding complication.
Our study has certain limitations. We did not evaluate other variables that contribute to the bleeding complications like medications, platelet transfusion before the procedure and others.45,46 We considered that a higher sample size for association analysis was necessary to evaluate the relationship between some variables and bleeding complications.
Conclusion
The frequency of bleeding complications associated with the insertion of central venous catheters in patients with BUN levels >70 mg/dl are common. It is not possible to determine a BUN cut-off value to predict complications and the association analysis is not conclusive.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Hedges SJ, Dehoney SB, Hooper JS, Amanzadeh J, Busti AJ. Evidence-based treatment recommendations for uremic bleeding. Nat Clin Pract Nephrol. 2007;3:138–153. doi:10.1038/ncpneph0421
2. Galbusera M, Remuzzi G, Boccardo P. Treatment of bleeding in dialysis patients. Semin Dial. 2009;22:279–286. doi:10.1111/j.1525-139X.2008.00556.x
3. Steiner RW, Coggins C, Carvalho AC. Bleeding time in uremia: a useful test to assess clinical bleeding. Am J Hematol. 1979;7:107. doi:10.1002/ajh.2830070203
4. Eknoyan G, Wacksman SJ, Glueck HI, Will JJ. Platelet function in renal failure. N Engl J Med. 1969;280:677. doi:10.1056/NEJM196903272801301
5. Zachee P, Vermylen J, Boogaerts MA. Hematologic aspects of end-stage renal failure. Ann Hematol. 1994;69:33–40. doi:10.1007/BF01757345
6. Thekkedath UR, Chirananthavat T, Leypoldt JK, Cheung AK, Mohammad SF. Elevated fibrinogen fragment levels in uremic plasma inhibit platelet function and expression of glycoprotein IIb-IIIa. Am J Hematol. 2006;81:915–926. doi:10.1002/ajh.20720
7. Escolar G, Cases A, Bastida E, et al. Uremic platelets have a functional defect affecting the interaction of von Willebrand factor with glycoprotein IIb-IIIa. Blood. 1990;76:1336. doi:10.1182/blood.V76.7.1336.1336
8. Benigni A, Boccardo P, Galbusera M, et al. Reversible activation defect of the platelet glycoprotein IIb-IIIa complex in patients with uremia. Am J Kidney Dis. 1993;22:668. doi:10.1016/S0272-6386(12)80429-X
9. Mannucci PM, Tripodi A. Hemostatic defects in liver and renal dysfunction. Hematol Educ Progr Am Soc Hematol Soc Hematol Progr. 2012;2012:168–173. doi:10.1182/asheducation.V2012.1.168.3798232
10. Lin B-S, Huang T-P, Tang G-J, Der-Cherng Tarng C-WK, Kong C-W. Ultrasound-guided cannulation of the internal jugular vein for dialysis vascular access in uremic patients. Nephron. 1998;78(4):423–428. doi:10.1159/000044971
11. Tomizawa M, Shinozaki F, Hasegawa R, et al. Laboratory test variables useful for distinguishing upper from lower gastrointestinal bleeding. World J Gastroenterol. 2015;21(20):6246–6251. doi:10.3748/wjg.v21.i20.6246
12. Zia Ziabari SM, Rimaz S, Shafaghi A, et al. Blood urea nitrogen to creatinine ratio in differentiation of upper and lower gastrointestinal bleedings; a diagnostic accuracy study. Arch Acad Emerg Med. 2019;7(1):e30.
13. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162(3):205–213. doi:10.7326/M14-1589
14. Van de Weerdt EK, Biemond BJ, Baake B, et al. Central venous catheter placement in coagulopathic patients: risk factors and incidence of bleeding complications. Transfusion. 2017;57(10):2512–2525. doi:10.1111/trf.14248
15. Ray CE, Shenoy SS. Patients with thrombocytopenia: outcome of radiologic placement of central venous access devices. Radiology. 1997;204(1):97–99. doi:10.1148/radiology.204.1.9205228
16. Agarwal SK, Dash SC, Dash SC, Dash SC. A prospective randomized study to compare ultrasound-guided with nonultrasound-guided double lumen internal jugular catheter insertion as a temporary hemodialysis access. Ren Fail. 2005;27(5):561–564. doi:10.1080/08860220500199084
17. Aydin Z, Gursu M, Uzun S, et al. Placement of hemodialysis catheters with a technical, functional, and anatomical viewpoint. Int J Nephrol. 2012;2012:5. doi:10.1155/2012/302826
18. Álvarez F. Accesos venosos centrales guiados por ultrasonido: ¿existe evidencia suficiente para justificar su uso de rutina? Rev Médica Clínica Las Condes. 2011;22(3):361–368. doi:10.1016/S0716-8640(11)70436-9
19. Jamale TE, Hase NK, Kulkarni M, et al. Earlier-start versus usual-start dialysis in patients with community-acquired acute kidney injury: a randomized controlled trial. Am J Kidney Dis. 2013;62:1116–1121. doi:10.1053/j.ajkd.2013.06.012
20. Bream PR. Update on insertion and complications of central venous catheters for hemodialysis. Semin Intervent Radiol. 2016;33(1):31–38. doi:10.1055/s-0036-1572547
21. Clark E, Kappel J, MacRae J, et al.; Canadian Society of Nephrology Vascular Access Work Group. Practical aspects of nontunneled and tunneled hemodialysis catheters. Can J Kidney Health Dis. 2016;3:2054358116669128. doi:10.1177/2054358116669128
22. Walser EM. Venous access ports: indications, implantation technique, follow-up, and complications. Cardiovasc Intervent Radiol. 2012;35(4):751–764. doi:10.1007/s00270-011-0271-2
23. Silva FS. Neck haematoma and airway obstruction in a patient with goitre: complication of internal jugular vein cannulation. Acta Anaesthesiol Scand. 2003;47(5):626–629. doi:10.1034/j.1399-6576.2003.00106.x
24. Sasvary F, Somlo P, Nwanosike N. Complications of central venous catheterization in hemodialysis patients. Bratisl Lek Listy. 2005;106(1):26–29.
25. Royo P, García-Testal A, Soldevila A, Panadero J, Cruz JM. Tunneled catheters. Complications during insertion. Nefrología. 2008;28(5):543–548.
26. Vats HS. Complications of catheters: tunneled and non tunneled. Adv Chronic Kidney Dis. 2012;19:188–194. doi:10.1053/j.ackd.2012.04.004
27. Napalkov P, Felici DM, Chu LK, et al. Incidence of catheter-related complications in patients with central venous or hemodialysis catheters: a health care claims database analysis. BMC Cardiovasc Disord. 2013;13:86. doi:10.1186/1471-2261-13-86
28. Weigand K, Encke J, Meyer FJ, et al. Low levels of prothrombin time (INR) and platelets do not increase the risk of significant bleeding when placing central venous catheters. Med Klin. 2009;104(5):331–335. doi:10.1007/s00063-009-1070-2
29. Hughes OU, Stella A, Samuel E. Complications of Internal jugular catheters in haemodialysis patients at a kidney care center in Nigeria. J Clin Nephrol. 2019;3:121–125. doi:10.29328/journal.jcn.1001037
30. Rabolink TJ, Zwaginga JJ, Koomans HA, Sixma JJ. Thrombosis and hemostasis in renal disease. Kidney Int. 1994;46:287–296. doi:10.1038/ki.1994.274
31. Mannucci PM, Remuzzi G, Pusineri F, et al. Deamino-8-d-arginine vasopressin shortens the bleeding time in uremia. N Engl J Med. 1983;308:8–12. doi:10.1056/NEJM198301063080102
32. Remuzzi G, Cavenaghi AE, Mecca G, Donati MB, De Gaetano G. Prostacyclin-like activity and bleeding in renal failure. Lancet. 1977;2:1195–1197. doi:10.1016/S0140-6736(77)90437-8
33. Remuzzi G, Pusineri F. Coagulation defects in uremia. Kidney Int. 1988;24:S13–17.
34. Noris M, Benigni A, Boccardo P, et al. Enhanced nitric oxide synthesis in uremia: implications for platelet dysfunction and dialysis hypotension. Kidney Int. 1993;44:445–450. doi:10.1038/ki.1993.264
35. Eknoyan G, Brown CH. Biochemical abnormalities of platelets in renal failure. Evidence for decreased platelet serotonin, adenosine diphosphate and Mg-dependent adenosine triphosphatase. Am J Nephrol. 1981;1:17–23. doi:10.1159/000166482
36. Benigni A, Boccardo P, Galbusera M, et al. Reversible activation defect of the platelet glycoprotein IIb—IIIa complex in patients with uremia. Am J Kidney Dis. 1993;22:668–676.
37. Napolitano M, Malato A, Raffaele F, et al. Ultrasonography-guided central venous catheterisation in haematological patients with severe thrombocytopenia. Blood Transfus. 2013;2013:1–5.
38. Haas B, Chittams JL, Trerotola SO. Large-bore tunneled central venous catheter insertion in patients with coagulopathy. J Vasc Interv Radiol. 2010;21:212–217. doi:10.1016/j.jvir.2009.10.032
39. Rabindranath KS, Kumar E, Shail R, Vaux EC. Ultrasound use for the placement of haemodialysis catheters. Cochrane Database Syst Rev. 2011;11:CD005279.
40. Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327:1–7. doi:10.1136/bmj.327.7411.361
41. Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters. Crit Care Med. 1996;24(12):2053–2058. doi:10.1097/00003246-199612000-00020
42. Fragou M, Gravvanis A, Dimitriou V, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med. 2011;39(7):1607–1612. doi:10.1097/CCM.0b013e318218a1ae
43. Wu SY, Ling Q, Cao LH, et al. Real-time two-dimensional ultrasound guidance for central venous cannulation: a meta-analysis. Anesthesiology. 2013;118(2):361–375. doi:10.1097/ALN.0b013e31827bd172
44. Brass P, Hellmich M, Kolodziej L, et al. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015;1:CD011447. doi:10.1002/14651858.CD011447
45. Rockholt MM, Thorarinsdottir HR, Lazarevic V, Rundgren M, Kander T. Central venous catheter-related complications in hematologic patients: an observational study. Acta Anaesthesiol Scand. 2022;66:473–482. doi:10.1111/aas.14020
46. Tercan F, Ozkan U, Oguzkurt L. US-guided placement of central vein catheters in patients with disorders of hemostasis. Eur J Radiol. 2008;65:253–256. doi:10.1016/j.ejrad.2007.04.002
47. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220–1229. doi:10.1056/NEJMoa1500964
48. Jodi B, Dzik WH; Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence‐based review. Transfusion. 2005;45:1413–1425. doi:10.1111/j.1537-2995.2005.00546.x
49. Schiffer CA, Bohlke K, Delaney M, et al. Platelet transfusion for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(3):283–299. doi:10.1200/JCO.2017.76.1734
50. Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727–736. doi:10.1016/j.jvir.2012.02.012
51. National Institute for Health and Care Excellence (UK). Blood Transfusion. National Institute for Health and Care Excellence (UK); 2015.
52. Rath CE, Mailliard JA, Schreiner GE. Bleeding tendency in uremia. N Engl J Med. 1957;257(17):808–811. doi:10.1056/NEJM195710242571704
53. Ferguson JH, Lewis JH, Zucker MB. Bleeding tendency in uremia. Blood. 1956;11(12):1073–1076. doi:10.1182/blood.V11.12.1073.1073
54. Molino D, De Lucia D, Gaspare De Santo N. Coagulation disorders in uremia. Semin Nephrol. 2006;26(1):46–51. doi:10.1016/j.semnephrol.2005.06.011
55. Weigert AL, Schafer AI. Uremic bleeding: pathogenesis and therapy. Am J Med Sci. 1998;316(2):94–104. doi:10.1097/00000441-199808000-00005
56. Zeck J, Schallheim J, Lew SQ, De Palma L. Whole blood platelet aggregation and release reaction testing in uremic patients. Biomed Res Int. 2013;2013:6. doi:10.1155/2013/486290
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
