Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Blastic Plasmacytoid Dendritic Cell Neoplasm with Lung Involvement and Cytopenia: A Case Report and a Literature Review
Authors Liu F , Qi F , Zhang J, Tan Y, Zhang X
Received 10 May 2023
Accepted for publication 1 August 2023
Published 11 August 2023 Volume 2023:16 Pages 2211—2216
DOI https://doi.org/10.2147/CCID.S414326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Fang Liu,1 Fei Qi,2 Jingya Zhang,1 Yaqi Tan,1 Xiuying Zhang1
1Department of Dermatology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Dermatology, Peking Union Medical College Hospital, Beijing, People’s Republic of China
Correspondence: Fang Liu, Department of Dermatology, Beijing Chaoyang Hospital, Capital Medical University, 8 Gongti South Road, Chaoyang District, Beijing, 100020, People’s Republic of China, Tel +86-10-85231688, Fax +86-10-85231217, Email [email protected]
Abstract: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a hostile cutaneous malignancy with dismal prognosis and unknown etiology with rarity. Most patients received traditional chemotherapy only has one year of median survival time. This article reports an 81-year-old male patient with BPDCN who presented with skin manifestations and was diagnosed with positive CD4, CD56, and CD123 immunohistochemical results. Systematic examination revealed lung involvement and cytopenia.
Keywords: blastic plasmacytoid dendritic cell neoplasm, pathological feature, lung involvement, cytopenia, treatment, prognosis
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is regarded as an organ cutaneous malignancy with unknown etiology.1 A number of victims with BPDCN usually suffered from skin lesions with or without myeloid involvement.1 Despite a small proportion of patients showed effective response to a variety of chemotherapies, the inevitable relapses and a median of 1-year overall survival (OS) hindered the steps of recovery in BPDCN patients.2 In this case, we present an aged male patient with BPDCN with lung involvement and cytopenia.
Case Report
A previously healthy 81-years old male admitted to our clinic due to “bruised-like” patches with pruritis on his face and trunk for two months. Physical exam was notable for violaceous infiltrated plaques on his face (Figure 1) and multiple purple nodules on his chest (Figure 2). He presented palpable enlarged lymph nodes in the neck. Laboratory values of complete blood count indicated cytopenia (WBC 1.95*109/L ↓, PLT 77*109/L ↓, RBC 3.35*1012/L ↓, HGB 99g/L ↓). The final diagnosis was made through skin biopsy and immunochemistry. The epidermis was spared under microscope. A clear Grenz zone was noticed between epidermis and dermis. The extensively infiltrated of lymphocytes can be observed within the entire dermis (Figure 3). High magnification of the lymphocytes in the dermis demonstrated nuclear atypia and immature nuclear chromatin pattern with occasional nucleoli (Figure 4). Followed immunochemistry showed positive for CD4 (Figure 5), CD56 (Figure 6), CD123 (Figure 7), Ki67 with the positive rate >90% (Figure 8). And the immunochemistry showed negative for CD3, CD8, CD20, CD68. The patient refused the following recommended therapeutic plan and complained cough, hemoptysis and expectoration on his last visit. The PET-CT was ordered which indicated pulmonary metastasis. We wanted to admit him for further care, unfortunately, the patient died due to multiple organ failure 9 months in the department of Emergency after the first visit.
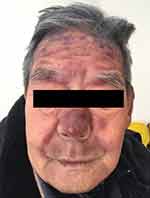 |
Figure 1 Violaceous infiltrated plaques on his face. |
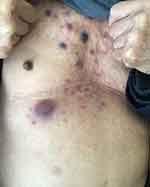 |
Figure 2 Multiple purple nodules on his chest. |
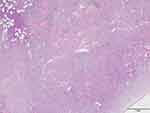 |
Figure 3 The epidermis was spared. Grenz zone can be observed between epidermis and dermis (x20). |
 |
Figure 4 High magnification of the dermis demonstrated nuclear atypia and immature nuclear chromatin pattern with occasional nucleoli (x100). |
 |
Figure 5 CD4(+) (x100). |
 |
Figure 6 CD56 (+) (x100). |
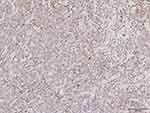 |
Figure 7 CD123(+) (x100). |
 |
Figure 8 Ki67>90% (X100). |
Discussion
In this article, we report a very rare tumor called BPDCN with lung involvement and cytopenia at the time of diagnosis. BPDCN is regarded as a myeloid-derived malignancy originating from plasmacytoid dendritic cells (pDCs).3 Given its blastic appearance and the positive CD56 expression, it was previously termed “blastic NK cell lymphoma”. Considering its rarity and changing nomenclature, the exact rate of BPDCN is difficult to estimate.4 No known environmental or genetic factors were found in the pathogenesis of BPDCN.5 BPDCN could take place in all age groups with old male patients’ predominance. Studies reported that the median age of patients at diagnosis was 65 to 67 years old.
More than 90% of patients with this disease had skin lesions as the initial manifestation, which could be accompanied by changes in lymph nodes and bone marrow. Sixty-four percent of patients had anemia at the time of diagnosis, 75% have thrombocytopenia, and 38% have neutropenia.2 This patient presented with skin manifestations accompanied by lymph node involvement. A blood routine examination showed that the patient had multiple manifestations of decreased blood cells, which is considered to be bone marrow involvement. It is recommended that the patient should receive bone marrow puncture, but the patient refused. Finally, we diagnosed the patient through the skin tissue pathological biopsy method.
It was believed that BPDCN originated from the forerunner of myeloid-derived, resting plasmacytoid dendritic cells (pDCs), the type 2 dendritic cells. A retrospective, observational study with 86 BPDCN patients demonstrated the existence of markers related to plasmacytoid dendritic cell origin (HLA-DRhigh, CD303+, CD304+, and cTCL1+) plus CD4 and CD56 and elevated expression of specific markers from the myeloid, B-, and T-lymphoid lineages, whereas other specific markers (myeloperoxidase, CD14, cCD3, CD19, and cCD22) were not expressed.2 We confirmed the diagnosis through skin biopsy. After diagnosis, a detailed systemic examination is necessary to understand the patient’s systemic involvement. Due to the patient’s refusal, we did not conduct further examination.
This disease progresses rapidly and has a poor prognosis; therefore, in-depth research is needed to improve patient survival rate. The number of molecular studies such as high-throughput sequencing (techniques) has been growing with increasing the uncovering of BPDCN in the field of pathogenesis, diagnosis, and therapies. Sapienza et al confirmed BPDCN had epigenetic modifier genes recurrently mutated and methylation signatures overexpressed. Signaling from the E-box transcription factor, TCF4, is critical for sustaining the oncogenic program for BPDCN.6 Cytogenetics also illustrated no specific chromosomal abnormalities found in BPDCN, while the genomic rearrangements spotted the involvement of the MYB family genes and MYC. Approximately 30% of BPDCN cases carried the 8q24 rearrangement; meanwhile, 6p21 harboring RUNX2, which is strongly associated with immunoblastoid cytomorphology and MYC gene expression, was more easily noticed. Moreover, in one-fifth of BPDCN patients, MYB rearrangement is detected.7 Studies also found overexpressed interleukin-3 receptor α (IL-3R α) and CD123 in patients with hematologic malignancies, particularly in acute BPDCN, indicating that CD123 might have the potential to become an attractive immunotherapeutic target. As a result, several biologics targeting CD123 pathway have been investigated to treat BPDCN.8
The FDA approved tagraxofusp (SL-401), a CD123-directed cytotoxin, containing human interleukin-3 fused to truncated diphtheria toxin, for adults and pediatric patients older than 2 years who have either treatment-naïve or relapsed/refractory BPDCN.9 An open-label, multi-cohort study with 47 patients with untreated or relapsed BPDCN recruited, evaluated the efficacy of intravenous injection of tagraxofusp at a dose of 7 μg or 12 μg per kilogram of body weight on days 1 to 5 of each 21-day, with over 90% overall response rate.10
Although it is known BPDCN may have an unsatisfied outcome, with which a large proportion of cases died when treated with conventional chemotherapies within one year, a bunch of emerging novel therapeutics based on advanced technologies, such as karyotyping, gene expression profiling, and high-throughput sequencing, had revealed the ambiguous pathogenetic progress of BPDCN and brought hopes with the introduction of new drugs. For instance, the introduction of SL-401, anti-CD123 immunotherapies, venetoclax, BET-inhibitors, and demethylating agents brings promising therapeutic options for BPDCN.11
Conclusion
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is an extremely rare, aggressive cutaneous malignancy with dismal prognosis and unknown etiology. Most patients sought treatment based on skin manifestations and require a dermatologist to quickly diagnose and conduct detailed systemic examinations to assess systemic involvement. Patients received traditional chemotherapy only had one year of median survival time. Therefore, early recognition, intervention, and treatment are essential to avoid further development. However, the treatment of BPDCN remains challenging for dermatologists.
Ethics Approval and Informed Consent
Written informed consent was signed by the patient’s daughter to approve publishing the case details and any accompanying images. Publication of the case details was approved by the ethical board of Beijing Chaoyang Hospital, Capital Medical University.
Acknowledgments
We thank the patient and physicians for participating in our study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (Fund No. 82273551).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sweet K. Blastic plasmacytoid dendritic cell neoplasm: diagnosis, manifestations, and treatment. Curr Opin Hematol. 2020;27(2):103–107. doi:10.1097/MOH.0000000000000569
2. Garnache-Ottou F, Vidal C, Biichle S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3(24):4238–4251. doi:10.1182/bloodadvances.2019000647
3. Hirner JP, O’Malley JT, LeBoeuf NR. Blastic plasmacytoid dendritic cell neoplasm: the dermatologist’s perspective. Hematol Oncol Clin North Am. 2020;34(3):501–509. doi:10.1016/j.hoc.2020.01.001
4. Pagano L, Valentini CG, Grammatico S, Pulsoni A. Blastic plasmacytoid dendritic cell neoplasm: diagnostic criteria and therapeutical approaches. Br J Haematol. 2016;174(2):188–202. doi:10.1111/bjh.14146
5. Cheng W, Yu TT, Tang AP, He Young K, Yu L. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41(3):405–419. doi:10.1007/s11596-021-2393-3
6. Sapienza MR, Pileri S. Molecular features of blastic plasmacytoid dendritic cell neoplasm: DNA mutations and epigenetics. Hematol Oncol Clin North Am. 2020;34(3):511–521. doi:10.1016/j.hoc.2020.01.002
7. Sakamoto K, Takeuchi K. Cytogenetics of blastic plasmacytoid dendritic cell neoplasm: chromosomal rearrangements and DNA copy-number alterations. Hematol Oncol Clin North Am. 2020;34(3):523–538. doi:10.1016/j.hoc.2020.01.003
8. Xue T, Budde LE. Immunotherapies targeting CD123 for blastic plasmacytoid dendritic cell neoplasm. Hematol Oncol Clin North Am. 2020;34(3):575–587. doi:10.1016/j.hoc.2020.01.006
9. Zhang Y, Sokol L. Clinical Insights into the management of blastic plasmacytoid dendritic cell neoplasm. Cancer Manag Res. 2022;14:2107–2117. doi:10.2147/CMAR.S330398
10. Pemmaraju N, Lane AA, Sweet KL, et al. Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 2019;380(17):1628–1637. doi:10.1056/NEJMoa1815105
11. Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers. 2019;11(5):595. doi:10.3390/cancers11050595
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
