Back to Journals » International Journal of Nanomedicine » Volume 18
Black Phosphorus – A Rising Star in the Antibacterial Materials
Authors Zhang L, You J, Lv H, Liu M, Quni S , Liu X, Zhou Y
Received 27 September 2023
Accepted for publication 3 November 2023
Published 10 November 2023 Volume 2023:18 Pages 6563—6584
DOI https://doi.org/10.2147/IJN.S438448
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Lu Zhang,1,2 Jiaqian You,1 Huixin Lv,1 Manxuan Liu,1 Sezhen Quni,1 Xiuyu Liu,1 Yanmin Zhou1,2
1Jilin Provincial Key Laboratory of Tooth Development and Bone Remodeling, Hospital of Stomatology, Jilin University, Changchun, People’s Republic of China; 2School of Stomatology, Jilin University, Changchun, People’s Republic of China
Correspondence: Yanmin Zhou, Email [email protected]
Abstract: Antibiotics are the most commonly used means to treat bacterial infection at present, but the unreasonable use of antibiotics induces the generation of drug-resistant bacteria, which causes great problems for their clinical application. In recent years, researchers have found that nanomaterials with high specific surface area, special structure, photocatalytic activity and other properties show great potential in bacterial infection control. Among them, black phosphorus (BP), a two-dimensional (2D) nanomaterial, has been widely reported in the treatment of tumor and bone defect due to its excellent biocompatibility and degradability. However, the current theory about the antibacterial properties of BP is still insufficient, and the relevant mechanism of action needs to be further studied. In this paper, we introduced the structure and properties of BP, elaborated the mechanism of BP in bacterial infection, and systematically reviewed the application of BP composite materials in the field of antibacterial. At the same time, we also discussed the challenges faced by the current research and application of BP, which laid a solid theoretical foundation for the further study of BP in the future.
Keywords: black phosphorus, antibacterial, nanometer material, photothermal therapy
Introduction
Microorganisms can cause serious tissue damage in the body, and severe inflammatory reactions can lead to delayed wound repair. Therefore, bacterial infection is one of the most critical challenges in the wound healing process.1 Antibiotics are the main strategy used to treat bacterial diseases,2 while ESKAPE pathogens (E. faecalis, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter) are able to evade currently used antibiotics through evolutionary mechanisms and become multidrug-resistant (MDR) bacteria.3,4 The emergence of MDR bacteria has become a global crisis, increasing morbidity and mortality among those infected.5 According to the World Health Organization, about 25,000 people in Europe and 700,000 worldwide die from drug-resistant bacterial infections each year, and by 2050, bacterial infections will cause 10 million deaths a year, far more than the current number of deaths from cancer.6,7 Moreover, treating antibiotic-resistant infections is expensive, costing society an estimated $20 billion a year and placing a burden on social healthcare systems.8 The misuse and abuse of antibiotics and the lack of new treatments exacerbate this crisis,9 creating an urgent need to develop new antimicrobial materials for use in everyday life and healthcare.
Nanomaterials, including metals -, metal oxides -, carbon -, quantum dots - etc., show promise in the fight against antimicrobial resistance.10 First of all, compared with traditional antibiotics, nanomaterials can obtain new antibacterial methods against pathogens, such as destruction of cell wall and membrane, oxidative damage, and destruction of intracellular components. Second, nanomaterials tend to have a high surface area to volume ratio and are considered to be an excellent drug carrier, which can change the delivery mode and enhance the bactericidal effect. Secondly, nanomaterials have a large surface area to volume ratio and are considered to be an excellent drug carrier, which can change the mode of drug delivery and enhance the bactericidal effect.11–13 Among them, two-dimensional nanomaterials such as graphene, transition metal disulfide (TMD), hexagonal boron nitride (hBN), etc. have been successfully applied as new antibacterial agents in the biomedical field due to their excellent electrical conductivity, mechanical properties, and wide applicability. However, they have some limitations. For example, graphene lacks a band gap, lacks light absorption in the visible region, and molybdenum disulfide (MoS2) has low carrier mobility, which limits their application.14,15 Therefore, scholars are looking for more perfect two-dimensional nanomaterials to reduce or eliminate the limitations of the above biomedical applications.
Black phosphorus, also known as phosphorene, is a rising star in two-dimensional nanomaterials and is considered a promising candidate to compensate for the shortcomings of graphene and TMD. In 1914, Bulk BP was synthesized from allotrope (white phosphorus or red phosphorus), which is more stable than white phosphorus or red phosphorus.16 However, little research was done on BP for more than 100 years. BP nanosheets (BPNSs) and BP quantum dots (BPQDs) attracted widespread interest in the scientific community until 2014, when BPNSs were first spun off from bulk BP. Compared with other two-dimensional nanomaterials, BP has high anisotropy of structure, adjustable band gap width, high hole mobility and electron mobility, which give BP unique properties such as electrical conductivity, photothermal, photodynamic, unusual mechanical behavior (negative Poisson’s ratio), etc.17–20 Among them, the near-infrared light response of BP makes it show remarkable effect in photothermal/photodynamic antibacterial.21,22 In addition, BPNSs has a high surface-to-volume ratio due to its unique layered structure, which makes it an excellent drug carrier for the loading and delivery of other antimicrobial nanomaterials or drugs.23–25 Therefore, the successful synthesis of layered BP has brought the application of BP in the field of antibacterial into a new era.
So far, the application of BP nanomaterials in biomedicine, especially in the treatment of anti-tumor and bone defect, has been well reviewed.26–28 In recent years, the application of BP in antibacterial field has attracted more and more attention and research. In this paper, the research progress of BP-based composites in antibacterial field in recent years was reviewed, including structure, safety, antibacterial mechanism and the application of various BP-based composites in antibacterial field, in order to lay a theoretical foundation for the further use of BP in antibacterial field (Figure 1).
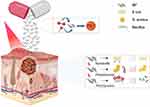 |
Figure 1 Characterization and antibacterial mechanism of BP. |
Structure and Synthesis Methods
Structure
BP is a new member of the two-dimensional nanomaterials family, which, like graphene, is made of a single element. Bulk BP is made up of many single layers. The phosphorus atoms in each layer are connected to three adjacent atoms via sp orbitals to form a tetrahedron (P4), where the shorter bond with a bond length of 0.2224 nm connects the phosphorus atoms in the same layer, while the longer bond of 0.2244 nm connects the top and bottom phosphorus atoms in the single layer.29 The non-planar double-layer structure formed by P-P covalent bonds is regarded as the armchair direction in the X-axis, and the Y-axis is often referred to as the zigzag direction. The bulk BP lattice parameters in the two directions are different, 3.30A and 4.53A, respectively.30,31 This hybrid produces a ruffle-honeycomb structure that breaks a single bond of P4, resulting in two different bond angles, 96.300° (θ1) and 102.095° (θ2), which are close to 109.5° (a perfect quadrangular structure), and thus BP is the most stable of the phosphorus family.32 In addition, the fourth orbital of each phosphorus atom carries a pair of lone electrons, further stabilizing the distorted structure of BP.33 The P-P distance (5.500A) between adjacent layers is greater than the covalent bond length of phosphodiene, indicating that these layers are stacked together by weak van der Waals forces rather than bond interactions,34 so BP can easily strip from the surface of the bulk crystal to obtain a single two-dimensional nanosheet. It is the second basic monolayer material after graphene that can be stripped from bulk crystals (Figure 2).28 BPNSs has better performance than bulk BP in all aspects, and its hole mobility is much higher than bulk BP, which is similar to silicon. It also has a wider tunable direct band gap than bulk BP, thus showing a wider absorption spectrum.32 In addition to two-dimensional layered structures, one-dimensional (1D) phosphene nanoribbons were also explored. Their electronic properties are highly sensitive to edge structure, edge type and edge width. Recently, Zhang et al prepared another BP nanostructure——zero-dimensional BP quantum dots from BP crystals for the first time, which have a higher band-gap width, ultra-small size, higher specific surface area and more active edge position, and have been widely concerned in the biomedical field, greatly promoting the research and development of BP.35,36
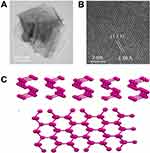 |
Figure 2 Morphology and structure of BP. (A) A TEM image of the few-layer BP. (B) Atomic resolution dark field TEM image of the few-layer BP, showing the crystal structure of the few-layer BP. Adapted with permission from Zhang T, Xie H, Xie S, et al. A Superior Two-Dimensional Phosphorus Flame Retardant: Few-Layer Black Phosphorus. Molecules. 2023;28(13):5062. Copyright © 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).37 (C) Schematic representation of single-layer black phosphorus nanosheet, side view and top view. Adapted with permission from Erande MB, Pawar MS, Late DJ. Humidity Sensing and Photodetection Behavior of Electrochemically Exfoliated Atomically Thin-Layered Black Phosphorus Nanosheets. ACS Appl Mater Interfaces. 2016;8(18):11548–11556, Copyright 2016, with permission from American Chemical Society (ACS).38 |
Synthesis Methods
At present, the preparation methods of BP nanosheets can be divided into two categories, one is “top-down method”, which mainly includes mechanical stripping, liquid stripping and so on. Mechanical stripping is a scotch tape-based mechanical exfoliation method that was first used to produce atomic layers of graphene from highly oriented pyrolytic graphite (HOPG), which is then repeatedly stripped away. The first successful stripping of single-layer BP nanosheets was also achieved by Li et al through this method.39 However, due to some shortcomings of this method, such as low yield, complicated process, and inability to control the size and thickness of nanosheets, it is impossible to expand, so scholars have subsequently developed a series of methods to improve.40,41 The liquid exfoliation is carried out by the solvent such as N-methyl-2-pyrrolidone (NMP) and the layer BP surface energy equilibrium phase. The method is simple and easy to operate, and the thickness and size of the nanosheet can be controlled, and BP can be stably dispersed in solvent after stripping (Figure 3).42,43 However, these “top-down methods” can not achieve large-scale production, the large-scale synthesis method of thin-film BP has become the future development direction, so another class of “bottom-up methods”, such as chemical vapor deposition (CVD), pulsed laser deposition, epitaxy, etc., began to enter people’s vision.44–46 Joshua et al first proposed the in-situ chemical vapor deposition method for the preparation of large area two-dimensional BP. They first prepared red phosphorus (RP) film on silicon substrate under vacuum condition, and then transferred it into quartz centrifuge tube, adding mineralizer Sn and SnI4, and realized the conversion of RP film to BP film under certain temperature program and pressure. Epitaxial method is another method that can generate thin layer BP on a large scale. Xu et al deposited Au films on Si/SiO2 substrate and heated the excess RP and Sn in a vacuum quartz glass tube to 750°C to form dispersed Au3SnP7 on Si/SiO2 substrate. After adding SnI4 as a mineralizer, nucleation and growth of BP films on Au3SnP7 were enabled (Figure 4). Transmission electron microscopy shows that BP has obvious layered structure, and the size and thickness of BP can be controlled by controlling temperature.47
 |
Figure 3 Liquid exfoliation. Bulk BP was added to NMP, stripped with ultrasonic assistance, then centrifuged to remove the supernatant, washed and redispersed in water. |
 |
Figure 4 Epitaxial method of BPNSs preparation. From the formation of Au3SnP7, to the epitaxial nucleation of BP, and finally the small BP nanosheets on the substrate fuse into large BP nanosheets. Adapted with permission from Xu Y, Shi X, Zhang Y, et al. Epitaxial nucleation and lateral growth of high-crystalline black phosphorus films on silicon. Nat Commun. 2020;11(1):1330. Copyright © The Author(s) 2020. http://creativecommons.org/licenses/by/4.0/.47 |
Biocompatibility and Biodegradability
Biocompatibility
Biocompatibility is an important topic in the research of biomaterials. Studies have shown that the cytotoxicity of BP is lower than that of graphene, but higher than that of transition metal dihalides such as molybdenum disulfide and tungsten disulfide.48 The entry of BPNSs into cells will cause a series of cellular reactions, including morphological change, plasma membrane disorder, oxidative stress, DNA damage and cell death,49 but these reactions are closely related to the size, concentration, oxidation degree of BP, action time and cell type.48–50 In vitro toxicity experiments, the toxicity of BP was positively correlated with the concentration, size and thickness. For example, when the concentration of BP was less than 50μg/ml, it had almost no toxicity to cells, while when the concentration reached 200 μg/ml, it had obvious apoptotic effect on HeLa cells; 48,50,51 BP with a transverse size of about 200 nm is much less cytotoxic to mammals than BP with a transverse size of 400nm.50,52 Moreover, studies have shown that unmodified BP can show significant cytotoxicity.53 In vivo toxicity tests, Mu et al investigated the metabolic distribution of BP in various organs. After injecting BP into the abdominal cavity of mice, they analyzed the phosphorus concentration in the organs of mice 1 day and 30 days later, and found that the phosphorus content only increased significantly in the bladder on the first day, and no significant BP accumulation was observed in other organs. What’s more, about 80% BPQDs was excreted through feces and urine 10 hours after administration. Huang et al conducted a venous circulation experiment, and the results of blood, pathology and biochemistry showed that BP had no significant damage to important organs, and the metabolic distribution results of BP were consistent with the toxicity results.54 The scholars also found that although BP can cause oxidative stress in mice, resulting in acute toxicity, the inflammatory response can gradually recover over time without causing pathological damage to the organs of the mice.55,56 Therefore, BP is considered to be a biocompatible nanomaterial, in order to further ensure the safety of BP in biomedical applications, the size, concentration, oxidation and other parameters of BP, as well as the action time in different cells need to be explored and optimized.
Biodegradability
In biorelated applications, the degradability of nanomaterials is of great importance in ensuring their safety to biological tissues.27 However, currently developed photothermic agents are generally slow to degrade and difficult to remove from the human body, resulting in poor biocompatibility.57–59 Studies have shown that BP is biodegradable, because BP is a nanostructure composed of a single phosphorus element, it has a special layered structure, and the layers are connected by weak van der Waals forces, resulting in its nature is more active, and it is easy to react with water and oxygen. The lone pair electrons on the surface of BP are easily taken away by oxygen molecules, so that the outer layer is oxidized, and the oxide layer is rapidly degraded by reacting with water, exposing the unreacted BP in the lower layer, and this cycle until the BP is completely degraded.28,60 Shao et al placed BPQDs in a phosphate buffer to simulate its degradability in vivo, and they demonstrated the degradation process by changing the shape of BPQDs in scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images, and BPQDs was almost completely degraded after 8 weeks.61 Wang et al further studied the biodegradation rate of layered BP under different atmospheres. They continuously irradiated the BP suspension for about 2 hours under three atmospheres of Ar, air and O2, and the results showed that the degradation rate of BP was the fastest in the oxygen environment.62 This proves that O2 is an essential element for BP photodegradation. The biodegradability of BP reduces cytotoxicity due to material accumulation in the body. In addition, the final degradation product of BP, PO43-, is an essential component of the human body and an important part of hydroxyapatite crystals in bone, which is not only safe, but also can promote bone formation as a source of mineralization.63
Antibacterial Mechanism
Damage the Integrity of the Cell Membrane
Mechanical destruction of bacterial membranes using rough surfaces and sharp edges is considered to be one of the main mechanisms of antimicrobial activity associated with nanomaterials.64 The layered structure of two-dimensional nanomaterials such as graphene can interact directly with cell membranes, causing physical damage. Damage to the cell wall causes an imbalance in cell infiltration, so cytoplasm accumulates in the surrounding space and eventually causes cell death.65,66 When Sun et al studied the antibacterial activity of BP, they found that although block BP inhibited the growth of bacteria, the killing rate of bacteria was lower than 60%, while the killing rate of stripped BPNSs on Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) was as high as 99.2%, indicating that the state of BP was closely related to the antibacterial activity, and the layered structure was more conducive to killing bacteria.67 To investigate further, Zhang et al treated cells with layered BP and found that BP accumulates on the cell surface, and they hypothesized that the deposition of BP might damage the cell membrane. They interacted layered BP with a model cell membrane composed of zwitterionic 1, 2-dioleyl-n-glycerol-3-phosphate (DOPC) vesicles, which were monitored by QCM-D. The results showed that the layered BP caused physical damage to the cell membrane, resulting in the release of water in the vesicles.50 What’s more, the larger the diameter of BPNs, the more serious the physical damage to the cell membrane. Phakatkar et al studied the antibacterial mechanism of BP under transmission electron microscopy, and observed that E. coli cells without BPNSs treatment had complete morphology, and after interaction with BPNSs, the cell plasma membrane separated from the bacterial cell wall, resulting in bacterial cell wall damage, cytoplasmic leakage, and bacterial rupture and death (Figure 5A). This is consistent with the results of Zhang et al.68 In addition to SEM images, Xiong et al also performed LDH assays to determine the leakage of cytoplasmic enzymes from bacterial cells into the medium, which can reflect the extent of cell membrane damage. The results showed that the LDH activity of both E. coli and Bacillus subtilis was significantly higher than that of the control group, and the LDH release of the experimental group increased with the passage of time, indicating that the membrane destruction was partly responsible for the bactericidal effect of BPNSs.69 Liu et al took a reverse approach, using dialysis membranes to separate BP and E. coli to prevent direct contact between them. After 3 hours of dialysis, no reduction in E. coli survival was detected, but when the two were in direct contact, even in the absence of light, E. coli survival decreased. This suggests that direct physical contact between BP and the bacteria causes the bacteria to die.70 The above results show that the physical damage of bacterial cell membrane caused by the sharp edge of BP is one of its important antibacterial mechanisms.
Promote Local Tissue Temperature Increase
Near Infrared Ray (NIR) light can penetrate the surface tissue to the deep tissue, and convert the light energy into heat energy to directly act on the surrounding tissue, promote blood circulation, accelerate metabolism, and facilitate tissue repair. Photothermal therapy (PTT) is to heat up photothermal agents (PTA) under the irradiation of NIR, which causes the surface of bacteria to heat up. When the temperature rises above 45°C, protein denaturation will be caused, which will lead to the death of bacteria. PTT is an effective method to combat MDR bacteria. Compared to other 2D nanomaterials, BP also has an adjustable bandgap width ranging from 0.3 eV (crystal) to 2.0 eV (monolayer), giving it wider light absorption in the UV and NIR regions.71 At room temperature, the electron mobility and hole mobility of bulk BP are 220 cm2 and 350 cm2 V−1 s−1 while the highest hole mobility of layered BP can reach ≈1000 cm2 V−1 s−1.18 BP has a high near-infrared extinction coefficient and photothermal conversion due to its unique structure, and it increases with the increase of irradiation power and BP concentration (Figure 5B). Sun et al synthesized BPQDs (transverse size about 2.6 nm, thickness about 1.5 nm) by liquid stripping method combined with probe ultrasound and water bath ultrasound, with extinction coefficient up to 14.8 Lg−1cm−1, much higher than gold nanords (AuNRs) (3.9 Lg−1cm−1) and oxidized graphene (3.6 Lg−1cm−1).56 Zhang et al synthesized quaternary aminated chitosan-black phosphorus nanocomposites, and calculated that the photothermal conversion rate of the composite was as high as 55.7%, which was significantly higher than that of traditional Au nanoshells (13%) and AuNRs (21%).62,72 Huang et al prepared a BPQDs hydrogel for the treatment of diabetic ulcer. After 808 nm laser irradiation, the temperature of the BPQDs hydrogel group increased by 35°C to 50°C within 4 minutes, compared with the pure hydrogel group, which only increased by 3°C. The photothermal antibacterial effect of the BPQDs hydrogel group was significantly higher than that of the pure hydrogel group.54 Although BP has excellent photothermal conversion, its thermal stability is greatly reduced due to its rapid degradation, which limits its application in PTT. Therefore, Hu et al loaded Cu2+ onto BP, and Cu2+ occupied the lone pair electrons of BP, preventing oxygen from reacting with it. Therefore, the BP@Cu nanocomposite has enhanced photothermal stability and avoids the accumulation of Cu2+ in the body, which solves the PTA problem mentioned above.73 Wang et al combined BPNSs, catechol-modified chitosan (CA-CS), and oxyglucan (Odex) to reduce its exposure to air and water. BP-based composites still showed good photothermal stability after three cycles of irradiation.74 In summary, BP is a promising PTT nanomaterial after certain treatment.
Enhance the Release of Reactive Oxygen Species (ROS)
While PTT is considered an excellent antimicrobial strategy, exposure to NIR light at high temperatures (>60 °C) and for long periods (>10 min) can cause irreversible damage to surrounding tissues.75,76 Photodynamic therapy (PDT) is a photosensitizer that produces ROS after exposure to light. Excessive accumulation of ROS not only consumes the antioxidant glutathione in bacteria, but also acts on bacterial cell membranes, resulting in lipid peroxidation and increased permeability of cell membranes. Malondialdehyde, a byproduct of lipid oxidation, can form complexes with proteins, resulting in cell membrane rupture and protein leakage. Therefore, PDT can kill bacteria at low temperatures, so as to avoid tissue damage at high temperatures.33,71,77 BP-based composites have demonstrated excellent ability to promote ROS production, including singlet oxygen (1O2), hydroxyl radical (-OH), superoxygen (O2−), and hydrogen peroxide (H2O2) (Figure 5B). Wang et al first demonstrated that BPNSs acts as a good metal-free photosensitiser to stimulate 1O2 production under near-infrared light. In their study, BPNSs had a 1O2 quantum yield of about 0.91, which is higher than most PDT reagents that have been reported.62 Shaw’s study of ROS species produced by small layers of BP using dyes found that 3.5 μg BP produced 200±1 nM of O2− and 375±0.3 nM of 1O2 after 2 hours of treatment under dark conditions. When they further investigated the mechanism of ROS production by BP, they found that the surface of defective BP could directly reduce O2 to produce O2− and 1O2.78 Li et al interestingly found that the in vivo antibacterial rate of the stent containing BPNSs was as high as 99.06%, while the in vitro antibacterial rate was only 82.13%. They hypothesized that this difference may be due to the fact that BPNSs coculture with bacteria in vitro for a much shorter period of time than in vivo, there is insufficient ROS production, and thus low antibacterial rate.79 Mo found that ROS production in macrophage-like cells treated with BPNSs increased significantly in a time-dependent manner, confirming Li’s conjecture.80 Moreover, Huang et al used DCFH-DA probe to monitor the production of ROS when exploring the antibacterial mechanism of BP, and found that the amount of ROS in BP group under NIR was higher than that in BP group under darkness. Further study found that after exposure to NIR, the high temperature generated by PTT inactivated the respiratory chain related proteins in the bacterial membrane structure, further promoting the production of ROS, and the two antibacterialsynergies.54 In order to determine the correlation between 1O2 produced by BP and bacterial death, Liu et al added ROS scrubber (NaN3) to the antibacterial system and found that bacterial survival rate increased after the addition of NaN3 compared with the original BPNs group. When the dosage of NaN3 was increased to 5 mg, the survival rate of bacteria recovered to the initial level.70 In summary, we conclude that ROS plays a key role in the antibacterial effect of BP.
 |
Figure 5 Antibacterial mechanism of BP. (A) The cell membrane density decreased, the cell membrane separated from the cytoplasm, and the cell membrane ruptured after contact with BP. Adapted with permission from Phakatkar AH, Firlar E, Alzate L, et al. TEM Studies on Antibacterial Mechanisms of Black Phosphorous Nanosheets. IJN. 2020;Volume 15:3071–3085, Copyright © 2020 Phakatkar et al. This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution – Non Commercial (unported, v3.0) License (http://creativecommons.org/licenses/by-nc/3.0/).69 (B) BP-based composites showed good photothermal effect and photodynamic effect. The photothermal effect is positively correlated with BP concentration and irradiation power. Adapted with permission from Xu D, Liu J, Wang Y, Jian Y, Wu W, Lv R. Black Phosphorus Nanosheet with High Thermal Conversion Efficiency for Photodynamic/Photothermal/Immunotherapy. ACS Biomater Sci Eng. 2020;6(9):4940–4948, Copyright 2021, with permission from American Chemical Society (ACS);81 Zhang M, Wang W, Cui Y, Zhou N, Shen J. Near-infrared light-mediated photodynamic/photothermal therapy nanoplatform by the assembly of Fe3O4 carbon dots with graphitic black phosphorus quantum dots. IJN. 2018;Volume 13:2803–2819. Dove press © 2018 Zhang et al This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution – Non Commercial (unported, v3.0) License (http://creativecommons.org/licenses/by-nc/3.0/).83 |
Application of BP in Antibacterial Field
In order to overcome antibiotic resistance, scholars have proposed the development of hybrid antibacterial materials that attack bacteria simultaneously through multiple mechanisms, thereby preventing the development of resistance.64 At the same time, because of the existence of lone pair electrons on the surface of BP, it is extremely easy to react with oxygen and water to degrade, and this instability limits the application of BP. The development of BP-based composites is expected to improve the stability of BP while realizing multi-mechanism antibacterial. At present, scholars mainly modify the BP through polymer surface modification, chemical surface modification, membrane embedding, doping other elements, loaded drugs and other ways to improve the stability of BP.83 Table 1 summarizes the main methods and mechanisms for modifying BP at present. In this paper, the materials that can modify BP are mainly divided into polymer, drug, metal nanomaterials, and the application of BP in the field of antibacterial is described according to this classification (Figure 6).
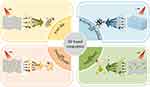 |
Figure 6 Application of BP-based composites. The main antibacterial strategies of pure BP, polymer-modified BP, drug-doped BP and metal-doped BP. |
 |
Table 1 Summary of the Modification of BP |
Antibacterial Activity of Pure BP
BP without any modification is often applied in the antibacterial field in the form of a coating. Shaw et al investigated the antibacterial effectiveness of BP as a surface coating material through surface functionalization technology, and the results showed that few-layer BP has high antibacterial activity against a broad spectrum of bacteria and fungi, including E. coli, Pseudomonas aeruginosa, MRSA, Salmonella typhimurium, and B. cereus, and the fungal strains, C. albicans, etc., which provides a direction for solving the infection problem of implants (Figure 7).78 Then Sun et al mixed BP powder into their traditional material——polyetheretherketone(PEEK)—— to solve the problem of infection by and poor wear resistance. The result showed that BP endowed the composite with excellent biological wettability and antibacterial properties, and the antibacterial rate was as high as 99.9%.94 In addition, the warming reaction caused by the near-infrared stimulation of BP also plays an important role in the antibacterial process. Yuan et al doped BP onto a hydroxyapatite (HA) -coated titanium implant to construct a BPs@HA composite coating. It was found that under the irradiation of NIR, the addition of BP could not only destroy the formed biofilm, but also damage the movement system of bacteria, which further prevented the formation and diffusion of biofilm. The final antibacterial rate reached 99%.95 Therefore, BP is considered as a potential biomedical material.
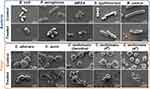 |
Figure 7 SEM images of bacteria and fungi. The top row was not BP treated, and the morphology of bacteria and fungi was normal; The next row of BP-treated bacteria and fungi crumbled and even burst. Adapted with permission from Shaw ZL, Kuriakose S, Cheeseman S, et al. Broad-Spectrum Solvent-free Layered Black Phosphorus as a Rapid Action Antimicrobial. ACS Appl Mater Interfaces. 2021;13(15):17340–17352, Copyright 2021, with permission from American Chemical Society (ACS).33 |
Antibacterial Activity of Polymer-Modified BP
In the biomedical field, the modification of polymers can not only neutralize the charge on the surface of nanoparticles, prevent the agglomeration of nanoparticles, and realize the slow release of drugs,96 but also cooperate with materials to jointly complete treatment. As shown in Figure 8, the polymers modified with BP can be divided into: (1) Improve the stability of BP; (2) Enhance the photothermal effect of BP; (3) Promote BP to produce more ROS; (4) Directly kill bacteria.
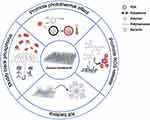 |
Figure 8 The main functions of polymers used to modify BP. These include protecting BP, enhancing photoheat, improving photodynamics and killing bacteria. |
The simplest and most feasible method to improve the stability of BP is hydrogel carrier. Miao et al doped BPNSs into gelatin methacryloyl (GelMA). The surface charge and high surface area of BPNSs promoted the strong interaction with polymer chains, further strengthened the crosslinking network formed after ultraviolet irradiation, and optimized the mechanical properties of the hydrogel. Moreover, the hydrogel containing BP showed excellent antibacterial properties compared with the pure hydrogel group, and the bactericidal rate against Staphylococcus aureus was as high as 98%.97 Huang et al added BPQD to PVA (polyvinyl alcohol) and ALG (sodium alginate) matrix to prepare BP hydrogel for promoting the healing of diabetic infected wounds. Under the synergistic effect of PTT and PDT, the effective bacteriostasis rate against MARS reached 90%, and the hydrogel could also regulate the expression of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) to promote wound healing.54 In addition, Li et al combined BP and PLGA to prepare a scaffold. On the one hand, the modification of PLGA isolated BP from water and oxygen. On the other hand, the inclusion of BP made the scaffold form a pore structure conducive to cell growth. After three weeks of co-culture in vitro under NIR, the scaffold doped with BP had almost no bacterial colonies, and the antibacterial rate reached 99.06.79
However, due to the weak penetration of NIR, the sensitivity of BP to light decreases when applied in vivo. In order to improve the photothermal conversion rate of BP, scholars have combined other PTA with BP to increase the absorption of NIR. For example, Zeng et al modified BP with polydopamine (PDA), and formed a BP-PDA coating on the surface of titanium implants with the help of the characteristics of PDA adhesion to almost all types of solid surfaces. The modification of PDA not only increases the biocompatibility and stability of BP, but also increases the absorbance from 220 nm to 800 nm. The strong light absorption ability gives the composites excellent photothermal properties. Under the synergistic action of photothermal effect and sound dynamic effect, 96.6% of Staphylococcus aureus could be killed.98 Zhao et al prepared an antibacterial scaffold for the treatment of infectious bone defects, which was inspired by chloroplast structure. They integrated BP with a network of chitosan and polycaprolactone fibers, coated with PDA. BP is similar to the thylakoid membrane and stromal layer in the chloroplast, and polydopamine is similar to the envelope, which ultimately forms the chloroplast scaffold. Due to the protection of PDA envelope, chloroplast scaffolds showed better photothermal stability than those without the protection of PDA envelope, the ratio of photothermal induction temperature increase was higher, and the antibacterial effect was more significant.99
In addition to enhancing photoheat, Xu et al covalent conjugated tea polyphenols (EGCG) containing hydroxyl to P of BPQDs, and the modification of EGCG promoted the production of more ROS by BPQDs, partly due to the stimulation of ROS production by the body when EGCG is spontaneously released. In addition, their synergistic effect promotes the expression of basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) in epithelial cells to promote the healing of infected wounds.100 However, long-term light exposure can cause phototoxicity, resulting in tissue damage, and once the light is stopped, the residual microorganisms will regrow and multiply, so Tan et al designed an antibacterial film that can still function in the absence of light. The film consists of BPNSs and poly (4-pyridine methyl styrene) (PPMS), and part of the 1O2 produced by BPNSs is stored in the form of PPMS-EPO under visible light irradiation at 660 nm. Under light, the heat generated by PTT of BP accelerates the decomposition of PPMS-EPO into PPMS and 1O2 for sterilization, while in darkness, it slowly decomposes and releases 1O2 for sterilization. The process is reversible. In vitro antibacterial experiments showed that the antibacterial rates of E. coli and S. aureus were 76.5% and 69.7% under the condition of no light, and the antibacterial rates increased to more than 99% after 10min of light.101
There are some polymers in nature that have antibacterial ability in themselves, such as natural cationic antibacterial polymers, quaternary ammonium salts, N-halogenated amine polymers, phosphine and sulfonium salts polymers, guanidine salts polymers, etc. These polymers not only modify BP, but also cooperate with BP antibacterial. Fu et al constructed polylysine engineered BPNSs (BP@ε-Pl) through a simple electrostatic interaction. The ε-PL coating has a broad spectrum of antibacterial activity, and it can tightly anchor to the bacterial surface through strong interactions with negatively charged bacterial cell membranes, resulting in membrane disintegration. Subsequently, in situ hyperthermia generated by BP under NIR can further eradicate pathogenic bacteria. Not only that, the coating also improves the biocompatibility and stability of BP.102 Using the same principle, Zhao et al used positively charged quaternary ammonium salt chitosan (QCS) and hyaluronic acid, assembled BPNSs and hemoglobin (Hb) on electrospun polylactic acid (PLLA) nanofibers, and prepared a dressing for diabetic wound healing. QCS is a kind of spectral hemostatic antibacterial material, and BP-derived PTT increases the sensitivity of bacteria to QCS. Finally, the dressing can effectively kill more than 98% of MRSA and more than 90% of E. coli, and can inhibit bacterial reproduction. The addition of Hb also endows the dressing with the function of transporting oxygen, alleviating the hypoxia caused by diabetic microangiopathopathy and promoting wound healing.103 Zhang et al modified BP with Elaeagnus mollis polysaccharides extracted from matsutake polysaccharides. The resulting compound showed higher activity and stability. Further studies showed that ROS produced by photocatalysis and active polysaccharide acted on the cell membrane together, resulting in cell deformation and death, and the bactericidal rate was as high as 99.999% after 60min of light. Not only that, the composite material can also reduce the expression of the virulence factors of Staphylococcus aureus hemolysin and thrombin, inhibit the formation of staphylococcus aureus biofilm, and avoid the generation of drug resistance. 104
In summary, the polymers not only modify BP and increase its stability, but also enhance the antibacterial properties of the material through its own antibacterial properties and synergistic photothermal and photodynamic properties.
Antibacterial Activity of Drug-Doped BP
BP is widely used as a drug delivery system because of its π-electron rich surface and large surface area to volume ratio.25,105 BP is negatively charged in water with A layer distance of ~5.24 Å,106 and positively charged small molecule drugs can be encapsulated in the interlayer space through electrostatic interactions. By combining the advantages of drug loading and inherent antibacterial activity, BP can be functionalized with drugs. On the one hand, the synergistic effect of different sterilization mechanisms can reduce the therapeutic dose and improve the therapeutic effect. On the other hand, the precise release of drugs can be achieved by controlling the light time, avoiding the misuse and overuse of drugs, so as to better protect the health of patients.
Since only relying on PTT antibacterial often needs to increase the local temperature to more than 45°C, which will cause damage to the surrounding tissues, so the antibacterial function of drugs loaded on BP, the synergistic antibacterial effect of the two can not only reduce the temperature requirements, but also reduce the use of drugs.107 Zheng et al loaded physcion (Phy) onto BPNSs. The physical cutting sterilization of BPNSs, photothermal sterilization and pharmaceutical sterilization of Phy worked together to achieve the bactericidal efficacy of BPNSs@Phy against S. aureus and Pseudomonas aeruginosa up to 99.5% and 99.8%.108 Song et al developed a novel bonding and light-responsive microparticle (MP) delivery system that incorporates BP and minocycline hydrochloride for the treatment of periodontitis by microfluidic electrospray. Not only can the system directly kill porphyromonas gingivalis, but the near-infrared light response of BP also enables controlled and effective drug release.109 However, widespread use of the drug can cause irreversible damage to the surrounding tissues. Therefore, Guo et al for the first time used galactose-black phosphorus nanosheets (GAL-BPNSs) as a transport platform for kanamycin, killing Pseudomonas aeruginosa (PAO1) in combination with photoheat. (Gal)3NH2 molecules can specifically recognize and interact with PAO1 surface receptors, so as to drive the internalization of Gal-BP NSs@Kana, and finally achieve targeted therapy.110 In addition, Chen et al functionalized the antimicrobial peptide derivative, nitric oxide (NO) donor S-nitrosocysteamine (SNO) with BPQDs, and the PTT effect of BPQDs led to the release of NO in near-infrared response, achieving the purpose of sterilization. The combination of antimicrobial peptide derivatives and BPQDs enhanced the antibacterial ability of each other, and finally the bactericidal rate of the composite material was close to 100% in the subcutaneous abscess model of Staphylococcus aureus infection.111
Bacterial infections not only exacerbate the inflammatory response and produce persistent pain, but also cause impaired angiogenesis and delay wound healing. Therefore, in addition to combining with antibacterial drugs, scholars also modify BP with other drugs to give the composite new properties.112 For example, Ding et al modified BPNSs with 4-octylitaconate (4OI), which has antioxidant and anti-inflammatory properties, to prepare a new composite material with antibacterial and antioxidant properties. Under light, the antibacterial rate of the composite was as high as 90%. Not only that, it also mitigated the excessive damage of ROS to endothelial cells by clearing ROS, thereby reducing the inflammatory response.113 Ouyang et al added lidocaine hydrochloride (Lid) to BP-based hydrogel to accelerate microcirculation blood flow and release Lid at the same time as PTT, so as to relieve pain, kill bacteria and reduce inflammation.114 Zhou et al prepared a novel asymmetric wettable film containing ginsenosides and BP, which can promote neovascularization. Further study of the mechanism revealed that the membrane can up-regulate Ki67, CD31, α-SMA and transforming growth factor-β1, and down-regulate tumor necrosis factor-α, IL-1β and IL-6 by triggering phosphatidylinositol 3-kinase (P-PI3K/PI3K) and protein kinase B phosphorylation (P-AKT/AKT) signaling pathways. It can promote the polarization of macrophage M2, inhibit the polarization of macrophage M1, and finally reduce the inflammatory response.115 Liu et al applied nano-sprays containing astragaloside (AS) and BP to the healing of infected wounds. The sharp edge of BPNSs and the local high temperature generated by NIR can effectively kill S. aureus and E. coli, with an antibacterial rate of up to 99%. At the same time, local high temperature can also promote the release of AS, promote the proliferation of fibroblasts and the formation of blood vessels, and facilitate the healing of infected wounds.116
Antibacterial Activity of Metal-Doped BP
Ag, Au, Cu and some alloyed form containing those metals have been widely used in various fields because of their excellent antibacterial properties, and their antibacterial mechanism mainly includes five aspects: (1) Induce cell wall and/or membrane damage by electrostatic binding to negative charges on bacterial surfaces.117,118 (2) Release metal ions with antimicrobial properties.119,120 (3) Induce photothermal effect produces local high temperature.120,121 (4) Induce ROS production and trigger oxidative stress.122,123 (5) Disrupt intracellular composition and metabolic balance.124 BP is combined with metal nanoparticles through orbital hybridization, and the combined metal nanoparticles can enhance the active center of BP, reduce the band gap, significantly improve the photocatalytic ability of BP, and then promote the photothermal and photodynamic effects, and complete the synergistic antibacterial (Figure 9).125–128
Silver is known to be the most effective of the metal nanoparticles against bacteria and other microorganisms, and is highly biocompatible, easily making it useful in medical applications.129 However, the use of Ag+ is often limited due to instability in the environment and cytotoxicity caused by sudden large dose release.130 Silver nanoparticles (AgNPs) alone disrupt cell signaling functions and thus become powerful inducers of cell death.131 Therefore, it is very important to find a carrier that can achieve Ag+ slow release for the application of Ag+ in the field of antibacterial. Scholars have found that there are lone pair electrons on the surface of BP, and positively charged Ag+ can be loaded onto the surface of BP through electrostatic action, and is reduced to AgNPs. With the degradation of BP, Ag+ is gradually released.93,132–134 Zhao et al prepared an injectable and self-healing hydrogel for the healing of infected soft tissues by in-situ reduction deposition. The -OH of BP is coordinated with Ag+, and Ag+ also acts as a crosslinking agent to promote the gluing of sodium thiosulfate (HA-SH) polymer chain segments. The addition of BP not only enhances the mechanical properties of hydrogel, but also its photothermal action and the antibacterial action of Ag+ play simultaneously, which greatly improves the antibacterial rate. In vitro antibacterial experiment, the antibacterial area of the hydrogel group containing only Ag+ was 2.223 ± 0.033 cm2, and after adding BP, the antibacterial area reached 2.267±0.024 cm2. On this basis, the increase of NIR irradiation showed the best antibacterial effect, and the antibacterial area was as high as 2.639±0.069 cm2. In addition, the hydrogel also promoted collagen fiber deposition and angiogenesis by down-regulating the expression of inflammatory factor TNF-a in infected wound tissue.132 When Deng et al added BP and Ag+ to PLLA scaffolds at the same time, scanning electron microscopy showed that the bacteria showed more severe morphological atrophy and the integrity of the membrane was damaged compared with other groups.93 Therefore, the combination of BP and Ag not only solves the limitation of Ag use, but also realizes synergistic antibacterial.
Conventional gold nanoparticles (AuNPs) are inert to bacteria, but once they are reduced in size to the nanocluster (NC) dimension (≤2 nm), they show strong antibacterial activity.135 Using BP as a reducing agent to prepare AuNPs is a simple and green synthesis method. AuNP of different sizes and densities can be prepared by adjusting the ratio of the two to avoid adding other toxic reducing agents and surfactants into the system.136 In addition, as a solid carrier, the large specific surface area of BP can support high density AuNPs, providing multiple sites for interaction with bacteria. At the same time, AuNPs can enhance the photothermal effect of the composite and amplify the oxidative stress response of the bacteria. The two complement each other and greatly improve the antibacterial efficiency.137 Li et al first used AuNPs to enhance BP as a signal component of a photothermal sensing antibody probe, and found that the photothermal conversion efficiency of BP-AU was 12.9% higher than that of BP alone.138 Because PTT often requires multiple NIR irradiation, the light stability of the material is required to be high. Yang et al prepared BP-AUNSs by in-situ reduction method, and the photothermal conversion efficiency was as high as 43.57%, which was much higher than that of BPNSs (35.03%). Moreover, the addition of Au increased the photostability of BP, and there was no obvious temperature change after five times of heating and cooling cycles.139 Aksoy et al compared the antibacterial properties of the BP group and the BP-AU group, and the results showed that the BP group could not completely kill bacteria under NIR, but when AuNPs was added, the antibacterial rate against Enterococcus faecalis was as high as 100%.137
In addition to the common gold and silver, BP can be combined with other metals and metal compound. Due to the surface defects of 2D BP, it is easy to decompose under water and oxygen conditions, so metal oxides can enhance the antibacterial and improve the stability of BP. For example, Zhang et al designed to use bismuth oxide (Bi2O3) to occupy the defect site of BP, form BP/Bi2O3 heterojunction, and modify polylysine on its surface for the treatment of diabetic infection wounds. Under NIR irradiation, the composite had 100% inhibition effect on Pseudomonas aeruginosa, S. aureus and E. coli.140 Li et al loaded titanium aminobenzenesulfonic acid (called Ti-SA4), a metal complex with antibacterial activity, onto BP through strong P-Ti coordination. Without NIR irradiation, the compound can directly kill most bacteria within 3h, and the antibacterial effect is far more than Ti-SA4.141 Zhang et al loaded Cu onto BP by a one-step reduction method, and the process of transferring lone pair electrons on the surface of BP to Cu led to the surge of reactive oxygen species (ROS), which greatly improved the antibacterial ability of the composite. In order to determine the types of reactive oxygen species, they did further research, and the results showed that the excellent antibacterial properties of BP/Cu nanocomposites are due to the generation of hydroxyl radicals.127
The addition of metal nanomaterials (Mg, Zn, etc.) with other properties has greatly enriched the function of BP-based composites. For example, Jing et al mixed magnesium modified BP (BP@Mg) into methacrylate gelatin (GelMA) to prepare photosensitive conductive hydrogel. The hydrogel has strong antibacterial activity, which can improve the inflammatory microenvironment and reduce the bone tissue damage induced by bacteria. In addition, the conductive nanosheets and bioactive ions released by BP@Mg can cooperate to promote the migration and secretion of Schwann cells, solving the problem of local nerve fiber necrosis caused by bacterial infection.142 Bose et al prepared a BP-ZnO hybrid coating for the surface of a titanium implant and showed an approximately 41.5% increase in ROS levels of BP-ZnO NH (S. aureus) and 36.3% (E. coli) under NIR irradiation compared to samples not exposed to NIR and a group treated with a single nanoagent. Not only that, the photothermal effect also accelerated the release of Zn2+, and the antibacterial rate of S. aureus was as high as 98%, and the antibacterial rate of E. coli was as high as 97%.143 In addition, the release of Zn2+ is involved in the immune regulation of the full-layer wound infected by bacteria, thus greatly reducing the inflammatory response during the process of wound repair.144
In summary, by binding BP and metal ions to the surface of bacterial cell membranes, generating local high temperature and promoting ROS generation, causing cell membrane damage, protein synthesis obstruction and irreparable DNA damage, the composites show enhanced antibacterial activity and other excellent properties, which is a very potential antibacterial strategy.
Finally, we summarized the application of BP-based composites in different fields and the antibacterial content in Table 2.
 |
Table 2 Application of BP-Based Composites in Antibacterial |
Conclusion and Challenge
Since the layered BP was first stripped from bulk BP in 2014, BPNSs have become the most promising 2D nanomaterials after graphene with excellent properties such as high carrier mobility, wide light absorption and adjustable band gap and thermal properties, and are also an important platform for manufacturing multi-functional materials in the field of nanotechnology. Scholars have made extensive exploration on the performance of BP and its application in various fields. In the biomedical field, BP has been applied in medical imaging, tumor therapy, bone tissue engineering and so on. In recent years, scholars have gradually realized that BP has better properties than graphene in the field of antibacterial, and there are more and more related studies on BP in antibacterial aspects. In this paper, the research progress of BP-based composites in antibacterial activity in recent years was reviewed, including structure, properties, antibacterial mechanism and application of BP nanocomposites in antibacterial activity. BP’s unique cellular folding structure gives it adjustable band gap width, high electron mobility, and is closely related to antibacterial properties. BP has a high safety mainly due to its good biocompatibility, and does not cause obvious toxicity to cells and organs. Moreover, BP can be degraded into PO43- which is non-toxic and harmless in the body. Its antibacterial mechanism mainly includes: (1) Damage the integrity of bacterial cell membranes through sharp edges; (2) Increase surface temperature and protein denaturation through local PTT; (3) Aggravate the oxidative stress response of bacteria by promoting ROS production. However, BP is easily degraded by reaction with oxygen and water, which is extremely unstable and seriously affects the antibacterial performance. Therefore, researchers introduced polymers, drugs, metal nanoparticles and other materials into BP to prepare BP-based composites. On the one hand, polymers such as hydrogels can modify BP and increase its photothermal stability. On the other hand, the introduction of antibacterial drugs and metal nanoparticles has added new antibacterial mechanisms, and the combined action of multiple antibacterial mechanisms is conducive to reducing the generation of bacterial resistance, which significantly promotes the application of BP in the antibacterial field.
Although BP has various excellent properties and has been studied in many fields, compared with graphene, BP research is still in the preliminary stage, and there are still many shortcomings and challenges. First of all, the current preparation of BPNSs needs to be carried out in a strict environment such as high temperature and high pressure without oxygen. Not only is the technology complex, but also requires expensive auxiliary materials or solvents. This leads to high costs and cannot achieve mass production. In addition, during the preparation process, BP is inevitably degraded by oxidation, which greatly affects its yield and quality. Second, compared with antibiotics, the biggest advantage of BP-based composites is that it can effectively avoid the generation of bacterial resistance. However, this conclusion is based on the laboratory bacterial passage experiment, and whether BP-based composites will produce resistance after being widely used as antibacterial drugs is unknown. Therefore, it is necessary to study the exact mechanism of resistance and elucidate the evolution and development process from the protein and gene level of bacteria, so as to prevent and control bacterial resistance. Finally, despite extensive toxicological studies on BP, the results are still varied. Most of the studies on the good safety of BP are limited to the macro level and short-term effects of the body. We need to pay more attention to the impact of BP on various systems in the body and the impact on the ecosystem. Studies have found that macrophages are one of the main cells involved in nanomaterials, and the amount of nanomaterials they ingests is closely related to the immune response and inflammation of the body.80 When BP enters the blood, it will rapidly adsorb plasma proteins to form a protein crown, which will increase the phagocytosis of macrophages for BP. In addition, the products of BP and protein can promote the secretion of inflammatory cytokines by macrophages, activate the NF-kB pathway, disturb the body’s immune system, and aggravate inflammation.149,150 Zhuang et al also found that BP has anti-angiogenic properties, which disrupt the endothelial NO synthase (eNOS)/nitric oxide (NO) signaling pathway associated with endothelial dysfunction.151 In addition, BP can also block insulin signaling (PI3K/AKT), disrupt glucose homeostasis and induce insulin resistance in muscle, leading to metabolic glucose disorders and induce cardiac inflammation.152 These results indicate that BP has potential toxicity to various systems in the body. BP is considered safe because its degradation product PO43- is non-toxic, but when the concentration of PO43- in the cell increases rapidly in a short period of time, it will also have a negative reaction to the body. Not only that, the degradation behavior of BP in vivo is more complex than in vitro. Due to changes in the surrounding environment (such as PH), the degradation behavior of BP will change, and the shape and size will also change during the degradation process.153 On the one hand, the interaction between BP and cells will be affected; on the other hand, the intermediate products and final products produced in different environments may have different toxicity to the body. In addition, considering that after the extensive application of BP, a large number of non-degraded nanomaterials will enter the aquatic environment, which is likely to pose a threat to the ecological environment, scholars have studied the impact of BP on aquatic organisms. It has been shown that BP is not only toxic to the aquatic organisms such as T. thermophila and Zebrafish, but also reduces their reproductive rate and causes fetal malformation.154 Therefore, BP may pose a certain threat to the ecosystem.
In summary, BP is a new star in antibacterial materials, but there are still too many unknown challenges about it. In order to realize the commercialization of BP, we not only need to further study more simple, efficient and affordable synthesis methods to reduce the cost of BP preparation, but also need more comprehensive research on its long-term impact in vivo and its impact on the ecological environment. Although the research road is full of challenges, we are optimistic that we can overcome it and eventually apply BP to various fields.
Acknowledgments
We acknowledge funding by the National Natural Science Foundation of China (No. 82371006). We are very grateful to Yanmin Zhou for the critical reading of the manuscript and helpful comments.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shi L, Chen J, Teng L, et al. The antibacterial applications of graphene and its derivatives. Small. 2016;12(31):4165–4184. doi:10.1002/smll.201601841
2. Ciofi Degli Atti MI, D’Amore C, Gagliotti C. Strategies to control antibiotic resistance: results from a survey in Italian children’s hospitals. Annali di igiene medicina preventiva e di comunità. 2019;31(1):3–12. doi:10.7416/ai.2019.2253
3. Mulani MS, Kamble EE, Kumkar SN, Tawre MS, Pardesi KR. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol. 2019;10:539. doi:10.3389/fmicb.2019.00539
4. Lopes BS, Strachan NJC, Ramjee M, et al. Nationwide stepwise emergence and evolution of multidrug-resistant Campylobacter jejuni sequence type 5136, United Kingdom. Emerg Infect Dis. 2019;25(7):1320–1329. doi:10.3201/eid2507.181572
5. Gupta A, Mumtaz S, Li CH, Hussain I, Rotello VM. Combatting antibiotic-resistant bacteria using nanomaterials. Chem Soc Rev. 2019;48(2):415–427. doi:10.1039/C7CS00748E
6. Willyard C. Drug-resistant bacteria ranked. Nature. 2017;543(7643):15. doi:10.1038/nature.2017.21550
7. Carvalho IT, Santos L. Antibiotics in the aquatic environments: a review of the European scenario. Environ Int. 2016;94:736–757. doi:10.1016/j.envint.2016.06.025
8. Naylor NR, Atun R, Zhu N, et al. Estimating the burden of antimicrobial resistance: a systematic literature review. Antimicrob Resist Infect Control. 2018;7(1):58. doi:10.1186/s13756-018-0336-y
9. ho SJ, Mizuno S, Okuno T, et al. Nationwide multicenter questionnaire surveys on countermeasures against antimicrobial resistance and infections in hospitals. BMC Infect Dis. 2021;21(1):234. doi:10.1186/s12879-021-05921-2
10. Zhao J, Huang S, Ravisankar P, Zhu H. Two-dimensional nanomaterials for photoinduced antibacterial applications. ACS Appl Bio Mater. 2020;3(12):8188–8210. doi:10.1021/acsabm.0c00950
11. Makabenta JMV, Nabawy A, Li CH, Schmidt-Malan S, Patel R, Rotello VM. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol. 2021;19(1):23–36. doi:10.1038/s41579-020-0420-1
12. Alabresm A, Chandler SL, Benicewicz BC, Decho AW. Nanotargeting of resistant infections with a special emphasis on the biofilm landscape. Bioconjugate Chem. 2021;32(8):1411–1430. doi:10.1021/acs.bioconjchem.1c00116
13. Khorsandi K, Hosseinzadeh R, Sadat Esfahani H, Keyvani-Ghamsari S, Ur Rahman S. Nanomaterials as drug delivery systems with antibacterial properties: current trends and future priorities. Expert Rev Anti Infect Ther. 2021;19(10):1299–1323. doi:10.1080/14787210.2021.1908125
14. Szunerits S, Boukherroub R. Antibacterial activity of graphene-based materials. J Mater Chem B. 2016;4(43):6892–6912. doi:10.1039/C6TB01647B
15. Chen F, Luo Y, Liu X, et al. 2D molybdenum sulfide‐based materials for photo‐excited antibacterial application. Adv Healthcare Mater. 2022;11(13):2200360. doi:10.1002/adhm.202200360
16. Bridgman PW. Two new modifications of phosphorus. J Am Chem Soc. 1914;36(7):1344–1363. doi:10.1021/ja02184a002
17. Gui R, Jin H, Wang Z, Li J. Black phosphorus quantum dots: synthesis, properties, functionalized modification and applications. Chem Soc Rev. 2018;47(17):6795–6823. doi:10.1039/C8CS00387D
18. Tran V, Soklaski R, Liang Y, Yang L. Layer-controlled band gap and anisotropic excitons in few-layer black phosphorus. Phys Rev B. 2014;89(23):235319. doi:10.1103/PhysRevB.89.235319
19. Ezawa M. Topological origin of quasi-flat edge band in phosphorene. New J Phys. 2014;16(11):115004. doi:10.1088/1367-2630/16/11/115004
20. Jing Y, Tang Q, He P, Zhou Z, Shen P. Small molecules make big differences: molecular doping effects on electronic and optical properties of phosphorene. Nanotechnology. 2015;26(9):095201. doi:10.1088/0957-4484/26/9/095201
21. Anju S, Ashtami J, Mohanan PV. Black phosphorus, a prospective graphene substitute for biomedical applications. Mater Sci Eng C. 2019;97:978–993. doi:10.1016/j.msec.2018.12.146
22. Xu Y, Chen S, Zhang Y, et al. Antibacterial black phosphorus nanosheets for biomedical applications. J Mater Chem B. 2023;11(30):7069–7093. doi:10.1039/D3TB00723E
23. Pandey A, Nikam AN, Fernandes G, et al. Black phosphorus as multifaceted advanced material nanoplatforms for potential biomedical applications. Nanomaterials. 2020;11(1):13. doi:10.3390/nano11010013
24. Ying-Yan M, Meng L, Jin-Da W, et al. NIR-triggered drug delivery system for chemo-photothermal therapy of posterior capsule opacification. J Contr Release. 2021;339:391–402. doi:10.1016/j.jconrel.2021.09.030
25. Zhang F, Peng F, Qin L, et al. pH/near infrared dual-triggered drug delivery system based black phosphorus nanosheets for targeted cancer chemo-photothermal therapy. Colloids Surfaces B. 2019;180:353–361. doi:10.1016/j.colsurfb.2019.04.021
26. Soman S, Kulkarni S, Pandey A, et al. 2D hetero-nanoconstructs of black phosphorus for breast cancer theragnosis: technological advancements. Biosensors. 2022;12(11):1009. doi:10.3390/bios12111009
27. Wang Z, Liu Z, Su C, et al. Biodegradable black phosphorus-based nanomaterials in biomedicine: theranostic applications. CMC. 2019;26(10):1788–1805. doi:10.2174/0929867324666170920152529
28. Jing X, Xiong Z, Lin Z, Sun T. The application of black phosphorus nanomaterials in bone tissue engineering. Pharmaceutics. 2022;14(12):2634. doi:10.3390/pharmaceutics14122634
29. He X, Zhu Y, Ma B, et al. Bioactive 2D nanomaterials for neural repair and regeneration. Adv Drug Deliv Rev. 2022;187:114379. doi:10.1016/j.addr.2022.114379
30. Tian R, Gu L, Ji Y, et al. Black phosphorus photodetector enhanced by a planar photonic crystal cavity. ACS Photonics. 2021;8(10):3104–3110. doi:10.1021/acsphotonics.1c01168
31. Yang S. Seeking novel low-symmetry 2D materials with strong in-plane anisotropy. MatLab. 2022;1. doi:10.54227/mlab.20220033
32. Li B, Lai C, Zeng G, et al. Black phosphorus, a rising star 2D nanomaterial in the post-graphene era: synthesis, properties, modifications, and photocatalysis applications. Small. 2019;15(8):1804565. doi:10.1002/smll.201804565
33. Qu G, Xia T, Zhou W, et al. Property–activity relationship of black phosphorus at the nano–bio interface: from molecules to organisms. Chem Rev. 2020;120(4):2288–2346. doi:10.1021/acs.chemrev.9b00445
34. Du Y, Ouyang C, Shi S, Lei M. Ab initio studies on atomic and electronic structures of black phosphorus. J Appl Phys. 2010;107(9):093718. doi:10.1063/1.3386509
35. Zhang X, Xie H, Liu Z, et al. Black phosphorus quantum dots. Angew Chem Int Ed. 2015;54(12):3653–3657. doi:10.1002/anie.201409400
36. Baker SN, Baker GA. Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed. 2010;49(38):6726–6744. doi:10.1002/anie.200906623
37. Zhang T, Xie H, Xie S, et al. A superior two-dimensional phosphorus flame retardant: few-layer black phosphorus. Molecules. 2023;28(13):5062. doi:10.3390/molecules28135062
38. Erande MB, Pawar MS, Late DJ. Humidity sensing and photodetection behavior of electrochemically exfoliated atomically thin-layered black phosphorus nanosheets. ACS Appl Mater Interfaces. 2016;8(18):11548–11556. doi:10.1021/acsami.5b10247
39. Li L, Yu Y, Ye GJ, et al. Black phosphorus field-effect transistors. Nature Nanotech. 2014;9(5):372–377. doi:10.1038/nnano.2014.35
40. Liu H, Neal AT, Zhu Z, et al. Phosphorene: an unexplored 2D semiconductor with a high hole mobility. ACS Nano. 2014;8(4):4033–4041. doi:10.1021/nn501226z
41. Clark N, Nguyen L, Hamer MJ, et al. Scalable patterning of encapsulated black phosphorus. Nano Lett. 2018;18(9):5373–5381. doi:10.1021/acs.nanolett.8b00946
42. Liu G, Tsai HI, Zeng X, et al. Black phosphorus nanosheets-based stable drug delivery system via drug-self-stabilization for combined photothermal and chemo cancer therapy. Chem Eng J. 2019;375:121917. doi:10.1016/j.cej.2019.121917
43. Li Q, Zhou Q, Shi L, Chen Q, Wang J. Recent advances in oxidation and degradation mechanisms of ultrathin 2D materials under ambient conditions and their passivation strategies. J Mater Chem A. 2019;7(9):4291–4312. doi:10.1039/C8TA10306B
44. Qiu M, Ren WX, Jeong T, et al. Omnipotent phosphorene: a next-generation, two-dimensional nanoplatform for multidisciplinary biomedical applications. Chem Soc Rev. 2018;47(15):5588–5601. doi:10.1039/C8CS00342D
45. Yang Z, Hao J, Yuan S, et al. Field-effect transistors based on amorphous black phosphorus ultrathin films by pulsed laser deposition. Adv Mater. 2015;27(25):3748–3754. doi:10.1002/adma.201500990
46. Wang Y, Yu Q, Li J, Wang J, Zhang K. Insight into the growth mechanism of black phosphorus. Front Phys. 2023;18(4):43603. doi:10.1007/s11467-023-1265-7
47. Xu Y, Shi X, Zhang Y, et al. Epitaxial nucleation and lateral growth of high-crystalline black phosphorus films on silicon. Nat Commun. 2020;11(1):1330. doi:10.1038/s41467-020-14902-z
48. Latiff NM, Teo WZ, Sofer Z, Fisher AC, Pumera M. The cytotoxicity of layered black phosphorus. Chem Eur J. 2015;21(40):13991–13995. doi:10.1002/chem.201502006
49. Zhang X, Donskyi IS, Tang W, et al. Biological effects of black phosphorus nanomaterials on mammalian cells and animals. Angew Chem Int Ed. 2023;62(6):e202213336.
50. Zhang X, Zhang Z, Zhang S, et al. Size effect on the cytotoxicity of layered black phosphorus and underlying mechanisms. Small. 2017;13(32):1701210. doi:10.1002/smll.201701210
51. Mu X, Wang J, Bai X, et al. Black phosphorus quantum dots induced oxidative stress and toxicity in living cell and mice. ACS Appl Mater Interfaces. 2017;9(24):20399–20409. doi:10.1021/acsami.7b02900
52. Kenry LCT, Lim CT. Biocompatibility and Nanotoxicity of Layered Two-Dimensional Nanomaterials. ChemNanoMat. 2017;3(1):5–16. doi:10.1002/cnma.201600290
53. Qu G, Liu W, Zhao Y, et al. Improved biocompatibility of black phosphorus nanosheets by chemical modification. Angew Chem Int Ed. 2017;56(46):14488–14493. doi:10.1002/anie.201706228
54. Huang S, Xu S, Hu Y, et al. Preparation of NIR-responsive, ROS-generating and antibacterial black phosphorus quantum dots for promoting the MRSA-infected wound healing in diabetic rats. Acta Biomaterialia. 2022;137:199–217. doi:10.1016/j.actbio.2021.10.008
55. He C, Ruan F, Jiang S, et al. Black phosphorus quantum dots cause nephrotoxicity in organoids, mice, and human cells. Small. 2020;16(22):2001371. doi:10.1002/smll.202001371
56. Sun Y, Fan S, Fan S, et al. In vitro and in vivo toxicity of black phosphorus nanosheets. J Nanosci Nanotechnol. 2020;20(2):659–667. doi:10.1166/jnn.2020.16922
57. Zha Z, Yue X, Ren Q, Dai Z. Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells. Adv Mater. 2013;25(5):777–782. doi:10.1002/adma.201202211
58. Zou Q, Abbas M, Zhao L, Li S, Shen G, Yan X. Biological photothermal nanodots based on self-assembly of peptide–porphyrin conjugates for antitumor therapy. J Am Chem Soc. 2017;139(5):1921–1927. doi:10.1021/jacs.6b11382
59. Chen Y, Wang L, Shi J. Two-dimensional non-carbonaceous materials-enabled efficient photothermal cancer therapy. Nano Today. 2016;11(3):292–308. doi:10.1016/j.nantod.2016.05.009
60. Ziletti A, Carvalho A, Campbell DK, Coker DF, Castro Neto AH. Oxygen Defects in Phosphorene. Phys Rev Lett. 2015;114(4):046801. doi:10.1103/PhysRevLett.114.046801
61. Shao J, Xie H, Huang H, et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat Commun. 2016;7(1):12967. doi:10.1038/ncomms12967
62. Wang H, Yang X, Shao W, et al. Ultrathin black phosphorus nanosheets for efficient singlet oxygen generation. J Am Chem Soc. 2015;137(35):11376–11382. doi:10.1021/jacs.5b06025
63. Im SH, Kim CY, Jung Y, Jang Y, Kim SH. Biodegradable vascular stents with high tensile and compressive strength: a novel strategy for applying monofilaments via solid-state drawing and shaped-annealing processes. Biomater Sci. 2017;5(3):422–431. doi:10.1039/C7BM00011A
64. Zhang C, Wang Y, Ma J, et al. Black phosphorus for fighting antibiotic-resistant bacteria: what is known and what is missing. Sci Total Environ. 2020;721:137740. doi:10.1016/j.scitotenv.2020.137740
65. Zhang Y, Ali SF, Dervishi E, et al. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 Cells. ACS Nano. 2010;4(6):3181–3186. doi:10.1021/nn1007176
66. Hu W, Peng C, Lv M, et al. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano. 2011;5(5):3693–3700. doi:10.1021/nn200021j
67. Sun Z, Zhang Y, Yu H, et al. New solvent-stabilized few-layer black phosphorus for antibacterial applications. Nanoscale. 2018;10(26):12543–12553. doi:10.1039/C8NR03513J
68. Phakatkar AH, Firlar E, Alzate L, et al. TEM studies on antibacterial mechanisms of black phosphorous nanosheets. IJN. 2020;15:3071–3085. doi:10.2147/IJN.S237816
69. Xiong Z, Zhang X, Zhang S, et al. Bacterial toxicity of exfoliated black phosphorus nanosheets. Ecotoxicol Environ Saf. 2018;161:507–514. doi:10.1016/j.ecoenv.2018.06.008
70. Liu W, Zhang Y, Zhang Y, Dong A. Black phosphorus nanosheets counteract bacteria without causing antibiotic resistance. Chem Eur J. 2020;26(11):2478–2485. doi:10.1002/chem.201905134
71. Maleki A, He J, Bochani S, Nosrati V, Shahbazi MA, Guo B. Multifunctional photoactive hydrogels for wound healing acceleration. ACS Nano. 2021;15(12):18895–18930. doi:10.1021/acsnano.1c08334
72. Zhang Y, Qu XF, Zhu CL, et al. A stable quaternized chitosan-black phosphorus nanocomposite for synergetic disinfection of antibiotic-resistant pathogens. ACS Appl Bio Mater. 2021;4(6):4821–4832. doi:10.1021/acsabm.1c00054
73. Hu K, Xie L, Zhang Y, et al. Marriage of black phosphorus and Cu2+ as effective photothermal agents for PET-guided combination cancer therapy. Nat Commun. 2020;11(1):2778. doi:10.1038/s41467-020-16513-0
74. Wang X, Tang X, Li N, et al. A multifunctional black phosphorus-based adhesive patch intrinsically induces partial EMT for effective burn wound healing. Biomater Sci. 2023;11(1):235–247. doi:10.1039/D2BM01625G
75. Turcheniuk K, Hage CH, Spadavecchia J, et al. Plasmonic photothermal destruction of uropathogenic E. coli with reduced graphene oxide and core/shell nanocomposites of gold nanorods/reduced graphene oxide. J Mater Chem B. 2015;3(3):375–386. doi:10.1039/C4TB01760A
76. Pan WY, Huang CC, Lin TT, et al. Synergistic antibacterial effects of localized heat and oxidative stress caused by hydroxyl radicals mediated by graphene/iron oxide-based nanocomposites. Nanomedicine. 2016;12(2):431–438. doi:10.1016/j.nano.2015.11.014
77. Yuan Z, Lin C, He Y, et al. Near-infrared light-triggered nitric-oxide-enhanced photodynamic therapy and low-temperature photothermal therapy for biofilm elimination. ACS Nano. 2020;14(3):3546–3562. doi:10.1021/acsnano.9b09871
78. Shaw ZL, Kuriakose S, Cheeseman S, et al. Broad-spectrum solvent-free layered black phosphorus as a rapid action antimicrobial. ACS Appl Mater Interfaces. 2021;13(15):17340–17352. doi:10.1021/acsami.1c01739
79. Li W, Li S, Zhang J, et al. Fabrication and evaluation of bone morphogenetic protein-2 microspheres coated black phosphorus nanosheets@polylactic-glycolic acid copolymers scaffold: a multifunctional antibacterial photothermal scaffold for bone regeneration. Int J Biol Macromol. 2022;210:350–364. doi:10.1016/j.ijbiomac.2022.05.028
80. Mo J, Xie Q, Wei W, Zhao J. Revealing the immune perturbation of black phosphorus nanomaterials to macrophages by understanding the protein Corona. Nat Commun. 2018;9(1):2480. doi:10.1038/s41467-018-04873-7
81. Xu D, Liu J, Wang Y, Jian Y, Wu W, Lv R. Black phosphorus nanosheet with high thermal conversion efficiency for photodynamic/photothermal/immunotherapy. ACS Biomater Sci Eng. 2020;6(9):4940–4948. doi:10.1021/acsbiomaterials.0c00984
82. Liang X, Ye X, Wang C, et al. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J Contr Release. 2019;296:150–161. doi:10.1016/j.jconrel.2019.01.027
83. Zeng G, Chen Y. Surface modification of black phosphorus-based nanomaterials in biomedical applications: strategies and recent advances. Acta Biomaterialia. 2020;118:1–17. doi:10.1016/j.actbio.2020.10.004
84. Yang X. Functional black phosphorus nanosheets for mitochondria-targeting photothermal/photodynamic synergistic cancer therapy. Chem Sci. 2019;10(13):3779–3785.
85. Deng L, Xu Y, Sun C, et al. Functionalization of small black phosphorus nanoparticles for targeted imaging and photothermal therapy of cancer. Science Bulletin. 2018;63(14):917–924. doi:10.1016/j.scib.2018.05.022
86. Huang XW, Wei JJ, Zhang MY, et al. Water-based black phosphorus hybrid nanosheets as a moldable platform for wound healing applications. ACS Appl Mater Interfaces. 2018;10(41):35495–35502. doi:10.1021/acsami.8b12523
87. Pan W, Dai C, Li Y, et al. PRP-chitosan thermoresponsive hydrogel combined with black phosphorus nanosheets as injectable biomaterial for biotherapy and phototherapy treatment of rheumatoid arthritis. Biomaterials. 2020;239:119851. doi:10.1016/j.biomaterials.2020.119851
88. Kumar V, Brent JR, Shorie M, et al. Nanostructured aptamer-functionalized black phosphorus sensing platform for label-free detection of myoglobin, a cardiovascular disease biomarker. ACS Appl Mater Interfaces. 2016;8(35):22860–22868. doi:10.1021/acsami.6b06488
89. Qi J, Xiong Y, Cheng K, et al. Heterobifunctional PEG-grafted black phosphorus quantum dots: “Three-in-One” nano-platforms for mitochondria-targeted photothermal cancer therapy. Asian J Pharm Sci. 2021;16(2):222–235. doi:10.1016/j.ajps.2020.09.001
90. Lim Y, Zhou W, Li G, et al. Black phosphorus nanomaterials regulate the aggregation of amyloid‐β. ChemNanoMat. 2019;5(5):606–611. doi:10.1002/cnma.201900007
91. Li Z, Guo T, Hu Y, et al. A highly effective π–π stacking strategy to modify black phosphorus with aromatic molecules for cancer theranostics. ACS Appl Mater Interfaces. 2019;11(10):9860–9871. doi:10.1021/acsami.9b00374
92. Ye X, Liang X, Chen Q, et al. Surgical tumor-derived personalized photothermal vaccine formulation for cancer immunotherapy. ACS Nano. 2019;13(3):2956–2968. doi:10.1021/acsnano.8b07371
93. Deng F, Wu P, Qian G, et al. Silver-decorated black phosphorus: a synergistic antibacterial strategy. Nanotechnology. 2022;33(24):245708. doi:10.1088/1361-6528/ac5aee
94. Sun X, Yu C, Zhang L, Cao J, Kaleli EH, Xie G. Tribological and antibacterial properties of polyetheretherketone composites with black phosphorus nanosheets. Polymers. 2022;14(6):1242. doi:10.3390/polym14061242
95. Yuan B, Zhou X, Li Y, et al. Black-phosphorus-nanosheet-reinforced coating of implants for sequential biofilm ablation and bone fracture healing acceleration. ACS Appl Mater Interfaces. 2022;14(41):47036–47051. doi:10.1021/acsami.2c13566
96. Lukyanov AN, Torchilin VP. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliv Rev. 2004;56(9):1273–1289. doi:10.1016/j.addr.2003.12.004
97. Miao Y, Shi X, Li Q, et al. Engineering natural matrices with black phosphorus nanosheets to generate multi-functional therapeutic nanocomposite hydrogels. Biomater Sci. 2019;7(10):4046–4059. doi:10.1039/C9BM01072F
98. Zeng J, Gu C, Geng X, Lin K, Xie Y, Chen X. Combined photothermal and sonodynamic therapy using a 2D black phosphorus nanosheets loaded coating for efficient bacterial inhibition and bone-implant integration. Biomaterials. 2023;297:122122. doi:10.1016/j.biomaterials.2023.122122
99. Zhao Y, Peng X, Wang D, et al. Chloroplast‐inspired scaffold for infected bone defect therapy: towards stable photothermal properties and self‐defensive functionality. Adv Sci. 2022;9(31):2204535. doi:10.1002/advs.202204535
100. Xu S, Chang L, Zhao X, et al.. Tea polyphenol modified, photothermal responsive and ROS generative black phosphorus quantum dots as nanoplatforms for promoting MRSA infected wounds healing in diabetic rats. J Nanobiotechnology. 2021;19(1). doi:10.1186/s12951-021-01106-w
101. Tan L, Li J, Liu X, et al. In situ disinfection through photoinspired radical oxygen species storage and thermal-triggered release from black phosphorous with strengthened chemical stability. Small. 2018;14(9):1703197. doi:10.1002/smll.201703197
102. Fu J, Liu T, Feng X, et al. A perfect pair: stabilized black phosphorous nanosheets engineering with antimicrobial peptides for robust multidrug resistant bacteria eradication. Adv Healthcare Mater. 2022;11(10):2101846. doi:10.1002/adhm.202101846
103. Zhao Y, Tian C, Liu Y, et al. All-in-one bioactive properties of photothermal nanofibers for accelerating diabetic wound healing. Biomaterials. 2023;295:122029. doi:10.1016/j.biomaterials.2023.122029
104. Zhang Z, Chen R, Mao S, et al. A novel strategy to enhance photocatalytic killing of foodborne pathogenic bacteria by modification of non-metallic monomeric black phosphorus with Elaeagnus mollis polysaccharides. Int J Biol Macromol. 2023;242:125015. doi:10.1016/j.ijbiomac.2023.125015
105. Cao J, Qi J, Lin X, et al. Biomimetic black phosphorus nanosheet-based drug delivery system for targeted photothermal-chemo cancer therapy. Front Bioeng Biotechnol. 2021;9:707208. doi:10.3389/fbioe.2021.707208
106. Tayari V, Hemsworth N, Fakih I, et al. Two-dimensional magnetotransport in a black phosphorus naked quantum well. Nat Commun. 2015;6(1):7702. doi:10.1038/ncomms8702
107. Zhang X, Li Q, Li L, et al. Bioinspired mild photothermal effect-reinforced multifunctional fiber scaffolds promote bone regeneration. ACS Nano. 2023;17(7):6466–6479. doi:10.1021/acsnano.2c11486
108. Zheng H, Li H, Deng H, et al. Near infrared light-responsive and drug-loaded black phosphorus nanosheets for antibacterial applications. Colloids Surfaces B. 2022;214:112433. doi:10.1016/j.colsurfb.2022.112433
109. Song C, Huang D, Zhao C, Zhao Y. Abalone‐inspired adhesive and photo‐responsive microparticle delivery systems for periodontal drug therapy. Adv Sci. 2022;9(30):2202829. doi:10.1002/advs.202202829
110. Guo Z, He J, Mahadevegowda SH, Kho SH, Chan‐Park MB, Liu X. Multifunctional glyco‐nanosheets to eradicate drug‐resistant bacteria on wounds. Adv Healthcare Mater. 2020;9(10):2000265. doi:10.1002/adhm.202000265
111. Chen M, Zhou J, Ran P, et al. Photoactivated release of nitric oxide and antimicrobial peptide derivatives for synergistic therapy of bacterial skin abscesses. Adv Healthcare Mater. 2022;11(12):2200199.
112. Ma Y, Jiang L, Hu J, Zhu E, Zhang N. Developing a versatile multiscale therapeutic platform for osteosarcoma synergistic photothermo-chemotherapy with effective osteogenicity and antibacterial capability. ACS Appl Mater Interfaces. 2022;14(39):44065–44083. doi:10.1021/acsami.2c10772
113. Ding Q, Sun T, Su W, et al. Bioinspired multifunctional black phosphorus hydrogel with antibacterial and antioxidant properties: a stepwise countermeasure for diabetic skin wound healing. Adv Healthcare Mater. 2022;11(12):2102791. doi:10.1002/adhm.202102791
114. Ouyang J, Ji X, Zhang X, et al. In situ sprayed NIR-responsive, analgesic black phosphorus-based gel for diabetic ulcer treatment. Proc Natl Acad Sci USA. 2020;117(46):28667–28677. doi:10.1073/pnas.2016268117
115. Zhou L, Liu N, Feng L, et al.. Multifunctional electrospun asymmetric wettable membrane containing black phosphorus/Rg1 for enhancing infected wound healing. Bioeng Transl Med. 2022;7(2). doi:10.1002/btm2.10274
116. Liu L, Wang W, Hong W, et al. Photothermal 2D nanosheets combined with astragaloside IV for antibacterial properties and promoting angiogenesis to treat infected wounds. Front Bioeng Biotechnol. 2022;9:826011. doi:10.3389/fbioe.2021.826011
117. Xie Y, Liu Y, Yang J, et al. Gold nanoclusters for targeting methicillin-resistant Staphylococcus aureus in vivo. Angew Chem Int Ed. 2018;57(15):3958–3962. doi:10.1002/anie.201712878
118. Yang P, Pageni P, Rahman MA, et al. Gold nanoparticles with antibiotic‐metallopolymers toward broad‐spectrum antibacterial effects. Adv Healthcare Mater. 2019;8(6):1800854. doi:10.1002/adhm.201800854
119. Zhang S, Dong H, He R, et al.et al. Hydro electroactive Cu/Zn coated cotton fiber nonwovens for antibacterial and antiviral applications. Int J Biol Macromol. 2022;207:100–109. doi:10.1016/j.ijbiomac.2022.02.155
120. Zhu J, Liu S, Zhang T, et al. Porous gold layer coated silver nanoplates with efficient antimicrobial activity. Colloids Surf B Biointerfaces. 2020;186:110727. doi:10.1016/j.colsurfb.2019.110727
121. Wang H, Song Z, Li S, Wu Y, Han H. One stone with two birds: functional gold nanostar for targeted combination therapy of drug-resistant Staphylococcus aureus Infection. ACS Appl Mater Interfaces. 2019;11(36):32659–32669. doi:10.1021/acsami.9b09824
122. Kim T, Zhang Q, Li J, Zhang L, Jokerst JV. A gold/silver hybrid nanoparticle for treatment and photoacoustic imaging of bacterial infection. ACS Nano. 2018;12(6):5615–5625. doi:10.1021/acsnano.8b01362
123. Xie YY, Hu XH, Zhang YW, et al. Development and antibacterial activities of bacterial cellulose/graphene oxide-CuO nanocomposite films. Carbohydr Polym. 2020;229:115456. doi:10.1016/j.carbpol.2019.115456
124. Athinarayanan J, Periasamy VS, Krishnamoorthy R, Alshatwi AA. Evaluation of antibacterial and cytotoxic properties of green synthesized Cu2O/Graphene nanosheets. Mater Sci Eng C. 2018;93:242–253. doi:10.1016/j.msec.2018.07.073
125. Sun L, Du T, Hu C, et al. Antibacterial activity of graphene Oxide/g-C3N4 composite through photocatalytic disinfection under visible light. ACS Sustain Chemi Engi. 2017;5(10):8693–8701. doi:10.1021/acssuschemeng.7b01431
126. Liu J, Rojas-Andrade MD, Chata G, et al. Photo-enhanced antibacterial activity of ZnO/graphene quantum dot nanocomposites. Nanoscale. 2018;10(1):158–166. doi:10.1039/C7NR07367D
127. Zhang D, Liu HM, Shu X, et al. Nanocopper-loaded Black phosphorus nanocomposites for efficient synergistic antibacterial application. J Hazard Mater. 2020;393:122317. doi:10.1016/j.jhazmat.2020.122317
128. Li X, Zhu J, Wei B. Hybrid nanostructures of metal/two-dimensional nanomaterials for plasmon-enhanced applications. Chem Soc Rev. 2016;45(11):3145–3187. doi:10.1039/c6cs00195e
129. Qing Y, Cheng L, Li R, et al. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. IJN. 2018;13:3311–3327. doi:10.2147/IJN.S165125
130. Dedman CJ, Newson GC, Davies GL, Christie-Oleza JA. Mechanisms of silver nanoparticle toxicity on the marine cyanobacterium Prochlorococcus under environmentally-relevant conditions. Sci Total Environ. 2020;747:141229. doi:10.1016/j.scitotenv.2020.141229
131. Quevedo AC, Lynch I, Valsami-Jones E. Silver nanoparticle induced toxicity and cell death mechanisms in embryonic zebrafish cells. Nanoscale. 2021;13(12):6142–6161. doi:10.1039/D0NR09024G
132. Zhao Y, Chen Z, Shao W, et al. Black phosphorus-enhanced injectable hydrogel for infected soft tissue healing. APL Bioeng. 2023;7(1):016103. doi:10.1063/5.0121241
133. Liang M, Zhang M, Yu S, et al. Silver‐laden black phosphorus nanosheets for an efficient in vivo antimicrobial application. Small. 2020;16(13):1905938. doi:10.1002/smll.201905938
134. Ouyang J, Liu RY, Chen W, et al. A black phosphorus based synergistic antibacterial platform against drug resistant bacteria. J Mater Chem B. 2018;6(39):6302–6310. doi:10.1039/C8TB01669K
135. Zheng K, Setyawati MI, Leong DT, Xie J. Antimicrobial gold nanoclusters. ACS Nano. 2017;11(7):6904–6910. doi:10.1021/acsnano.7b02035
136. Naskar A, Lee S, Kim KS. Au–ZnO conjugated black phosphorus as a near-infrared light-triggering and recurrence-suppressing nanoantibiotic platform against Staphylococcus aureus. Pharmaceutics. 2021;13(1):52. doi:10.3390/pharmaceutics13010052
137. Aksoy İ, Küçükkeçeci H, Sevgi F, Metin Ö, Hatay Patir I. Photothermal antibacterial and antibiofilm activity of black phosphorus/gold nanocomposites against pathogenic bacteria. ACS Appl Mater Interfaces. 2020;12(24):26822–26831. doi:10.1021/acsami.0c02524
138. Li S, Zhang Y, Wen W, et al. A high-sensitivity thermal analysis immunochromatographic sensor based on au nanoparticle-enhanced two-dimensional black phosphorus photothermal-sensing materials. Biosens Bioelectron. 2019;133:223–229. doi:10.1016/j.bios.2019.03.039
139. Yang G, Liu Z, Li Y, et al. Facile synthesis of black phosphorus–Au nanocomposites for enhanced photothermal cancer therapy and surface-enhanced Raman scattering analysis. Biomater Sci. 2017;5(10):2048–2055. doi:10.1039/C7BM00414A
140. Zhang Y, Chen W, Feng W, Fang W, Han X, Cheng C. Multifunctional chondroitin sulfate based hydrogels for promoting infected diabetic wounds healing by chemo-photothermal antibacterial and cytokine modulation. Carbohydr Polym. 2023;314:120937. doi:10.1016/j.carbpol.2023.120937
141. Li Z, Wu L, Wang H, et al. Synergistic antibacterial activity of black phosphorus nanosheets modified with titanium aminobenzenesulfanato complexes. ACS Appl Nano Mater. 2019;2(3):1202–1209. doi:10.1021/acsanm.8b02065
142. Jing X, Xu C, Su W, et al. Photosensitive and conductive hydrogel induced innerved bone regeneration for infected bone defect repair. Adv Healthcare Mater. 2023;12(3):2201349. doi:10.1002/adhm.202201349
143. Bose S, Surendhiran D, Chun BS, et al. Facile synthesis of black phosphorus-zinc oxide nanohybrids for antibacterial coating of titanium surface. Colloids Surf B Biointerfaces. 2022;219:112807. doi:10.1016/j.colsurfb.2022.112807
144. Zhou L, Zhou L, Wei C, Guo R. A bioactive dextran-based hydrogel promote the healing of infected wounds via antibacterial and immunomodulatory. Carbohydr Polym. 2022;291:119558. doi:10.1016/j.carbpol.2022.119558
145. Li Z, Luo G, Hu W, et al. Mediated drug release from nanovehicles by black phosphorus quantum dots for efficient therapy of chronic obstructive pulmonary disease. Angew Chem Int Ed. 2020;59(46):20568–20576. doi:10.1002/anie.202008379
146. Fang J, Wan Y, Sun Y, et al. Near-infrared-activated nanohybrid coating with black phosphorus/zinc oxide for efficient biofilm eradication against implant-associated infections. Chem Eng J. 2022;435:134935. doi:10.1016/j.cej.2022.134935
147. Xu H, Xu H, Ma S, et al. Bifunctional electrospun poly (L-lactic acid) membranes incorporating black phosphorus nanosheets and nano-zinc oxide for enhanced biocompatibility and antibacterial properties in catheter materials. J Mech Behav Biomed Mater. 2023;142:105884. doi:10.1016/j.jmbbm.2023.105884
148. Li X, Ren S, Song L, et al. Combined black phosphorus nanosheets with ICG/aPDT is an effective anti-inflammatory treatment for periodontal disorders. IJN. 2023;18:813–827. doi:10.2147/IJN.S394861
149. Mo J, Xu Y, Wang X, Wei W, Zhao J. Exploiting the protein Corona: coating of black phosphorus nanosheets enables macrophage polarization via calcium influx. Nanoscale. 2020;12(3):1742–1748. doi:10.1039/C9NR08570J
150. Chen J, Lu L, Zhang C, Zhu X, Zhuang S. Endothelial dysfunction and transcriptome aberration in mouse aortas induced by black phosphorus quantum dots and nanosheets. Nanoscale. 2021;13(19):9018–9030. doi:10.1039/D1NR01965A
151. Zeng J, Ruan F, Wu M, et al. Black phosphorus quantum dots cause glucose metabolism disorder and insulin resistance in mice. Ecotoxicol Environ Saf. 2022;246:114168. doi:10.1016/j.ecoenv.2022.114168
152. Zhang S, Zhang X, Lei L, et al. pH‐dependent degradation of layered black phosphorus: essential role of hydroxide ions. Angewandte Chemie. 2019;131(2):477–481. doi:10.1002/ange.201809989
153. Wu Q, Yao L, Zhao X, et al. Cellular uptake of few-layered black phosphorus and the toxicity to an aquatic unicellular organism. Environ Sci Technol. 2020;54(3):1583–1592. doi:10.1021/acs.est.9b05424
154. Yang X, Liang J, Wu Q, et al. Developmental toxicity of few-layered black phosphorus toward Zebrafish. Environ Sci Technol. 2021;55(2):1134–1144. doi:10.1021/acs.est.0c05724
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.