Back to Journals » Drug Design, Development and Therapy » Volume 13
Bisperoxovanadium protects against spinal cord injury by regulating autophagy via activation of ERK1/2 signaling
Authors Tang Y, Li K, Yang C, Huang K, Zhou J, Shi Y, Xie K, Liu J
Received 17 September 2018
Accepted for publication 17 December 2018
Published 1 February 2019 Volume 2019:13 Pages 513—521
DOI https://doi.org/10.2147/DDDT.S187878
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Cristiana Tanase
Yu-jin Tang,1,* Kai Li,2,* Cheng-liang Yang,1 Ke Huang,1 Jing Zhou,3 Yu Shi,1 Ke-gong Xie,1 Jia Liu1
1Department of Orthopaedics, Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, Guangxi, China; 2Academy of Orthopedics, The Third Affiliated Hospital of Southern Medical University, Guangzhou, Guangdong, China; 3Department of Anatomy, Youjiang Medical University for Nationalities, Baise, Guangxi, China
*These authors contributed equally to this work
Background: Spinal cord injury (SCI) is a disease of the central nervous system with few restorative treatments. Autophagy has been regarded as a promising therapeutic target for SCI. The inhibitor of phosphatase and tensin homolog deleted on chromosome ten (PTEN) bisperoxovanadium (bpV[pic]) had been claimed to provide a neuroprotective effect on SCI; but the underlying mechanism is still not fully understood.
Materials and methods: Acute SCI model were generated with SD Rats and were treated with control, acellular spinal cord scaffolds (ASC) obtained from normal rats, bpV(pic), and combined material of ASC and bpV(pic). We used BBB score to assess the motor function of the rats and the motor neurons were stained with Nissl staining. The expressions of the main autophagy markers LC3B, Beclin1 and P62, expressions of apoptosis makers Bax, Bcl2, PARP and Caspase 3 were detected with IF or Western Blot analysis.
Results: The bpV(pic) showed significant improvement in functional recovery by activating autophagy and accompanied by decreased neuronal apoptosis; combined ASC with bpV(pic) enhanced these effects. In addition, after treatment with ERK1/2 inhibitor SCH772984, we revealed that bpV(pic) promotes autophagy and inhibits apoptosis through activating ERK1/2 signaling after SCI.
Conclusion: These results illustrated that the bpV(pic) protects against SCI by regulating autophagy via activation of ERK1/2 signaling.
Keywords: bisperoxovanadium, spinal cord injury, autophagy, apoptosis, ERK1/2 signaling
Introduction
Spinal cord injury (SCI) is a serious central traumatic condition, which involves primary and secondary mechanisms of injury.1–3 Although therapeutic intervention for primary injury is difficult, secondary injury mechanisms may be manipulated, providing invaluable therapeutic targets for curing SCI.4 Secondary injury often incorporates apoptosis, hypoxia, oxidative stress, and inflammation and is believed to have a more significant impact on neurofunctional recovery after SCI.5,6 Previous studies have demonstrated that apoptosis of neural cells occurs in secondary SCI and is closely associated with recovery after SCI.7–10 Therefore, a thorough elucidation of the mechanisms responsible for secondary injury is important to understand neurodegenerative disorders and to determine an appropriate therapeutic method.
Autophagy plays an important role in intracellular homeostasis characterized by the degradation of cytoplasmic proteins and organelles during development and under stress conditions.11–13 Autophagy flux is also necessary for normal neuronal homeostasis, and its dysfunction contributes to neuronal cell death in several neurodegenerative diseases.14 It was reported that autophagy contributes to the inhibition of apoptosis; enhancing autophagy promotes the recovery of neurological functions by inhibiting apoptosis, while the inhibition of autophagy increases apoptosis of neurons and also causes neurodegeneration in mice.14–16 In SCI, activation of autophagy can protect against neuronal loss and clear intracellular damaged proteins to promote recovery of motor function.17 Upregulation of autophagy markers has been observed after SCI, but the precise mechanism of autophagy’s contribution in SCI is not fully understood.
The inhibitor of phosphatase and tensin homolog deleted on chromosome ten (PTEN), bisperoxovanadium (bpV(pic)), has been reported to protect nerves following trauma and ameliorate secondary injuries in SCI.18,19 As PTEN acts as an inhibitor of the AKT/mTOR (mechanistic target of rapamycin) pathway, inhibition of PTEN by bpV(pic) would lead to the activation of AKT/mTOR signaling. It is well accepted that mTOR is a central cell growth regulator that integrates growth factor and nutrient signals, and autophagy is inhibited by the mTOR signaling. In this regard, the impact of bpV(pic) on autophagy in SCI may be controversial and a systemic analysis is needed.
In this study, we treated SCI rats with a unique technique combining bpV(pic) with acellular spinal cord (ASC) scaffolds from normal rats. We provided sufficient evidence to demonstrate that bpV(pic) treatment significantly improved functional recovery by activating autophagy, accompanied by decreased neuronal apoptosis, and combined ASC with bpV(pic) could enhance these effects. Further, in vitro analysis with rat neuron stem cells (RNSCs) verified that bpV(pic) enhanced autophagy through activation of ERK1/2 signaling.
Materials and methods
Acute spinal cord injury model
Adult male Sprague Dawley (SD) rats (250–300 g) were purchased from the Animal Center of Youjiang Medical College for Nationalities. All animals were housed in standard temperature conditions with a 12-hour light/dark cycle and regularly fed with food and water. All surgical procedures were performed under anesthesia by intraperitoneal injection with 10% chloral hydrate (0.4 mL/100 g). The skin was incised to expose the vertebral column and to perform a laminectomy at the T9 level. Under a surgical microscope, two right-sided hemisections of the spinal cord were created using a microdissection scissor at levels T9 and T10. A gap of 2 mm width was produced, and tissue was removed with a 22-gauge ethylene tetrafluoroethylene needle. Animals that underwent laminectomy without SCI were used as a sham control (n=4). Animals with a hemisected SCI were randomly divided into four groups after SCI: animals treated with an ASC scaffold implantation (n=6), animals treated with poly-L-lactic acid (PLLA)/bpV(pic) implantation (n=6), animals treated with the implantation of an ASC scaffold with PLLA/bpV(pic) (n=6), and SCI only (n=6). To prevent infection, rats were treated with ampicillin (100 mg/kg) and gentamicin (12 mg/kg) subcutaneously once a day following surgery for 3 days. Manual bladder expression was performed twice a day until they regained bladder control, ~3–5 days after initial injury. All animal experiments were approved by the local ethics committee for animal research at Youjiang Medical College for Nationalities and were performed in accordance with international standards for animal welfare.20
ASC scaffold preparation
Spinal cord was harvested from SD rats after intraperitoneal injection of an overdose of anesthetic (10% chloral hydrate, 60 mL/kg) and immediately placed in PBS at 4°C. The thoracic spinal cords were harvested. The chemical extraction for ASC preparation was based on a procedure described previously.35 Briefly, thoracic spinal cords were treated with a series of detergents including distilled water, Triton X-100, and sodium deoxycholate solution. The spinal cord segments were then washed three times (1 hour each) in a 0.01% PBS solution and stored in the same solution at 4°C. The samples were cut into 0.2×0.1 cm blocks before being freeze dried, and all samples were sterilized by irradiation before use, as previously described.21 All samples were subsequently freeze-dried for 24 hours in a freeze dryer and sterilized by irradiation (3 kGy) with Cobalt-60 gamma rays; the ASC scaffold had been crosslinked before being used.
Locomotion recovery assessment
Motor function recovery was assessed using the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale. Individual rats were placed on an open field and observed for 4 minutes by two observers blind to the treatment condition. The score of each hind limb was recorded, and the averages were determined; results were scored on a scale of 0–21. The scores were graded according to the rat’s motor motion and rating scale. The test was carried out once a week after transplantation up to 6 weeks postsurgery.
Cell culture
The RNSC was obtained from Cyagen Biosciences (Cat NO RASNF-01001, Suzhou, China). The cells were maintained in the RNSC growth medium (Cat NO RASNF-90081, Cyagen Biosciences) at 37°C in a 5% CO2 humidified incubator, with the supplemental of 1% penicillin–streptomycin, 2% B27, 20 ng/mL epidermal growth factor, and 10 ng/mL basic fibroblast growth factor. For neurogenic differentiation, RNSCs were plated in 6-well tissue culture plates coated with poly-l-lysine (PLL)/laminin at 2×104 cells/cm2 in growth medium volume of 2 mL per well, and cells were incubated at 37°C in a 5% CO2 humidified incubator. After 2 days, the medium was changed to neural stem cell neurogenic differentiation medium (Cat NO RAXNX-90081, Cyagen Biosciences). The differentiation medium was replaced every 3 days, and 7 days later, the RNSCs were cocultured with control (DMSO), ASC, bpV(pic) (20 ng/mL), and ASC combined with bpV(pic) for 24 hours. Then the RNSCs were analyzed with Western blot and immunofluorescence analyses.
Cell survival assay
A cell survival assay was performed with the use of the cell counting kit-8 (CCK-8) according to the manufacturer’s recommendations (Beyotime Biotechnology, Shanghai, China). Briefly, NSCs were cultured in 96-well culture plates at 1×104 cells/well as a monolayer. After coculturing with saline, ASC scaffold only, PLLA/bpV(pic) microspheres only, and ASC scaffold combined with PLLA/bpV(pic) microspheres for 24 hours, RNSCs in all groups were rinsed twice with PBS, then 10 μL of CCK-8 solution was added to each well. After incubation for 4 hours, cell proliferation was evaluated by measuring absorbance at 450 nm using a microplate reader.
Western blot analysis
Tissues and cells were lysed using lysis buffer (62.5 mM Tris–HCl, pH 6.8), 10% glycerol, 2% SDS, 50 mM dithiothreitol, and 0.01% bromophenol blue at 96°C for 10 minutes. The samples were separated with SDS-PAGE for 70 minutes, and after electrophoresis, proteins were transferred onto membranes (Bio-Rad Laboratories, Hercules, CA, USA) using a wet transfer method. Each membrane was then incubated in primary antibodies overnight at 4°C in a shaker. After incubation in specific secondary antibodies, immunoblots were detected using an enhanced chemiluminescence kit (ECL kit; Proteintech Group, Inc., Rosemont, IL, USA).
Antibodies
Anti-p-PTEN (#9554; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-β-III-tubulin (ab78078, Abcam, Cambridge, UK), anti-NeuN (24,307T, Cell Signaling Technology), anti-LC3B (#3868, Cell Signaling Technology), anti-Beclin1 (GTX31722, GeneTex, Inc., Irvine, CA, USA), anti-p62 (ab56416, Abcam), anti-caspase-3 (ab44976, Abcam), anti-poly adenosinediphosphate-ribose polymerase (PARP) (#9532, Cell Signaling Technology), anti-pS6 (s235/236) (#4858, Cell Signaling Technology), anti-S6 (#2317, Cell Signaling Technology), anti-Bax (gtx109683, Genetex,), anti-Bcl2 (15071T, Cell Signaling Technology), anti-p-ERK1/2 (#4370, Cell Signaling Technology), anti-ERK1/2 (ab54230, Abcam), and anti-β-actin (#3700, Cell Signaling Technology) were used.
Immunofluorescence analysis
Tissue segments containing the lesion (1 cm on each side of the lesion) were removed and postfixed by immersion in 4% paraformaldehyde overnight, transferred to 30% sucrose solution until they sank, and cut into 10 μm thick transverse sections using a freezing microtome (Thermo Fisher Scientific, Waltham, MA, USA). Sections were rewarmed at room temperature for 30 minutes and blocked with sheep serum working solution at room temperature for 2 hours. The tissues were incubated in primary antibodies overnight at 4°C. The sections were subsequently washed three times and incubated in the corresponding fluorescent secondary antibodies (goat antirabbit 594 and goat antimouse fluorescein isothiocyanate, 1:500, Thermo Fisher Scientific) for 1 hour at room temperature. The sections were then stained with DAPI and observed by fluorescent microscopy (Olympus Corporation, Tokyo, Japan).
Nissl staining
Sections were rewarmed at room temperature for 30 minutes, then immersed in 100% ethanol, 75% ethanol, and distilled water for 30 seconds each, and then stained with a 0.1% Cresyl Violet solution at 37°C for 5 minutes. The sections were washed in distilled water, immersed in 95% ethanol for 30 seconds, and soaked in anhydrous ethanol, anhydrous ethanol, and xylene for 5 minutes each. The sections were sealed with neutral gum and observed under a microscope. Three different visual fields around the transplanting area of each section were used for the quantitative statistics analysis with ImageJ software (Media Cybernetics, Bethesda, MD, USA).
Statistical analysis
All experiments were performed at least three times. All data were presented as the mean ± SD using SPSS version 20.0 software for statistical analysis, with graphs generated in GraphPad Prism 6.0. The Student’s t-test was used to assess statistically significant differences in the data between groups. A P-value <0.05 was considered statistically significant.
Results
ASC combined with bpV(pic) improves functional recovery after SCI
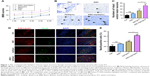
All rats had normal motor function with a BBB score of 22 before SCI. There was no locomotor dysfunction in rats from sham surgery, with a BBB score of 22 throughout the study. Serious limb locomotor dysfunction was found in rats after SCI in both the control and ASC-treated group, which was significantly improved by bpV(pic) (P<0.05). Moreover, rats treated with ASC combined with bpV(pic) demonstrated the highest improvement in motor function (Figure 1A). This showed that ASC combined with bpV(pic) promoted functional neurobehavioral recovery after SCI.
ASC combined with bpV(pic) decreases motor neuron loss in SCI
We analyzed the effect of all interventions on the loss of motor neurons after SCI in rats. Nissl staining was performed on slices from all groups at day 7 after contusion. Control rats demonstrated a great loss of large anterior horn cells, and the ASC-only-treated group exhibited a similar result. However, in the bpV(pic) group, motor neurons in the anterior horns of rats were significantly preserved, and the numbers of intact motor neurons were more in the ASC combined with bpV(pic) group than the bpV(pic)-only group (Figure 1B and C). On confocal immunofluorescent analysis of the spinal tissues from all groups with β-III-tubulin and NeuN antibody, we noticed that the expression of the nuclear protein in the neurons with NeuN was stronger in the ASC combined with bpV(pic) group than the others (Figure 1D and E). In conclusion, ASC combined with bpV(pic) decreased motor neuron loss in rat spines after SCI.
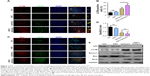
ASC combined with bpV(pic) upregulates autophagy and attenuates apoptosis in rats’ neurons after SCI
To determine the expression of autophagy in the spines of all groups, we detected the expression of the main autophagy marker microtubule-associated protein light chain 3B (LC3B) in neurons. As reported above, the expression of NeuN was strongest in the ASC combined with bpV(pic) group, while the group treated with bpV(pic) only also demonstrated an increase in the level of NeuN in comparison with the control and ASC-only groups. Impressively, the expression of LC3B also had the same tendency as NeuN, which was highest in the combined group. Treatment with bpV(pic) enhanced colocalized expression of LC3B and NeuN and combined with ASC resulted in a better effect than bpV(pic) only (Figure 2A and B). We also tested the expressions of antiapoptotic protein Bcl-2 and proapoptotic protein Bax with immunofluorescent analysis. Compared to the control and ASC-only groups, the expression of Bcl-2 was upregulated, while Bax was downregulated (the ratio of Bax/Bcl-2) in bpV(pic)- and ASC-combined-with-bpV(pic)-treated SCI rats. But the discrepancy between bpV(pic) and ASC combined with bpV(pic) was not significant (Figure 2C and D). We also used Western blot analysis to detect expression levels of the markers of autophagy and apoptosis in the spinal cords from SACI rats undergoing different treatments. Compared to the control and ASC-only groups, downregulated levels of p62 and elevated levels of LC3B and Beclin1 verified a pattern of increased autophagy in the bpV(pic) and ASC combined with bpV(pic) groups. Also, antiapoptotic protein Bcl-2 increased, and proapoptotic protein Bax decreased in the bpV(pic) and ASC combined with bpV(pic) groups, confirming the immunofluorescence results (Figure 2E). These data suggested that after SCI, rats treated with bpV(pic) or ASC combined with bpV(pic) upregulated autophagy and attenuated apoptosis in neurons. Moreover, the ASC combined with bpV(pic) had a better effect in promoting autophagy than bpV(pic) only.
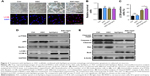
ASC combined with bpV(pic) enhanced autophagy and reduced apoptosis in rat neuronal stem cells
To investigate the effect of ASC and bpV(pic) in vitro, after incubation in neuronal differentiation induction media for 7 days, RNSCs were cocultured with control, ASC only, bpV(pic) only, and ASC combined with bpV(pic) for 24 hours. The survival rates of the RNSCs were comparable in all four groups (Figure 3B), while the differentiation of the RNSCs was enhanced after treated with bpV(pic) only and ASC combined with bpV(pic) (Figure 3A). The expressions of LC3B in the RNSCs bpV(pic) and the ASC combined with bpV(pic) cocultured groups were significantly enhanced compared with the other two groups in the immunofluorescent analysis. Also, ASC combined with bpV(pic) had the best effect on augmenting the expression of autophagy marker LC3B (Figure 3A and C). Moreover, Western blot analysis of the protein expression of autophagy markers replicated the enhancement of autophagy in RNSCs from the bpV(pic) and ASC combined with bpV(pic) groups. As the phosphorylation of PTEN was inhibited by bpV(pic), the expression of p62 decreased and the expressions of LC3B-II and Beclin1 increased (Figure 3D). And we also detected the expressions of apoptosis markers after coculturing with different materials. The bpV(pic) and ASC combined with bpV(pic) showed a parallel effect on inhibition of expression of cleaved PARP and caspase 3 and also decreased the ratio of Bax/Bcl2 (Figure 3E). Together, these data indicated that bpV(pic) and ASC combined with bpV(pic) enhanced autophagy and reduced apoptosis in cultured RNSCs.
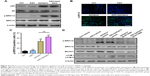
bpV(pic) enhanced autophagy through activating ERK1/2 signaling in neurons
To elucidate the underling mechanism of bpV(pic) on enhancing autophagy and reducing apoptosis in neurons, we next detected the expression of related targets of bpV(pic). With Western blot analysis, we noticed that the expression of downstream of mTORC1-pS6 level was enhanced by treating with bpV(pic), while the level of p-ERK1/2 was also increased in bpV(pic) cocultured RNSCs (Figure 4A). With immunofluorescent analysis, we confirmed enhanced expression of p-ERK1/2 in the spines of SCI rats treated with bpV(pic) and ASC combined with bpV(pic) (Figure 4B and C). It is known that autophagy can be inhibited by mTOR signaling, but here we found enhanced autophagy with activated mTOR. Thus, we speculate that bpV(pic) may promote autophagy in SCI through ERK1/2 signaling. Then, we stimulated the cocultured RNSCs with the inhibitor of ERK1/2 signaling, SCH772984 (20 nM). After adding SCH772984 to cocultured RNSCs for 24 hours, protein levels were tested with Western blot analysis. As shown in Figure 4D, enhanced expressions of p-ERK1/2 in bpV(pic) and ASC combined with bpV(pic) groups were reversed by SCH772984. The expressions of autophagy markers LC3B and Beclin1 were also reversed by the ERK1/2 inhibitor SCH772984 (Figure 4D). Thus, these data indicated that the enhanced autophagy caused by bpV(pic) occurred through activating ERK1/2 signaling.
Discussion
Previous studies have revealed that administration of PTEN inhibitors to the injured spinal cord has neuroprotective effects and promotes neurological functional recovery.22–26 We showed that inhibiting PTEN with bpV(pic) promoted autophagy and inhibited apoptosis in vitro and in vivo, and combined ASC with bpV(pic) resulted in a better effect on recovery of locomotor function and neuronal structure after SCI. With the inhibitor of ERK1/2, we confirmed that the enhanced autophagy caused by bpV(pic) occurred through activation of ERK1/2 signaling.
SCI is a major health concern and a cause of disability and mortality worldwide. Studies have proven that neuroprotection and neurorecovery play important roles in the treatment of SCI27–29 and that autophagy has been shown to exert a neuroprotective effect in rats with SCI.14,17 Recent studies have focused on the relationship between apoptosis and autophagy following acute SCI and claimed that autophagy activation resulted in decreased neuronal loss by inhibiting apoptosis after acute SCI.17 Therefore, we also noticed that apoptosis was diminished with lower expression of its markers, cleaved PARP, and caspase 3, as well as the ratio of Bax/Bcl2 with bpV(pic) treatment. These data indicated that with the treatment of bpV(pic) and ASC combined with bpV(pic), autophagy can be enhanced, and apoptosis can be inhibited in the neurons.
bpV(pic) compounds selectivity inhibited phosphorylated PTEN and had been reported to have a neuroprotective effect after central nervous system injury. The PI3K/AKT pathway and its downstream effector, the mTOR complex, have been well characterized as major inhibitory regulators of autophagy.30 PTEN, a natural antagonist of the PI3K/AKT pathway, has been found to positively regulate autophagy.31 In this study, we noticed that the level of the substrate of mTOR signaling ps6 was elevated as phosphorylated PTEN decreased, which meant that bpV(pic) activated mTOR signaling in SCI. However, autophagy was not inhibited by bpV(pic) treatment; to the contrary, it was enhanced in the bpV(pic)-treated and ASC-combined-bpV(pic)-treated groups. Hence, we propose that activation of mTOR with bpV(pic) would not affect the initiating process of autophagy and that there must be other potential mechanisms in regulating autophagy with bpV(pic), through inhibiting PTEN or not.
ERK1/2 belongs to the mitogen-activated protein kinase family, which consists of two downstream molecules, JNK and c-Jun, which regulate cell survival and neuroprotective function of the nervous system. ERK1/2 activation in the injured spinal cord regulates inflammatory pain in sensory neurons of the spinal cord.32,33 Blockade of ERK1/2 decreased sprouting of axons in the corticospinal tract around the lesioned area.34,35 In bpV(pic)-treated RNSCs and rat spines after SCI, ERK1/2 phosphorylation was elevated. Studies have suggested that increased ERK phosphorylation levels induce autophagy in cells. With inhibition of ERK1/2, we noticed that as the phosphorylation level of ERK1/2 fell, increased autophagy caused by bpV(pic) could be reversed by SCH772984. Thus, we speculated that bpV(pic) promoted autophagy through activation of ERK1/2 signaling.
Conclusion
Previously, we combined the ASC scaffold to blood-derived mesenchymal stem cells to bridge the spinal cord cavity and promote long-distance axonal regeneration and functional recovery in SCI rats.21 In this study, we confirmed that bpV(pic) promoted neurofunctional recovery after SCI, and combined ASC with bpV(pic) enhanced the neuroprotective effect. Further, we found that bpV(pic) enhanced autophagy through activation of ERK1/2 signaling, then inhibiting apoptosis and resulting in decreased neuronal loss. Therefore, our research may provide a better understanding of autophagy in repairing damaged spinal cords in the future.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (approval number: 81560213) and supported by 2018GXNSFAA138074.
Disclosure
The authors report no conflicts of interest in this work.
References
McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359(9304):417–425. | ||
Young W. Spinal cord regeneration. Cell Transplant. 2014;23(4–5):573–611. | ||
Carlson GD, Gorden C. Current developments in spinal cord injury research. Spine J. 2002;2(2):116–128. | ||
Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7(8):628–643. | ||
Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21(4):429–440. | ||
Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15(3):415–436. | ||
Charriaut-Marlangue C. Apoptosis: a target for neuroprotection. Thérapie. 2004;59(2):185–190. | ||
Green DR. Apoptotic pathways: the roads to ruin. Cell. 1998;94(6):695–698. | ||
Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1(2):120–130. | ||
Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348(14):1365–1375. | ||
Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. | ||
Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306(5698):990–995. | ||
Todde V, Veenhuis M, van der Klei IJ. Autophagy: principles and significance in health and disease. Biochim Biophys Acta. 2009;1792(1):3–13. | ||
Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441(7095):880–884. | ||
Liu S, Sarkar C, Dinizo M, et al. Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death Dis. 2015;6(1):e1582. | ||
Kanno H, Ozawa H, Sekiguchi A, Itoi E. Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death. Neurobiol Dis. 2009;33(2):143–148. | ||
Jing CH, Wang L, Liu PP, Wu C, Ruan D, Chen G. Autophagy activation is associated with neuroprotection against apoptosis via a mitochondrial pathway in a rat model of subarachnoid hemorrhage. Neuroscience. 2012;213:144–153. | ||
Walker CL, Xu XM, Xm X. PTEN inhibitor bisperoxovanadium protects oligodendrocytes and myelin and prevents neuronal atrophy in adult rats following cervical hemicontusive spinal cord injury. Neurosci Lett. 2014;573:64–68. | ||
Walker CL, Wang X, Bullis C, et al. Biphasic bisperoxovanadium administration and Schwann cell transplantation for repair after cervical contusive spinal cord injury. Exp Neurol. 2015;264:163–172. | ||
Webster J. International standards for farm animal welfare: science and values. Vet J. 2013;198(1):3–4. | ||
Liu J, Chen J, Liu B, et al. Acellular spinal cord scaffold seeded with mesenchymal stem cells promotes long-distance axon regeneration and functional recovery in spinal cord injured rats. J Neurol Sci. 2013;325(1–2):127–136. | ||
Danilov CA, Steward O. Conditional genetic deletion of PTEN after a spinal cord injury enhances regenerative growth of CST axons and motor function recovery in mice. Exp Neurol. 2015;266:147–160. | ||
Ohtake Y, Park D, Abdul-Muneer PM, et al. The effect of systemic PTEN antagonist peptides on axon growth and functional recovery after spinal cord injury. Biomaterials. 2014;35(16):4610–4626. | ||
du K, Zheng S, Zhang Q, et al. PTEN deletion promotes regrowth of corticospinal tract axons 1 year after spinal cord injury. J Neurosci. 2015;35(26):9754–9763. | ||
Zr H, Chen HY, Hu ZZ, Xie P, Liu QH. PTEN knockdown with the Y444F mutant AAV2 vector promotes axonal regeneration in the adult optic nerve. Neural Regen Res. 2018;13(1):135–144. | ||
Ohtake Y, Hayat U, Li S. PTEN inhibition and axon regeneration and neural repair. Neural Regen Res. 2015;10(9):1363–1368. | ||
Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21(10):1355–1370. | ||
Kamradt T, Rasch C, Schuld C, et al. Spinal cord injury: association with axonal peripheral neuropathy in severely paralysed limbs. Eur J Neurol. 2013;20(5):843–848. | ||
McKerracher L. Spinal cord repair: strategies to promote axon regeneration. Neurobiol Dis. 2001;8(1):11–18. | ||
Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584(7):1287–1295. | ||
Arico S, Petiot A, Bauvy C, et al. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276(38):35243–35246. | ||
Xu Q, Garraway SM, Weyerbacher AR, Shin SJ, Inturrisi CE. Activation of the neuronal extracellular signal-regulated kinase 2 in the spinal cord dorsal horn is required for complete Freund’s adjuvant-induced pain hypersensitivity. J Neurosci. 2008;28(52):14087–14096. | ||
Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp Neurol. 2006;199(2):397–407. | ||
Lee YS, Yoon HJ, Oh JH, et al. 1,3-dinitrobenzene induces apoptosis in TM4 mouse Sertoli cells: involvement of the c-Jun N-terminal kinase (JNK) MAPK pathway. Toxicol Lett. 2009;189(2):145–151. | ||
Xu C, Zhang H, Liu C, et al. Rapamycin inhibits ERK1/2-mediated neuronal apoptosis caused by cadmium. Oncotarget. 2015;6(25):21452–21467. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.