Back to Journals » International Medical Case Reports Journal » Volume 16
Birdshot Chorioretinopathy in Early Adulthood: Review of Current Literature and Case Report
Authors Pham BH , Uludag G, Hien DL , Than NTT, Hwang JJ , Akhavanrezayat A , Matsumiya W, Lajevardi S, Regenold J, Halim MS , Nguyen QD
Received 16 August 2023
Accepted for publication 20 October 2023
Published 11 December 2023 Volume 2023:16 Pages 815—831
DOI https://doi.org/10.2147/IMCRJ.S430981
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Brandon Huy Pham,1 Gunay Uludag,2 Doan Luong Hien,2 Ngoc Trong Tuong Than,2 Jaclyn Joyce Hwang,2 Amir Akhavanrezayat,2 Wataru Matsumiya,2 Sherin Lajevardi,2 Jonathan Regenold,2 Muhammad Sohail Halim,2 Quan Dong Nguyen2
1Stanford University School of Medicine, Palo Alto, CA, USA; 2Spencer Center for Vision Research, Byers Eye Institute at Stanford University, Palo Alto, CA, USA
Correspondence: Quan Dong Nguyen, Spencer Center for Vision Research, Byers Eye Institute at Stanford, 2370 Watson Court, Suite 200, Palo Alto, CA, 94303, USA, Tel +1 650-725-7245, Email [email protected]
Purpose: We describe the course of a patient diagnosed with birdshot chorioretinopathy (BSCR) in early adulthood and summarize clinical findings from similar BSCR patients reported in the literature.
Observations: A 37-year-old male presented to our tertiary uveitis facility with bilateral ocular discomfort, hazy vision, and floaters. Ocular examination was notable for vitritis, optic disc edema, and ovoid hypopigmented chorioretinal lesions, visible on indocyanine green chorioangiography as multiple hypocyanescent spots in the intermediate phase. Full-field electroretinography and visual evoked potential showed global retinal dysfunction and optic nerve dysfunction. Laboratory evaluations were notable only for human leukocyte antigen (HLA)-A29 positivity. The patient was diagnosed with BSCR and started on oral prednisone and eventually managed with infliximab.
Conclusions and Importance: BSCR can affect patients in early adulthood. Proper diagnostic work-up, including assessing HLA-A29 positivity, is needed to manage atypical cases.
Keywords: birdshot chorioretinopathy, early adulthood, human leukocyte antigen (HLA)-A29
Background
Birdshot chorioretinopathy (BSCR), also known as birdshot uveitis or vitiliginous chorioretinitis, is an uncommon type of uveitis first described in 1949 by Franceschetti and Babel.1 The disease is characterized by ovoid choroidal lesions that typically begin at the posterior pole and radiate out toward the periphery, forming a pattern that resembles gunshot spatter from birdshot. The distribution of these lesions, which sometimes varies significantly among patients, can be macula-predominant, macula-sparing, or asymmetric.2,3 Others may show diffuse involvement of the entire posterior pole and periphery. Regardless of the distribution, these lesions almost always affect the inferonasal area surrounding the optic disc. Typically, these changes manifest chronically and in both eyes.2
BSCR is uncommon with prevalence between 0.2 and 1.7 cases/100,000.4 BSCR represents about 0.5–1.5% of uveitis cases seen in tertiary practices.5 It is predominantly seen in Caucasian populations and is most commonly diagnosed in individuals of Northern European descent. BSCR predominantly affects individuals between the ages of 40 and 60 and is more common in females. A large systemic review reported a mean age of disease onset at 53-years-old with a female bias of 54.1%.6 While the disease primarily affects middle-aged individuals, younger patients can also be affected, though this is less common.
We herein report a case of a patient previously diagnosed with birdshot chorioretinopathy at age 34 and subsequently treated with methotrexate, cyclosporine, adalimumab, oral steroids, and infliximab, followed by a review of previously reported cases of BSCR that presented before the age of 40. Our case highlights the importance of maintaining a broad differential diagnosis that includes birdshot chorioretinopathy in the appropriate clinical context even in patients younger than the typical demographic.
Case Report
A 37-year-old male presented to our tertiary uveitis facility in January 2020 by referral for further vitreoretinal evaluation. His symptoms were flashes and photopsias predominantly in the right eye. He had previously been diagnosed with presumed bilateral BSCR in the summer of 2016. Initial laboratory evaluations, including complete blood count, complete metabolic panel, hemoglobin A1c and iron studies, were all unremarkable. Laboratory evaluations for infectious etiologies, including QuantiFERON-TB Gold (QFT), rapid plasma reagin (RPR), and fluorescent treponemal antibody absorption (FTA-ABS) tests, were all negative or non-reactive. Magnetic resonance imaging of the brain in September 2016 was unremarkable. In November 2016, the patient was treated with topical corticosteroid and intravitreal bevacizumab in the right eye (OD), as well as 60 mg daily oral prednisone. In December 2016, he was started on immunomodulatory therapy (IMT) with cyclosporine and methotrexate, which were both stopped in 2017 due to a possible lack of efficacy. The patient was then started on adalimumab, during which his symptoms (flashes and floaters) reportedly improved. After about 1 year of therapy, adalimumab was stopped due to reduced serum iron levels in early 2019. In October 2019, he received a fluocinolone acetonide intravitreal implant 0.18mg injection OD but noticed no subsequent improvement in visual symptoms.
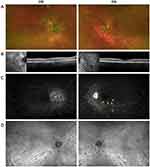
On initial exam in January 2020, the patient noted moderate ocular discomfort (OD > OS), hazy vision (OD > OS), and floaters (OU). Visual acuity was 20/20 OU. On initial evaluation, review of systems was negative from a systemic standpoint. Intraocular pressures were 13 mm Hg OD and 12 mm Hg OS. Anterior segment exam was notable for 1+ flare bilaterally, with mild posterior subcapsular cataract OD, but otherwise unremarkable. Dilated fundus exam was notable for optic disc edema (OD > OS) and peripapillary and peripheral ovoid hypopigmented chorioretinal lesions (OU). The right eye also showed temporal peripapillary atrophy, 1+ vitreous cells, macular retinal pigment epithelium pigmentary changes, and small retinal hemorrhage in the superotemporal quadrant periphery (Figure 1A). Spectral domain optical coherence tomography (SD-OCT) showed optic disc elevation in peripapillary intraretinal fluid with mild epiretinal membrane (ERM) OD > OS (Figure 1B). Wide-angle fluorescein angiography (FA) (OPTOS Plc, Dunfermline, UK) showed hazy media, disc leakage and peripapillary staining OD, and diffuse optic disc leakage with mild segmental staining of venules along the inferior temporal arcade OS. There was hyperfluorescence of several of the lesions in the peripapillary region and along the inferior temporal arcade without any sign of leakage OU (Figure 1C).
Two weeks later, full-field electroretinography (ffERG) showed mild-to-moderate global retinal dysfunction, with clear geographic regions of macular depression (OD > OS). The 30 Hz flicker implicit times revealed significant delay in both eyes (OS > OD). Visual evoked potential showed relatively symmetric delay for both small and large stimuli, suggesting mild optic nerve dysfunction. Goldmann visual fields (GVF) and Humphrey visual fields (HVF) showed blind spot enlargement OD and normal blind spot with no generalized constriction OS. Laboratory evaluations were notable only for human leukocyte antigen (HLA) A29 positivity. Angiotensin converting enzyme, lysozyme, and repeat QFT test results were all within normal limits.
At 1-month follow-up visit in February 2020, the patient noted stable visual acuity, some improvement in floaters in both eyes, and persistent haziness predominantly OD. Visual acuity remained 20/20 OU. Intraocular pressure was 15 mm Hg OD and 8 mm Hg OS. Anterior segment exam was notable for 1+ flare OU. Dilated fundus exam was notable for 0.5+ cells and 0.5+ haze in vitreous OU. Overall examination findings of the posterior pole were stable Findings in SD-OCT and wide-angle FA were relatively unchanged since the first visit. Wide-angle indocyanine green chorioangiography (ICG) showed clear media, multiple hypocyanescent spots in the posterior pole and midperiphery in the intermediate phase, and hypocyanescent spots faded in the late phase OU (Figure 1D). Fundus autofluorescence showed hypofluorescence around the optic disc and retina.
The patient was diagnosed with BSCR based on HLA-A29 positivity, typical fundus appearance, and negative evaluation for other autoimmune and infectious etiologies. Given the patient’s active disease as evidenced by optic disc edema associated with vitreous cells in both eyes, along with highly suggestive FA and ICG findings, the patient was begun on therapy with infliximab at a dose of 7.5 mg/kg monthly. He was also given 3 days of intravenous (IV) methylprednisolone at a dose of 1000 mg/day followed by a tapering course of oral prednisone.
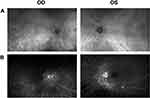
The patient did not come to follow-up visit until April 2021 and received eight cycles of 7.5 mg/kg infliximab infusions along with 1000 mg prednisone for 3 days during that period. VA declined to 20/40 OD due to posterior subcapsular cataract (PSC) formation and was 20/20 OS. No AC cell and flare were present in both eyes. Vitreous was hazy OD due to PSC and clear OS. Optic disc SD-OCT did not show significant changes. On FA, media was blurry at the center OD due to cataract formation. Partial optic disc staining was present in OU. There was no retinal vascular staining and capillary leakage in OU. Staining of peripapillary chorioretinal lesions in both the early and late frames of the FA remained the same OU (Figure 2). Four months later, the patient received additional four cycles of IFX infusions along with 1 day of 1000 mg methylprednisolone. VA decreased to 20/70 OD due to progression of PSC and was 20/20 OS. Ophthalmic examination, SD-OCT and FA findings remained unchanged. ICG was performed and hypocyanescent spots showed marked improvement with scattered few hypocyanescent spots at posterior pole (OS>OD) in both eyes on ICG (Figure 2). Electrophysiological testing including ffERG, mfERG, and VEP and visual field testing, including GVF and HVF, remained the same since the initial visit. Although there was significant improvement in ocular findings on examination and both in FA and ICG, mycophenolate mofetil 2000 mg daily was added to his treatment regimen due to the presence of optic disc edema and there remained a few hypocyanescent spots at the posterior pole in ICG.
Discussion
Generating a Diagnosis
We present a case of a 37-year-old male with bilateral BSCR diagnosed at age 34. We did not begin therapy immediately after the first visit, as our goal was to corroborate the correct diagnosis for the patient before beginning systemic treatment. The differential diagnosis for the patient’s condition on initial presentation included ocular sarcoidosis and masquerading syndrome, though less likely, among others. Infectious etiologies were less likely given the history of responsiveness to IMT and the time course of illness. Given the clinical presentation, in conjunction with HLA-A29 positivity and the characteristic hypopigmented ovoid chorioretinal lesions seen on fundus exam, the patient was diagnosed with BSCR.
How Does BSCR Develop?
The etiology of BSCR is thought to be related to autoimmunity, and HLA-A29 positivity has long been known to be a significant genetic risk factor.7 While only about 7% of the general population is HLA-A29 positive, between 80% and 98% of patients with BSCR show HLA-A29 positivity.8 Patients with symptoms suggestive of BSCR but with HLA-A29 negativity may have other disorders, such as Waldenstrom’s macroglobulinemia and common variable immune deficiency. Recent work9 has shown that the HLA-A29 gene is crucial in the pathophysiology of BSCR, including in transgenic mouse models.10 BSCR is also associated with IL-17, released from Th17 cells that play an important role in autoimmunity.11 Ongoing research is aimed at identifying different triggers of this autoimmune response.
The precise mechanism by which BSCR causes chorioretinopathy is not fully understood, and many theories exist. A high proportion of BSCR patients show in vitro responsiveness to retinal soluble antigen (S-Ag), suggesting that the pathophysiology of BSCR may be related to an autoimmune response to S-Ag.7 In another study, the biopsy of an eye with HLA-A29 positivity found many areas of lymphocytes at various levels of choroid and surrounding retinal blood vessels, suggesting that the disease may be primarily related to the choroid rather than the retina.12
How Does BSCR Present?
Initial symptoms of BSCR are typically decreased visual acuity, photopsias, and floaters. Patients may also exhibit decreased night vision, decreased color vision, light sensitivity, visual distortion, ocular pain, and loss of depth perception.6 The severity of these symptoms can vary from one individual to another. BSCR is a chronic disease with fluctuating symptoms and sometimes sudden flares. If left untreated, these flares can lead to macular edema and retinal atrophy that can lead to significant vision loss over time. Clinical manifestations of disease typically present symmetrically and bilaterally, though some patients show asymmetric patterns of disease.2
Retinal vascular leakage is often one of the first signs of BSCR, followed by hypopigmented ovoid birdshot lesions on a dilated fundus exam. Cystoid macular edema can present earlier but is usually a later finding and is uniquely more characteristic of BSCR than other types of uveitis; 84% of patients with BSCR show signs of cystoid macular edema compared to only 30% in other types of uveitis.13 BSCR is also characterized by retinal pigment epithelium changes, optic nerve atrophy, and subretinal neovascularization.14 While the disease is predominantly ocular, it has also been associated with systemic hypertension, mood disorders, hearing loss, and skin disorders including vitiligo and cancer.15
How is BSCR Diagnosed?
Unfortunately, the uncommon nature of BSCR occasionally leads to delays in clinical recognition, diagnosis, and treatment. A dilated fundus exam shows cream or orange oval-shaped chorioretinal lesions positioned in a “birdshot” pattern. These lesions may be absent on fundus exam but will be visible on ICG. Classification criteria for BSCR based on the standardization of uveitis nomenclature (SUN) working group are as follows: 1) characteristic bilateral multifocal choroiditis on ophthalmoscopy and 2) absent to mild anterior chamber inflammation and 3) absent to moderate vitritis or 4) multifocal choroiditis with positive HLA-A29 test and either characteristic “birdshot” spots on ophthalmoscopy or characteristic multifocal hypocyanescent spots on ICG without characteristic “birdshot” spots on ophthalmoscopy.16 Required diagnostic criteria, which were established in 2002, include ≥3 peripapillary birdshot lesions, <1+ anterior vitreous cells, <2+ vitreous haze, and bilateral ocular involvement.17 Supportive findings include HLA-A29 positivity, retinal vasculitis, and cystoid macular edema, though these are not required for diagnosis. Patients are not diagnosed with BSCR if they show signs of keratic precipitates or posterior synechiae, or if alternative causes for the disease are more likely (ie infectious, neoplastic, and inflammatory).17
Given that BSCR is uncommon, other causes of uveitis should be considered when evaluating a patient with characteristic symptoms. Patients should always be evaluated for syphilis (RPR test, FTS-ABS), sarcoidosis (angiotensin-converting enzyme, lysozyme, chest X-ray), and tuberculosis (purified protein derivative skin test, interferon-gamma release assays). Given that BSCR is strongly associated with HLA-A29 positivity, an HLA-A29 serology can also help to guide diagnosis.4
BSCR is typically monitored by clinical exam and multimodal imaging; however, subtle lesions can occasionally be missed on fundus exam, and fundus autofluorescence can help better identify areas of atrophy with hypoautofluorescence. FA will show hypofluorescent lesions with subtle late staining and is particularly useful for monitoring cystoid macular edema, optic disc leakage, and retinal vasculitis.18 ICG is most useful in diagnosis, especially in cases without obvious spots, as well as in the following disease.19 BSCR can also be monitored with electroretinogram, which shows prolonged 30Hz flicker implicit times and significantly reduced b-wave amplitudes.20 Visual field testing may show many foci of vision loss, including arcuate changes and blind spots. SD-OCT shows decreased reflectivity in macular photoreceptor bands and may show abnormal chorioretinal morphology and cystoid macular edema.
Current Treatments for BSCR
Few standardized treatment protocols for BSCR exist in part due to the uncommon nature of this disease. Currently, there is no cure for BSCR, but certain treatments aimed at suppressing inflammation may slow disease progression. Untreated patients often experience a progressive decline in visual function. Acute treatment for BSCR will involve high-dose steroids (>20mg/d) followed by a taper to control inflammatory changes in the eye. Oral steroids and intravitreal triamcinolone implants have both shown benefits in reducing cystoid macular edema. Systemic IMT (cyclosporine A, mycophenolate mofetil, azathioprine, methotrexate) is typically necessary, as BSCR is usually a disease with chronic activity.20 Biologics have been employed, typically in patients with inadequate response to conventional immunosuppressive therapy. The largest body of evidence exists for tumor necrosis factor (TNF)-alpha inhibitors, such as infliximab and adalimumab. Emerging therapies include biologics that inhibit IL-2 (daclizumab), IL-17 (secukinumab), and IL-6 (tocilizumab).21
Does BSCR in Adolescence and Young Adulthood Differ from BSCR in Late Adulthood?
We reviewed the literature for examples of patients who were diagnosed with BSCR before age 40 to assess how the clinical presentation and course of younger patients would compare to older patients. Table 1 summarizes the initial presentation, clinical course, and treatment of 24 patients identified in the literature who were diagnosed with BSCR before age 40. Studies that mentioned patients younger than 40 but did not describe sufficient clinical information about patients’ initial presentation, visual acuities, treatment, and clinical course were excluded. For example, a separate study22 that reported five cases of BSCR in children younger than age 16, but in which specific ages of individual patients and clinical course were not described, was not included in Table 1.
 |
Table 1 Case Report and Literature Review of Birdshot Chorioretinopathy Patients Younger Than Age 40 |
Of the 24 patients, 21 patients were HLA-A29-positive; the HLA-A29 status of the 3 patients in Ryan & Maumenee35 was not specified, but they all had the classic clinical presentation. Mean age at presentation was 33.9 years (median 35 years; range 17–39 years). There were 10 men and 14 women. Mean follow-up time where specified was 6.5 years (median 3 years; 0.2–32 years). Ten patients had ≥5 years of follow-up, and five patients had ≥10 years of follow-up. Five patients whose disease was refractory to first-line therapies who started treatment with tocilizumab generally had favorable outcomes with complete resolution of CME in one or both eyes.24–26 The complete details of the clinical course were not clearly described in some patients,7,28,30,31 and we were not able to determine the number of patients on specific types of treatments based on the information available. One patient received panretinal photocoagulation OD due to optic disc neovascularization and remained stable with visual acuity at 20/40 throughout 3 years of follow-up.33
Given the uncommon nature of BSCR, in conjunction with the fact that it is even less common in young populations, we do not have large enough sample sizes to conduct meaningful statistical analyses. However, based on our review, younger patients in general seem to present similarly to older patients. Aside from the 31-year-old in Trinh et al,29 36-year-old patient in Oh et al,32 38-year-old in Nussenblatt et al,7 and 35- and 36-year-old patients in Ryan & Maumenee,35 all patients generally had favorable visual outcomes, with final visual acuities better than about 20/40. Longer duration of follow-up seemed to correlate with decreased visual acuity; for example, the 31-year-old patient in Trinh et al followed for 32 years ended up with a visual acuity of 20/400 OD and 20/200 OS, and the 35- and 36-year-old patients in Ryan & Maumenee followed for 20 and 12 years, respectively, both ended up with visual acuities worse than 20/200 in both eyes. The youngest reported case of BSCR occurred in a 17-year-old female who was started on adalimumab and was recently published by Lee et al.23 While our analysis is limited by small sample size and confounding factors, these findings seem to indicate that longer total duration of disease may correlate with poorer visual outcomes, suggesting that it may be important to promptly recognize and treat patients with BSCR diagnosed at younger ages.
We noticed that patients with the worst prognoses were often in studies from the 1980s when treatment algorithms were considerably less advanced. For example, of the two patients with poor prognoses in Ryan & Maumenee,35 the 35-year-old patient received no treatment, with a visual acuity that worsened significantly from initial to final visit, and the 36-year-old patient received 400 rads of radiation to the posterior segment of both eyes. Their lack of proper treatment with immunomodulatory therapy for example, in conjunction with the long duration of disease, may have played a role in their poor final visual outcomes.
Conclusions
BSCR is an uncommon cause of posterior uveitis. It almost always affects individuals in middle to late adulthood but can also affect patients in early adulthood. Realizing that BSCR is not only limited to middle and late adulthood is important for proper diagnostic work-up, including assessing for HLA-A29 positivity, and management of atypical cases. Although the clinical features and treatment course of our case presented may not be particularly unique, our patient’s relatively young age for a diagnosis of BSCR highlights the importance of keeping a broad differential diagnosis in the appropriate clinical context. There may be benefit to prompt recognition and treatment of active inflammation to maintain vision in patients diagnosed with BSCR at younger ages.
Abbreviations
BSCR, Birdshot chorioretinopathy; CME, cystoid macular edema; ERM, epiretinal membrane; FA, fluorescein angiography; ffERG, full-field electroretinography; GVF, Goldmann visual fields; HLA, human leukocyte antigen; HVF, Humphrey visual fields; ICG, indocyanine green chorioangiography; IMT, immunomodulatory therapy; OD, right eye; OS, left eye; OU, both eyes; QFT, QuantiFERON-TB Gold; RPR, rapid plasma reagin; SD-OCT, Spectral domain optical coherence tomography; S-Ag, retinal soluble antigen.
Data Sharing Statement
The datasets used and/or analyzed in this study are available from the corresponding author upon reasonable request.
Consent for Publication
Informed consent to publish this case report has been obtained from the patient in writing. Institutional Board Review (IRB) approval was not required for the publication of this case report.
Acknowledgments
The authors acknowledge the medical providers at the Byers Eye Institute at Stanford for their contribution to the evaluation, diagnosis, and management of this patient.
Author Contributions
BHP and GU analyzed and interpreted the patient data. BHP wrote the manuscript with support from GU. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
Dr Quan Dong Nguyen reports grants from Acelyrin, Priovant, and Alumis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Franceschetti A, Babel J. Chorioretinitis With “Candle Spots”. a manifestation of Besnier-BoeckDisease. Ophthalmologica. 1949;118(4–5):701–710. doi:10.1159/000300769
2. Gasch AT, Smith JA, Withcup SM. Birdshot retinochoroidopathy. Br J Ophthalmol. 1999;83(2):241–249.
3. Rodriguez A, Calonge M, Pedroza-Seres M, et al. Referral patterns of uveitis in a tertiary eye care center. Arch Ophthalmol. 1996;114(5):593–599. doi:10.1001/archopht.1996.01100130585016
4. Minos E, Barry RJ, Southworth S, et al. Birdshot chorioretinopathy: current knowledge and new concepts in pathophysiology, diagnosis, monitoring and treatment. Text Orphanet J Rare Dis. 2016;11:61.
5. Jones NP. The Manchester uveitis clinic: the first 3000 patients--epidemiology and casemix. Ocul Immunol Inflamm. 2015;23(2):118–126. doi:10.3109/09273948.2013.855799
6. Shah KH, Yu F, Goldhardt R, et al. Birdshot chorioretinopathy. Surv Ophthalmol. 2005;50(6):519–541.
7. Nussenblatt RB, Ryan S, Green WR, Maumenee AE. Birdshot retinochoroidopathy associated with HLA-A29 antigen and immune responsiveness to retinal S-antigen. Am J Ophthalmol. 1982;94(2):147–158.
8. Kiss S, Stephen Foster C. Birdshot retinochoroidopathy. Int Ophthalmol Clin. 2020;46(2):39–55.
9. Donvito B, Monnet D, Tabary T, et al. Different HLA class IA region complotypes for HLA-A29.2 and -A29.1 antigens, identical in birdshot retinochoroidopathy patients or healthy individuals. Invest Ophthalmol Vis Sci. 2005;46(9):3227–3232. doi:10.1167/iovs.04-0858
10. Szpak Y, Vieville JC, Tabary T, et al. Spontaneous retinopathy in HLA-A29 transgenic mice. Proc Natl Acad Sci. 2001;98(5):2572–2576. doi:10.1073/pnas.051595998
11. Kuiper JJ, Mutis T, de Jager W, de Groot-Mijnes JD, Rothova A. Intraocular interleukin-17 and proinflammatory cytokines in HLA-A29-associated birdshot chorioretinopathy. Am J Ophthalmol. 2011;152(2):177–182. doi:10.1016/j.ajo.2011.01.031
12. Gaudio PA, Kaye DB, Crawford JB. Histopathology of birdshot retinochoroidopathy. Text Br J Ophthalmol. 2002;86(12):1439–1441. doi:10.1136/bjo.86.12.1439
13. Rothova A, Probst K, van Kooij B, Baarsma GS. Birdshot chorioretinopathy: long-term manifestations and visual prognosis. Ophthalmology. 2004;111(5):954–959.
14. Priem HA, Oosterhuis JA. Birdshot chorioretinopathy: clinical characteristics and evolution. Br J Ophthalmol. 1988;72(9):646–659. doi:10.1136/bjo.72.9.646
15. Pagnoux C, Mahr A, Aouba A, et al. Extraocular manifestations of birdshot chorioretinopathy in 118 French Patients. Presse Med. 2010;39(5):e97–e102. doi:10.1016/j.lpm.2009.12.005
16. Standardization of Uveitis Nomenclature Working G. Classification criteria for birdshot chorioretinitis. Am J Ophthalmol. 2021;228:65–71. doi:10.1016/j.ajo.2021.03.059
17. Levinson RD, Rothova A, Accorinti M, Holland GN. Research criteria for the diagnosis of birdshot chorioretinopathy: results of an international consensus conferences. Am J Ophthalmol. 2006;141(1):185–187.
18. Guex-Crosier Y, Herbort CP. Prolonged retinal arterio-venous circulation time by fluorescein but not by indocyanine green angiography in birdshot chorioretinopathy. Ocul Immunol Inflamm. 1997;5(3):203–206. doi:10.3109/09273949709116895
19. Fardeau C, Herbort CP, Kullmann N, Quentel G, LeHoang P. Indocyanine green angiography in birdshot chorioretinopathy. Ophthalmology. 1999;106(10):1928–1934. doi:10.1016/S0161-6420(99)90403-7
20. Mailhac A, Aptel F, Berthemy S, Bouillet L, Chiquet C. Five-year trends in multifocal electroretinogram for patients with birdshot chorioretinopathy. Am J Ophthalmol. 2019;200:138–149.
21. Menezo V, Taylor SR. Birdshot uveitis: current and emerging treatment options. Clin Ophthalmol. 2014;8:73–81. doi:10.2147/OPTH.S54832
22. Pivetti-Pezzi P. Uveitis in Children. Eur J Ophthalmol. 1996;2:6.
23. Lee J, Smith WM, Goldstein DA. Birdshot chorioretinopathy presenting in a teenager. Am J Ophthalmol Case Rep. 2020;19:100807. doi:10.1016/j.ajoc.2020.100807
24. Leclercq M, Le Besnerais M, Langlois V, et al. Tocilizumab for the treatment of birdshot uveitis that failed interferon alpha and anti-tumor necrosis factor-alpha therapy: two cases report and literature review. Clin Rheumatol. 2018;37(3):849–853. doi:10.1007/s10067-018-4007-4
25. Phasukkijwatana N, Iafe N, Sarraf D. Optical coherence tomography angiography of A29 birdshot chorioretinopathy complicated by retinal neovascularization. Retin Case Bri Rep. 2017;11(Suppl 1):S68–S72. doi:10.1097/ICB.0000000000000418
26. Calvo-Río V, Blanco R, Santos-Gómez M, et al. Efficacy of anti-IL6-receptor tocilizumab in refractory cystoid macular edema of birdshot retinochoroidopathy report of two cases and literature review. Ocul Immunol Inflamm. 2017;25(5):604–609. doi:10.1080/09273948.2016.1231331
27. Carlson RW, Theriault R, Schurman CM, et al. Phase II trial of anastrozole plus goserelin in the treatment of hormone receptor-positive, metastatic carcinoma of the breast in premenopausal women. J Clin Oncol. 2010;28(25):3917–3921. doi:10.1200/JCO.2009.24.9565
28. Keane PA, Allie M, Turner SJ, et al. Characterization of birdshot chorioretinopathy using extramacular enhanced depth optical coherence tomography. JAMA Ophthalmology. 2013;131(3):341–350. doi:10.1001/jamaophthalmol.2013.1724
29. Trinh L, Bodaghi B, Fardeau C, et al. Clinical features, treatment methods, and evolution of birdshot chorioretinopathy in 5 different families. Am J Ophthalmol. 2009;147(6):1042–1047. doi:10.1016/j.ajo.2008.12.035
30. Lim L, Harper A, Guymer R. Choroidal lesions preceding symptom onset in birdshot chorioretinopathy. Arch Ophthalmol. 2006;124(7):1057–1058. doi:10.1001/archopht.124.7.1057
31. Becker MD, Wertheim MS, Smith JR, Rosenbaum JT. Long-term follow-up of patients with birdshot retinochoroidopathy treated with systemic immunosuppression. Ocul Immunol Inflamm. 2005;13(4):289–293. doi:10.1080/09273940490912407
32. Oh KT, Christmas NJ, Folk JC. Birdshot retinochoroiditis: long term follow-up of a chronically progressive disease. Am J Ophthalmol. 2002;133(5):622–629. doi:10.1016/s0002-9394(02)01350-8
33. Martidis A, Duker JS, Puliafito CA. Intravitreal triamcinolone for refractory cystoid macular edema secondary to birdshot retinochoroidopathy. Archives of Ophthalmology. 2001;119(9):1380–1383.
34. Soubrane G, Bokobza R, Coscas G. Late developing lesions in birdshot retinochoroidopathy. Am J Ophthalmol. 1990;109(2):204–210. doi:10.1016/s0002-9394(14)75988-4
35. Ryan SJ, Maumenee AE. Birdshot retinochoroidopathy. Am J Ophthalmol. 1980;89(1):31–45. doi:10.1016/0002-9394(80)90226-3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.