Back to Journals » Clinical Ophthalmology » Volume 17
Biosimilar versus InnovAtor MoLecule of RAnibizumab in Neovascular Age-Related MaCular DEgeneration (The BALANCE Trial): Real-World Evidence
Authors Chakraborty D , Mondal S, Boral S, Das A, Sinha TK, Majumdar S, Bhattacharya R, Maitra R
Received 17 February 2023
Accepted for publication 28 March 2023
Published 8 April 2023 Volume 2023:17 Pages 1067—1076
DOI https://doi.org/10.2147/OPTH.S407219
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Debdulal Chakraborty,1 Soumen Mondal,1 Subhendu Boral,1 Arnab Das,1 Tushar Kanti Sinha,1 Saptorshi Majumdar,1 Ranabir Bhattacharya,2 Ritobroto Maitra3
1Department of Vitreoretinal Services, Disha Eye Hospitals, Kolkata, West Bengal, India; 2Department of MIS, Disha Eye Hospitals, Kolkata, West Bengal, India; 3Radical Health Tech, New Delhi, India
Correspondence: Debdulal Chakraborty, Department of Vitreoretinal services, Disha Eye Hospitals, Kolkata, West Bengal, India, Tel +91 33 66360000, Email [email protected]
Purpose: To analyse outcomes of innovator ranibizumab (IRM) (Lucentis) and biosimilar ranibizumab (BRM) (Razumab) in Indian eyes with neovascular age-related macular degeneration (nAMD).
Methods: Retrospective observational study in nAMD patients, who were treated with IRM or BRM (3 loading doses followed by pro re nata (PRN). Primary outcome measures were change in best corrected visual acuity (BCVA) and central macular thickness (CMT) along with safety analysis. Secondary outcomes measures were changes in the subretinal fluid (SRF) and intraretinal fluid (IRF).
Results: Inclusion criteria were satisfied in 164 eyes (60.74%). A total of 87 eyes were treated with IRM, and 77 eyes received BRM. Baseline BCVA was 0.57± 0.27 logMAR in IRM group and 0.61± 0.25 in the BRM group. At 3, 6, 9, and 12 months BCVA was 0.27± 0.22 (p< 0.0001), 0.34± 0.23 (p< 0.0001), 0.39± 0.25 (p< 0.0001), and 12 months 0.41± 0.23 (p< 0.0001) in the IRM group and 0.24± 0.16 (p< 0.0001), 0.27± 0.16 (p< 0.0001), 0.34± 0.17 (p< 0.0001), 0.38± 0.18 (p< 0.0001) in the BRM group. Baseline CMT was 420.39± 54.45 μm in IRM group and 407.82± 53.07 μm in BRM group. At 3, 6, 9, and 12 months, CMT decreased to 258.28± 20.4 μm (p< 0.0001), 268.38± 19.5 μm (p< 0.0001), 269.51± 32.41 μm (p< 0.0001), and 278.28± 16.56 μm (p< 0.0001) in the IRM group and 258.84± 17.47 μm (p< 0.0001), 265.69± 17.29 μm (p< 0.0001), 273.64± 23.13 μm (p< 0.0001), and 283.09± 19.66 μm (p< 0.0001) in the BRM group. Similar improvements in IRF and SRF levels in the patients were noted in both groups. Required number of doses of IRM and BRM was similar over the 12 month period in both groups. A similar profile of adverse events was noted in both the groups.
Conclusion: Intravitreal injection of IRM and BRM show similar efficacy and safety in Indian eyes with nAMD.
Keywords: neovascular age-related macular degeneration, anti-VEGF, ranibizumab, biosimilar ranibizumab
Introduction
Neovascular age-related macular degeneration (nAMD) is a major cause of loss of vision among people above fifty, with the prevalence constantly increasing with age.1 It is a progressive degenerative disease resulting in central vision impairment.2–4 nAMD is today a global problem that affects people across different countries and diverse ethnicities.5,6 It has been estimated that the number of nAMD-afflicted patients will be 288 million by 2040.7
Angiogenesis, induced by vascular endothelial growth factor (VEGF), plays a prominent role in the pathogenesis of nAMD.8 Therefore, treatment with anti-VEGF molecules is currently the preferred therapeutic mode against nAMD.9 Introduction of anti-VEGF drugs has resulted in significant improvement in nAMD prognosis over the years. Application of anti-VEGFs has significantly reduced almost certain blindness and has resulted in improved visual acuity in nAMD patients.10 Ranibizumab is one of the most effective anti-VEGF drugs that has been approved for nAMD treatment.11 This fully humanized monoclonal antibody fragment functions by binding to different VEGF-A isoforms.12 Multiple randomized controlled trials and real-world evidence have proven the ability of ranibizumab to significantly improve visual acuity in nAMD patients, and prevent loss of vision.13–16
A major obstacle to the widespread public use of innovative biological medicines such as ranibizumab is their high cost. Added to this, the fact that nAMD is a lifelong disease necessitates intravitreal ranibizumab injections at regular intervals, further enhancing the treatment cost.17 Despite the availability of ranibizumab at reduced rates in India, many patients still consider the drug unaffordable.17,18 Although bevacizumab is a cheaper off-label drug that can be used for retina disorders, the necessity to inject multiple patients from a single vial, the non-availability of compounding pharmacies for aliquoting, and its association with cluster endophthalmitis have been constant threats to its free use.19 Therefore, it is necessary to develop cheaper substitutes for ranibizumab other than bevacizumab.
In recent times, biosimilar compounds have been considered promising alternatives to expensive biological medicines.20,21 Biosimilar compounds are molecules that are very similar to an original biological product regarding clinical efficacy, immunogenicity, and biosafety. However, biosimilar compounds can have minute variations from the original compound, and safety and effectiveness can be reasons for concern. A safe and effective biosimilar compound can help treat the disease effectively, and also decrease the overall treatment cost for diseases such as nAMD. At present, biosimilar compounds for several therapeutic monoclonal antibodies are available in the market for treating various diseases.22
Razumab (INTAS pharmaceuticals, Gujarat, India) is a biosimilar ranibizumab.23 In 2015, Razumab was first approved for treating retinal vascular diseases in India.24 Its effectiveness in such disorders has so far been found satisfactory, and, in India alone, more than 100,000 intravitreal injections of Razumab have been done.25 The clinical effectiveness of Razumab has already been demonstrated in the treatment of different retinal vascular diseases.20,26–30 Our group has published the comparable efficacy of Razumab to innovator ranibizumab in the treatment of diabetic macular edema (DME).31 However, to the best of our knowledge, there are no comparative studies showing the efficacy of this biosimilar (BRM) with the innovator ranibizumab (IRM) molecule with up to 1-year follow-up.
Ours is a network of tertiary eye care centers and teaching hospitals in eastern India, and we cater to more than a million patients annually. We have been increasingly adopting the BRM in our institution over the past 7 years as confidence in this molecule grows; currently, more than half of all our patients receive BRM. In this study, we present a retrospective analysis of outcomes of BRM compared to IRM in eyes with nAMD in an Indian population.
Methods
This multicenter observational study was done at a group of eye hospitals in eastern India. The study adhered to the tenets of the Declaration of Helsinki, Good Clinical Practice guidelines, and International Council for Harmonization. Our protocol was approved by the Institutional Review Board of Disha Eye Hospitals (Regn Number ECR/846/Inst/WB/2016/RR-19: EC-CT-2020-138). Informed consent was obtained from all the participating patients who underwent intravitreal injections. We collected the data from 4 centers within our hospital network, and the patients were treated by 10 fellowship-trained retina specialists.
Participants were included if they were ≥50 years of age and had a previously untreated subfoveal choroidal neovascularisation (CNV) lesion secondary to nAMD in the study eye with evidence of activity as documented on OCT by the presence of subretinal fluid or intraretinal fluid, leakage from CNV detected by fluorescein angiography (FA), and having a baseline visual acuity of 20/40 or worse. They underwent intravitreal injection of IRM or BRM for the first time (treatment-naïve) across our hospital network between March 2021 and October 2021 and were followed up for twelve months from the day of first injection. Only patients that had received a loading dose of 3 injections and subsequently at least three injections in the PRN phase were entered into a datasheet.
Relevant information, including demographic data, was retrieved from the centralized electronic medical records system. Selected patients were followed up every month for a total period of twelve months starting from the initial injection. Patients with other vision-impairing diseases such as glaucoma or cataract, history of retinal laser within 6 months prior to the first injection, other co-existing retinal disease, and vitreoretinal surgery were excluded from the study. A flowchart of the selection process is shown in Figure 1.
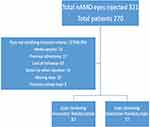 |
Figure 1 Flowchart of the selection process. |
Patient data such as demographics, BCVA (recorded in Snellen’s chart and converted to logMAR), intraocular pressure, fundus evaluation, and lens status as assessed by slit lamp examination were recorded. Optical coherence tomography (OCT) characteristics such as central macular thickness (CMT) and fluid status were noted using Spectralis OCT (Heidelberg, Germany). During each visit, we recorded the automated CMT display from scans with a minimum 50% image quality. Also, when available, we recorded the fundus fluorescein angiography findings from case files. Indocyanine green angiography (ICGA) could not be performed in all patients due to non-availability of equipment at some centers.
The selected patients were provided with all information on the two drugs IRM (Lucentis, Novartis, India) and BRM (Razumab, Intas Pharmaceuticals, India). We assigned the injections according to the choice and the affordability of the of the patient using standard procedure for intravitreal injections. Following our institutional protocol, after a loading dose of 3 intravitreal injections of IRM/BRM, we used a pro re nata (PRN) treatment regimen, in which the patients underwent monthly evaluations for a year. We advised repeat injections at any point during the evaluation period, if there was persistence or increase of subretinal or intraretinal fluid (SRF/IRF), the central macular thickness (CMT) was ≥300 μm or more, or there was increase of ≥100 μm of CMT. The primary outcome measure between IRM and BRM treatment was the change in visual acuity at month 3 (ie after 3 loading doses) and at 6, 9, and 12 month time points. Reduction of CMT, decrease in IRF and SRF levels, and the number of injections required to achieve clinical improvement were considered secondary outcome measures of the treatment.
Statistical variables were expressed as mean along with standard deviation, medians along with interquartile range (IQR), and number (%) for categorical data. Demographic characteristics of the two treatment groups were compared by the Wilcoxon rank-sum test and the chi-square test for equality of proportions (for categorical data). We analyzed differences in visual acuity and CMT at different time points for each treatment group by the Wilcoxon sign test. The improvements in visual acuity and CMT at different time points were compared between the two treatment groups by the Wilcoxon rank-sum test. McNemar’s chi-square test was used to analyze the differences in IRF and SRF levels (present/absent) between different time points for each treatment group, and IRF and SRF level improvement between the two treatment groups was analyzed by the chi-square test for equality of proportions. The baseline parameters of the two treatment groups were compared using the Wilcoxon rank-sum test. The number of doses of IRM and BRM used in the study were compared by the chi-square goodness of fit test.
We maintained all the data in Microsoft Excel and did the statistical analysis using the R statistical package version 3.5.3. We considered all p-values less than 0.05 as statistically significant.
Results
Out of a possible 321 eyes of 270 patients that received either the IRM or BRM during the study period, inclusion criteria were satisfied by 164 eyes (60.74%). Within this cohort, 87 eyes were treated with IRM and 77 eyes received BRM (Figure 1). The two groups did not have any statistically significant difference regarding demographic characteristics and baseline parameters such as BCVA, CMT, and the presence of intraretinal fluid (IRF) and subretinal fluid (SRF) (Table 1).
 |
Table 1 Demographic and Baseline Characteristics of Patients |
Both IRM and BRM treatments resulted in significant improvements in the BCVA of the patients. Compared to the baseline (0.57±0.27 logMAR), IRM-treated patients had significant improvements in BCVA after 3 months (0.27±0.22; p<0.0001), 6 months (0.34±0.23; p<0.0001), 9 months (0.39±0.25; p<0.0001), and 12 months (0.41±0.23; p<0.0001). BRM treatment also resulted in significant improvements in BCVA at 3 months (0.24±0.16; p<0.0001), 6 months (0.27±0.16; p<0.0001), 9 months (0.34±0.17; p<0.0001), and 12 months (0.38±0.18; p<0.0001) compared to the baseline (0.61±0.25). Both the drugs resulted in similar improvements in the visual acuity of the nAMD patients (Figure 2A and B). Comparative analysis between the two groups revealed no significant difference at any of the time points (Table 2).
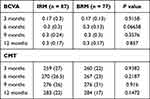 |
Table 2 Improvement in BCVA and CMT of Patients After Treatment |
 |
Figure 2 (A) Box plot showing Baseline and Treatment Period Visual Acuity (BCVA) for IRM. (B) Box plot showing Baseline and Treatment Period Visual Acuity (BCVA) for BRM. |
Both IRM and BRM injections also resulted in significant improvements in CMT among the nAMD patients. Compared to the baseline (420.39±54.45 μm), IRM injection led to significant reduction in CMT after 3 months (258.28±20.4 μm; p<0.0001), 6 months (268.38±19.5 μm; p<0.0001), 9 months (269.51±32.41 μm; p<0.0001), and 12 months (278.28±16.56 μm; p<0.0001). On the other hand, BRM treatment also reduced the CMT significantly after 3 months (258.84±17.47 μm; p<0.0001), 6 months (265.69±17.29 μm; p<0.0001), 9 months (273.64±23.13 μm; p<0.0001), and 12 months (283.09±19.66 μm; p<0.0001) compared to the baseline (407.82±53.07 μm; p<0.0001). Similar to BCVA, no significant difference was observed in CMT improvement between the two drugs (Figure 2A and B) (Table 2) at any of the time points. The baseline and treatment period CMT for the IRM and BRM group are presented in Figure 3A and B. No significant differences were observed between the two treatment groups with respect to improvements in IRF and SRF levels in the patients at any of the time points (Table 3).
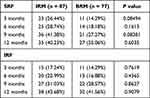 |
Table 3 SRF and IRF Levels in Patients After Treatment |
 |
Figure 3 (A) Box plot showing Baseline and Treatment Period CMT for IRM. (B) Box plot showing Baseline and Treatment Period CMT for BRM. |
Moreover, no significant variation was observed between the required number of doses of IRM and BRM over the period of 12 months (Table 4).
 |
Table 4 Distribution of IRM and BRM Doses During 12 Months Study |
Thus, we observed similar patterns of overall improvement in the condition of nAMD patients treated with either the innovator ranibizumab or its biosimilar derivative. No adverse side effects such as intraocular inflammation and endophthalmitis were found in any of the patients in this study. In the follow-up period, no drug-induced systemic adverse effects such as myocardial infarction, thrombosis, or stroke were observed.
Safety Analysis
The mean IOP at baseline was similar to the mean IOP after the last injection of each molecule (IRM: 14.92±3.27 mmHg vs 14.68±3.28 mmHg; p=0.81) (BRM: 14.45±3.42 vs 14.29±3.36 mmHg; p=0.43); (p=0.8). Non-serious adverse events (nSAE) such as mild ocular pain, burning sensation, subconjunctival hemorrhage, and watering were noted in both groups. Retinal pigment epithelial tear (RPE Tear) was noted in one eye in each group. Anterior chamber inflammation (cells/flare) and/or posterior chamber inflammation (vitritis/snow banking/snow balls/retinitis/retinal vasculitis) were not detected in any of the eyes in both the groups during any of the visits. No serious systemic adverse events were noted in any of the patients in each group. One eye in the IRM group had vitreous hemorrhage following injection which resolved conservatively. Table 5 summarizes the safety data of the study population.
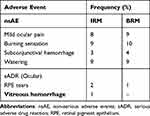 |
Table 5 Adverse Events |
Discussion
In this study, we compared the efficacy of innovator ranibizumab (IRM) (Lucentis) and a biosimilar ranibizumab (BRM) (Razumab) in the treatment of nAMD in Indian patients. We found the biosimilar drug to produce a similar pattern of improvement in the visual and anatomical outcomes as the innovator ranibizumab in nAMD patients, with comparative analysis between the two groups revealing no significant difference at any of the time points. For both drugs, the maximum improvement in visual acuity was observed at three months perhaps due to the loading dose of 3 monthly injections. Improvement of BCVA compared to baseline was maintained through 52 weeks, with both groups showing a similar pattern of improvement. Amongst the secondary treatment outcomes, significant improvements of CMT was noted in both groups at all time points. Both fluid compartments (SRF and IRF) showed similar improvement starting from the 3rd month after the initial three loading doses. The comparable efficacy of IRM to that of BRM is further asserted by similar number of injections required by the two drugs over the same period to achieve similar clinical improvement.
While a fixed or a treat-and-extend regimen can result in better anatomical and morphological improvement than a PRN regimen for the treatment of nAMD,32 in resource-poor settings a PRN regimen with strict follow-up can provide comparable results with much reduced expenses.20,33 A further reduction in the expenses can be brought about with the use of biosimilar ranibizumab.20 In real-life situations the burden of repeated injections may make it difficult to sustain treatments with biologics for long periods of time, more so with the higher cost of the innovator drug. Apart from the cost of treatment, several other factors such as the necessity of multiple hospital visits, availability of caregivers, low levels of basic education, lack of sufficient knowledge regarding disease and treatment, etc. have been found to be contributing factors toward patient non-compliance in developing countries.31,34 Non-adherence to treatment can significantly affect the outcome of anti-VEGF therapy in retinal vascular diseases, leading to inferior treatment outcomes in real-life scenarios compared to that from randomized clinical trials.35 A comparative study by Ehlken et al revealed that appropriate adherence to treatment and follow-up regimens results in better maintenance of visual acuity in DME or nAMD patients.36 To avoid non-adherence to treatment, we adopted the PRN protocol following the initial 3 loading doses with a strict monthly follow-up to ensure good disease control as well as to reduce the requirement of injections. Although the sample size in the present study is not very big, the strict inclusion criteria of patients ensured matching demographics and baseline parameters in each treatment group, allowing a fair comparison of the performances of the innovator and biosimilar ranibizumab in treating nAMD.
Our results are in concordance with other short-term studies of the recent past. Ratra et al, in their analysis, observed that the efficacy of biosimilar ranibizumab was comparable to that of innovator ranibizumab in retinal vascular disease.21 A significant improvement in patient condition was observed after the initial 3 loading doses, and the improvement persisted in the later follow-ups in their study. Sharma et al, in their pooled data retrospective study also observed significant and sustained improvement in BCVA after treatment with biosimilar ranibizumab.26 The BIRA study also revealed similar efficacy of both agents in the treatment of nAMD.28
Although anti-VEGF agents like ranibizumab have revolutionized the treatment outcomes of vision-threatening diseases such as nAMD, their high cost poses a significant hindrance to patient treatment in developing countries. Kelkar et al reported the cost of treatment to be an important factor for discontinuation of treatment and non-compliance in patients with retinal vascular disease.17 They reported 51.5% loss of follow-ups among nAMD and DME patients treated with intravitreal anti-VEGFs.17 In India, the problem of high treatment costs is often aggravated by the absence of healthcare benefits and insurance among patients. Therefore, lowering the price of the required medicines is believed to improve patient compliance as it would decrease the overall treatment cost. A major purpose of developing biosimilar drugs is to provide treatment at cheaper rates without compromising the treatment quality. The World Health Organization (WHO) has defined biosimilar drugs as biotechnological products with comparable quality as well as clinical and nonclinical evaluation with an approved reference product.36 In India, the biosimilar ranibizumab is about 30–40% less expensive than the innovator ranibizumab.37 Hence, biosimilar ranibizumab is gradually becoming popular in the country. Bevacizumab, another anti-VEGF monoclonal antibody, is often used in the treatment of retinal vascular disease. However, bevacizumab, originally developed as an anti-angiogenic in the treatment of solid tumors, is an off-label medication in retinal vascular disease.38 However, bevacizumab continues to be popular in treating nAMD due to its low cost. The availability of bevacizumab in a multi-dose vial has given rise to problems in aliquoting and storing bevacizumab, resulting in preventing its unrestricted use, as has been noted by the Vitreoretinal Society of India.39 While biosimilar ranibizumab can cut treatment costs to a great extent, its availability in single-dose vials offers the advantage of minimal chance of contamination during usage. These together have been instrumental in bringing about a gradual shift from the off-label use of bevacizumab to biosimilar ranibizumab in India.24
Ocular and systemic safety are always of concern when anti-VEGF therapy is being administered to the eye. The risk of endophthalmitis due to anti-VEGF injections in the eye has been reported as 0.35%.22 Previously, sterile endophthalmitis has been reported with BRM (Razumab).23 However, in a pooled safety analysis of 9406 BRM injections our group noted the incidence of infectious endophthalmitis as 0.01% and that of non-infectious vitritis as 0.02%,25 which is similar to what has been reported with other anti-VEGF agents in the literature.22 Arterial thromboembolic events were seen in 2.7% of patients in ANCHOR and 3.8% in MARINA,13,15 though a meta-analysis of real-world studies found an incidence of only 0.6%.40 The real-world data of BRM injections have reported the incidence of non-fatal myocardial infarction as 0.12% and that of non-fatal cerebrovascular accident as 0.09%.25 In the current study the safety profile of BRM (Razumab) appears to be consistent with the known ranibizumab profile; no new safety concerns were identified during the study period. It must be mentioned, however, that the current study was not powered to comprehensively analyze safety. Also immunogenicity of BRM (Razumab) in this study was not evaluated. The ASSET study, however, looked into the immunogenicity of Razumab and noted no difference with the innovator molecule.27
Biosimilar compounds are different from generic drugs because subtle structural differences may exist between the original biologic drug and its biosimilar.37 Such minor variations can arise in the monoclonal antibody structure due to its production inside living cells. Hence, the possibility of drug-related adverse events is there. However, biosimilar drug manufacturing occurs after thorough analytical studies on drug safety. For approval by the drug control authorities, biosimilar drugs must pass through several layers of clinical studies that investigate their safety and immunogenicity,41 thereby ensuring the safe use of these agents. Our group published the largest set of data on the safety of Razumab in 9406 eyes.25 There have been other studies with small cohorts and shorter periods evaluating the safety and efficacy of BRM (Razumab) in nAMD demonstrating efficacy and safety.27,30,42 The Bira study by Sharma et al had a follow-up of 6 months unlike the current study which had a follow-up of 1 year following the start of the first injection in both groups. Similar to other previous studies on biosimilar ranibizumab, in our current study we did not observe any serious adverse effects of the drug in patients at any point in time. The availability of biosimilars has added to our armamentarium of anti-VEGF drugs and enabled us to offer re-treatments at reduced costs.37 However, the lack of comparative studies with a significant period of follow-up with the innovator molecule has led to “nocebo effect” described by Sharma et al regarding the wider use of biosimilar ranibizumab. To that extent this current study will help provide additional real-world data on safety and efficacy. Given the financial constraints of long-term treatment with anti VEGF agents, the use of biosimilars with a good safety and efficacy profile, along with a PRN or observe-and-treat strategy, following 3 loading doses, strict follow-up, and timely reinjections can help minimize the number of injections without losing treatment benefits in eyes with nAMD in resource-poor settings. FDA approval of SB11, a biosimilar ranibizumab, is perhaps the beginning of a new paradigm in the use of biosimilar ranibizumab in the treatment of retinal vascular disease in general and nAMD in particular.43
The limitations of our study include its retrospective design and PRN protocol after 3 loading doses, a relatively small data set with which it may be difficult to comment on adverse events. All eyes did not undergo ICGA, and this can be considered a study limitation. However, it does give an insight into the efficacy of biosimilar ranibizumab (Razumab) compared to the innovator molecule in the treatment of nAMD. Long-term, prospective studies will enable us to better understand the efficacy and safety of BRM in nAMD management.
Conclusion
The present study has shown that BRM (Razumab) can improve visual acuity and reduce central macular thickness in patients of nAMD over a one-year period with an efficacy similar to that of innovator ranibizumab (Lucentis). Thus, intravitreal injection of BRM (Razumab) in a loading dose followed by a PRN protocol can be a viable strategy for treating nAMD. As biosimilar ranibizumab is much cheaper than the innovator product, its adoption on a larger scale can ensure better utilization of resources in the treatment of retinal and ophthalmic disease in the long run without compromising the quality of visual and anatomic outcomes in patients.
Consent to Participate
The authors confirm that all research participants provided informed consent for involvement in this study.
Acknowledgments
Dr Debasish Bhattacharya MS, Chairman, Disha Eye Hospitals, Kolkata, India
Disclosure
The authors report no conflicts of interest in this work.
References
1. Veritti D, Sarao V, Lanzetta P. Neovascular age-related macular degeneration. Ophthalmologica. 2012;227(Suppl 1):11–20. doi:10.1159/000337154
2. Cheung LK, Eaton A. Age-related macular degeneration. Pharmacotherapy. 2013;33(8):838–855. doi:10.1002/phar.1264
3. Gheorghe A, Mahdi L, Musat O. Age-related macular degeneration. Rom J Ophthalmol. 2015;59(2):74–77.
4. Handa JT, Verzijl N, Matsunaga H, et al. Increase in the advanced glycation end product pentosidine in Bruch’s membrane with age. Invest Ophthalmol Vis Sci. 1999;40(3):775–779.
5. Klein R, Klein BE, Cruickshanks KJ. The prevalence of age-related maculopathy by geographic region and ethnicity. Prog Retin Eye Res. 1999;18(3):371–389. doi:10.1016/s1350-9462(98)00025-1
6. Vingerling JR, Dielemans I, Hofman A, et al. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology. 1995;102(2):205–210. doi:10.1016/s0161-6420(95)31034-2
7. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi:10.1016/S2214-109X(13)70145-1
8. Barchitta M, Maugeri A. Association between vascular endothelial growth factor polymorphisms and age-related macular degeneration: an updated meta-analysis. Dis Markers. 2016;2016:8486406. doi:10.1155/2016/8486406
9. Veritti D, Sarao V, Soppelsa V, Danese C, Chhablani J, Lanzetta P. Managing neovascular age-related macular degeneration in clinical practice: systematic review, meta-analysis, and meta-regression. J Clin Med. 2022;11(2):325. doi:10.3390/jcm11020325
10. Ricci F, Bandello F, Navarra P, Staurenghi G, Stumpp M, Zarbin M. Neovascular age-related macular degeneration: therapeutic management and new-upcoming approaches. Int J Mol Sci. 2020;21(21):8242. doi:10.3390/ijms21218242
11. Schmidt-Erfurth U, Chong V, Loewenstein A, et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol. 2014;98(9):1144–1167. doi:10.1136/bjophthalmol-2014-305702
12. Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26(8):859–870. doi:10.1097/01.iae.0000242842.14624.e7
13. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi:10.1056/NEJMoa054481
14. Davis J, Olsen TW, Stewart M, Sternberg P Jr. How the comparison of age-related macular degeneration treatments trial results will impact clinical care. Am J Ophthalmol. 2011;152(4):509–514. doi:10.1016/j.ajo.2011.07.004
15. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi:10.1056/NEJMoa062655
16. Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 1. Am J Ophthalmol. 2008;145(2):239–248. doi:10.1016/j.ajo.2007.10.004
17. Kelkar A, Webers C, Shetty R, et al. Factors affecting compliance to intravitreal anti-vascular endothelial growth factor therapy in Indian patients with retinal vein occlusion, age-related macular degeneration, and diabetic macular edema. Indian J Ophthalmol. 2020;68(10):2143–2147. doi:10.4103/ijo.IJO_1866_19
18. Sengupta S. Current perspectives on use of anti-vascular endothelial growth factor agents for retinal disorders. Indian J Ophthalmol. 2021;69(2):209–210. doi:10.4103/ijo.IJO_72_21
19. Stewart MW, Narayanan R, Gupta V, Rosenfeld PJ, Martin DF, Chakravarthy U. Counterfeit avastin in India: punish the criminals, not the patients. Am J Ophthalmol. 2016;170:228–231. doi:10.1016/j.ajo.2016.05.023
20. Verma L, Thulasidas M, Purohit A, Gupta A, Narula R, Talwar D. Clinical efficacy and safety of Razumab® (CESAR) study: our experience with the world’s first biosimilar ranibizumab. Indian J Ophthalmol. 2021;69(2):347–351. doi:10.4103/ijo.IJO_2516_20
21. Ratra D, Roy K, Giridhar S, Madaan S; Sankara Nethralaya Vitreoretinal Study Group. Comparison between ranibizumab biosimilar, innovator ranibizumab and bevacizumab in a real-world situation. Ophthalmol Ther. 2022;11(1):135–149. doi:10.1007/s40123-021-00416-4
22. Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs. 2015;7(1):9–14. doi:10.4161/19420862.2015.989042
23. Griaud F, Winter A, Denefeld B, et al. Identification of multiple serine to asparagine sequence variation sites in an intended copy product of LUCENTIS® by mass spectrometry. MAbs. 2017;9(8):1337–1348. doi:10.1080/19420862.2017.1366395
24. Sheth JU, Stewart MW, Khatri M, et al. Changing trends in the use of anti-vascular endothelial growth factor (anti-VEGF) biosimilars: insights from the vitreoretinal society of India biosimilars of anti-VEGF survey. Indian J Ophthalmol. 2021;69(2):352–356. doi:10.4103/ijo.IJO_2703_20
25. Chakraborty D, Stewart MW, Sheth JU, et al. Real-world safety outcomes of intravitreal ranibizumab biosimilar (Razumab) therapy for chorioretinal diseases. Ophthalmol Ther. 2021;10(2):337–348. doi:10.1007/s40123-021-00345-2
26. Sharma S, Khan MA, Chaturvedi A; RE-ENACT Study Investigators Group. Real-life clinical effectiveness of razumab® (the world’s first biosimilar of ranibizumab) in retinal vein occlusion: a subgroup analysis of the pooled retrospective RE-ENACT study. Ophthalmologica. 2019;241(1):24–31. doi:10.1159/000488602
27. Sharma S, Gupta V, Maiti A, et al. Safety and efficacy of Razumab™ (world’s first biosimilar ranibizumab) in wet age-related macular degeneration: a post-marketing, prospective ASSET study. Int J Retin Vitr. 2021;7(1):24. doi:10.1186/s40942-021-00293-w
28. Sharma A, Kumar N, Parachuri N, Bandello F, Kuppermann BD, Loewenstein A. Ranibizumab biosimilar (Razumab) vs innovator ranibizumab (Lucentis) in neovascular age-related macular degeneration (n-AMD)- efficacy and safety (BIRA study). Eye. 2022;36(5):1106–1107. doi:10.1038/s41433-021-01616-9
29. Sameera VV, Apoorva AG, Joshi S, Guruprasad AS. Safety and efficacy of Razumab - the new biosimilar in India: our experience. Kerala J Ophthalmol. 2016;28:180. doi:10.4103/kjo.kjo_18_17
30. Sharma S, Khan MA, Chaturvedi A; RE-ENACT Study Investigators Group. Real life clinical effectiveness of Razumab® (world’s first biosimilar ranibizumab) in wet age-related macular degeneration: a subgroup analysis of pooled retrospective RE-ENACT study. Int J Ophthalmol Eye Sci. 2018;6:368–373.
31. Chakraborty D, Sengupta S, Mondal S, et al. Comparison of innovator vs. biosimilar ranibizumab in treating diabetic macular edema: a multicenter retrospective study. Ophthalmol Ther. 2022;11(2):629–638. doi:10.1007/s40123-022-00463-5
32. Hatz K, Prünte C. Treat and extend versus pro re nata regimens of ranibizumab in neovascular age-related macular degeneration: a comparative 12 month study. Acta Ophthalmol. 2017;95(1):e67–e72. doi:10.1111/aos.13031
33. Narayanan R, Kelkar A, Abbas Z, et al. Sub-optimal gain in vision in retinal vein occlusion due to under-treatment in the real world: results from an open-label prospective study of intravitreal ranibizumab. BMC Ophthalmol. 2021;21(1):33. doi:10.1186/s12886-020-01757-7
34. Weiss M, Sim D, Herold T, Et A. Compliance and adherence of patients with diabetic macular edema o intravitreal anti-vascular endothelial growth factor therapy in daily practice. Retina. 2018;38(12):2293–2300. doi:10.1097/Iae.0000000000001892
35. Ehlken C, Ziemssen F, Eter N, et al. Systematic review: non-adherence and non-persistence in intravitreal treatment. Graefes Arch Clin Exp Ophthalmol. 2020;258(10):2077–2090. doi:10.1007/s00417-020-04798-2
36. Ehlken C, Helms M, Böhringer D, Agostini HT, Stahl A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol. 2017;12:13–20. doi:10.2147/OPTH.S151611
37. Sharma A, Reddy P, Kuppermann BD, Bandello F, Lowenstein A. Biosimilars in ophthalmology: “Is there a big change on the horizon?”. Clin Ophthalmol. 2018;12:2137–2143. doi:10.2147/OPTH.S180393
38. Grisanti S, Ziemssen F. Bevacizumab: off-label use in ophthalmology. Indian J Ophthalmol. 2007;55(6):417–420. doi:10.4103/0301-4738.36474
39. Available from: https://vrsi.in/wp-content/uploads/2018/02/Avastin_Guidlines_Book.pdf.
40. Kim LN, Mehta H, Barthelmes D, Nguyen V, Gillies MC. Meta-analysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina. 2016;36(8):1418–1431. doi:10.1097/IAE.0000000000001142
41. Sharma A, Kumar N, Kuppermann BD, Bandello F, Loewenstein A. Understanding biosimilars and its regulatory aspects across the globe: an ophthalmology perspective. Br J Ophthalmol. 2020;104(1):2–7. doi:10.1136/bjophthalmol-2019-314443
42. Sharma A, Hafeez Faridi M, Kumar N, et al. Immunogenicity and efficacy after switching from original ranibizumab to a ranibizumab biosimilar: real-world data. Eye. 2020;34(6):1008–1009. doi:10.1038/s41433-019-0745-z
43. Bressler NM, Veith M, Hamouz J, et al. Biosimilar SB11 versus reference ranibizumab in neovascular age-related macular degeneration: 1-year Phase III randomised clinical trial outcomes. Br J Ophthalmol. 2023;107(3):384–391. doi:10.1136/bjophthalmol-2021-319637
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
