Back to Journals » International Journal of Nanomedicine » Volume 19
Biomimetic Nano-Drug Delivery System: An Emerging Platform for Promoting Tumor Treatment
Authors Han X , Gong C, Yang Q, Zheng K, Wang Z, Zhang W
Received 2 October 2023
Accepted for publication 12 December 2023
Published 18 January 2024 Volume 2024:19 Pages 571—608
DOI https://doi.org/10.2147/IJN.S442877
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Xiujuan Han,1,2,* Chunai Gong,3,* Qingru Yang,1,2 Kaile Zheng,1 Zhuo Wang,1,2 Wei Zhang4
1Department of Pharmacy, First Affiliated Hospital of Naval Medical University (Shanghai Changhai Hospital), Shanghai, 200433, People’s Republic of China; 2School of Life Sciences and Biopharmaceuticals, Shenyang Pharmaceutical University, Shenyang, 110016, People’s Republic of China; 3Department of Pharmacy, Shanghai Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, 201999, People’s Republic of China; 4Department of Pharmacy, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, 200433, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhuo Wang, Department of Pharmacy, First Affiliated Hospital of Naval Medical University (Shanghai Changhai Hospital), Shanghai, 200433, People’s Republic of China, Email [email protected] Wei Zhang, Department of Pharmacy, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, 200433, People’s Republic of China, Email [email protected]
Abstract: With the development of nanotechnology, nanoparticles (NPs) have shown broad prospects as drug delivery vehicles. However, they exhibit certain limitations, including low biocompatibility, poor physiological stability, rapid clearance from the body, and nonspecific targeting, which have hampered their clinical application. Therefore, the development of novel drug delivery systems with improved biocompatibility and high target specificity remains a major challenge. In recent years, biofilm mediated biomimetic nano-drug delivery system (BNDDS) has become a research hotspot focus in the field of life sciences. This new biomimetic platform uses bio-nanotechnology to encapsulate synthetic NPswithin biomimetic membrane, organically integrating the low immunogenicity, low toxicity, high tumor targeting, good biocompatibility of the biofilm with the adjustability and versatility of the nanocarrier, and shows promising applications in the field of precision tumor therapy. In this review, we systematically summarize the new progress in BNDDS used for optimizing drug delivery, providing a theoretical reference for optimizing drug delivery and designing safe and efficient treatment strategies to improve tumor treatment outcomes.
Keywords: nanoparticles, cell membrane, targeted therapy
Introduction
Malignant tumors, a significant threat to human health and life, exhibit high morbidity, metastasis, and a poor prognosis.1,2 With continuous advances in medical technology, progress has been made in tumor treatment. Despite these challenges, the treatment of malignant tumors remains a persistent challenge due to the unique characteristics of tumor cells, high recurrence rates, drug targeting issues, poor penetration of the biological barrier, short in vivo circulation, and the toxicity of traditional chemotherapy.3
Drug-targeted delivery systems based on nanomaterials and nanotechnology offer a promising solution. Nano-drug delivery system (NDDS) with nanoparticles (NPs) as drug carriers can enhance drug stability, solubility, enable targeted delivery and drug-controlled release, concentrating drugs at tumor sites while minimizing systemic side effects compared to free drugs.4–6 Although a substantial number of nanocarriers are currently under preclinical research, they still have limitations in clinical application.7 For example, it may be difficult to avoid cellular and autoimmune reactions in the blood of normal tissues when NPs enter the body.8 NPs exposed to biological fluids, such as blood, quickly interact with host proteins to form a protein corona that endows NPs with new characteristics in the biological environment and may cause unpredictable therapeutic outcomes.9 Moreover, the dense extracellular matrix in solid tumors limits the penetration of nanocarriers.10 Recent advances in nanotechnology focus on optimizing NP properties through surface modifications. For example, polyethylene glycol (PEG) is used to functionalize the surface of NPs to achieve immune escape and extend their biological half-life.11 Protein-modified nanocarriers also improve targeting.12–15 Although these materials exhibit promising prospects, there is still room for improvement in areas such as immune compatibility or targeting.16,17
In recent decades, a biomimetic camouflage strategy with natural cell membranes (CMs) has attracted increasing attention for promoting tumor-targeted therapy.18 A new intelligent biomimetic nano-drug delivery system (BNDDS) was constructed by encapsulating NPs with biologically derived CMs to preserve the biological activity of the source cells without damaging the physicochemical properties and drug-carrying capacity of the NPs.19 Therefore, this multifunctional biomimetic platform shows good prospects for tumor treatment because of its excellent biocompatibility, low immunogenicity, long cycle time, tissue homing characteristics, and ability to cross biological barriers.20,21 Over the past 20 years, researchers have explored biomimetic membrane-camouflaged nanocarriers, offering features such as immune escape, biological barrier crossing, pharmacokinetics, and pharmacodynamic regulation, for precise and personalized tumor treatment. Visualization analyses of keyword co-occurrence from 2018 to 2023 were performed using Pajek and VOS viewer bibliometric software (Figure 1). Research studies have extensively reported on the investigation and application of BNDDS within the academic field of tumor treatment. This paper summarizes BNDDS composition, preparation, characterization, and recent developments across various BNDDS. In addition, the challenges and outlook of BNDDS for translation into clinical practice are presented.
 |
Figure 1 Network diagram illustrating keyword co-occurrences on biomimetic NPs, CM, nano-drug delivery, targeted therapy, tumor treatment. |
Brief Description of BNDDS
Composition of BNDDS
BNDDS mainly consists of core particles with drug-loaded nanostructures and biomimetic outer membranes with biological activity (Figure 2). Nanomaterials for core particles are divided into three main categories: inorganic, organic, and inorganic-organic hybrids. Common organic NPs include lipid-based NPs, proteins, and polymers. These NPs exhibit good long-term safety and high biocompatibility. Since the 1990s, different liposomal and polymeric NPs have been approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA).22,23 Common inorganic materials include metals and related oxides, carbon-based NPs, mesoporous silica NPs (MSNs), hollow manganese dioxide (HMnO2), and other non-metallic nanomaterials.4,24 Inorganic NPs exhibit good biocompatibility and high drug loading rates due to their unique electrical, magnetic, and optical properties, as well as variability in size, structure, and geometric shape.25 Metal organic frameworks are the most common organic–inorganic hybrid nanomaterials that combine the synergistic effects of organic and inorganic materials.26 NPs play a decisive role in the drug release profile, pharmacokinetic behavior, and size and shape of drug delivery systems, and different core nanomaterials have different functions. Therefore, personalized designs are required for different clinical research purposes.24,27 Table 1 summarizes the advantages and disadvantages of common NPs. Biomimetic materials play an important role in the packaging, protection, targeting, and improvement of NP biocompatibility. Common biomimetic materials are divided into natural CMs, CM derivatives (extracellular vesicles), functionalized CMs, and CM mimics. The fate of a BNDDS is affected by two factors: the body’s environment and its own characteristics. Tumor microenvironment (TME) abnormalities hinder BNDDS dispersion and penetration in solid tumors, whereas BNDDS characteristics, preparation, and drug loading affect their transport.28,29
 |
Figure 2 Biomimetic membrane coated nanoparticles for cancer therapy. |
 |
Table 1 The Advantages and Disadvantages of Common NPs |
Self-Assembly of BNDDS
An intelligent BNDDS camouflaged by a biomimetic membrane typically involves three primary steps for self-assembly. (1) Biomimetic membrane preparation. The preparation of biomimetic membrane involves the extraction of CMs from different cell types. Anucleated cells, such as mature red blood cells (RBCs) and platelets, can be easily removed through hypotonic treatment or freeze-thaw cycles. However, the extraction of CMs from nucleated cells, such as cancer and T cells, is more complex. This requires the separation of plasma membranes from organs and proteins. The preparation of functionalized and hybrid CMs is complicated. In addition, extracellular vesicles derived from some cells described above have also been used as membrane coatings for NPs. However, large-scale production of BNDDS remains a major challenge for clinical translation. (2) The synthesis of drug-loaded nanoplatform cores. A variety of materials are used for NP preparation (as previously described), which can confer a high cargo capacity or excellent electrical, magnetic, and optical properties to the nanocarriers. Therefore, researchers can load drugs onto selected NPs through noncovalent encapsulation or covalent binding according to different needs and research purposes to form drug-loaded nanocores that determine the morphology, in vivo drug release performance, and pharmacokinetics of the BNDDS. (3) Wrapping the biomimetic membrane-based shell onto drug-loaded NPs is the most important and final step that determines the perfect preparation of biomimetic carriers. Commonly used packaging methods include co-incubation, stirring, mixing, mechanical extrusion, ultrasonication, microfluidic electroporation, and CM-templated polymerization.43
Characterization of BNDDS
The chemical structure and surface proteins of these carriers play crucial roles in immune escape and internal circulation. Consequently, characterization becomes imperative post-preparation, encompassing three key objectives: (1) Verifying the complete encapsulation of NPs and biomimetic membrane. (2) Determining fundamental characteristics such as morphology, particle size, and alterations in surface charge of the modified NPs. This analysis typically employs instruments, including transmission electron microscopy and dynamic light scattering. (3) Ensuring the functionality and safety of BNDDS, including assessments of release, efficacy, and toxicity of functional proteins and nanocore drugs. However, the characterization of biomimetic CM-modified NPs remains challenging owing to the heterogeneity of the TME and the dynamic and complex nature of BNDDS entering the body.
Biological Barriers to Be Crossed by NDDS in the Body
Nano-drugs require a multistep cascade process after intravenous injection to exert their effects, including passing through the blood barrier, accumulating at the tumor site, infiltrating the tumor tissue, endocytosis, intracellular transport, and drug release. Any low-efficiency steps can affect the anti-tumor effect of the drug. Biomimetic nano-drug delivery platforms driven by nanotechnology have the potential to overcome biological barriers and achieve precise drug delivery. This section outlines key biological obstacles faced by NDDS when delivering drugs to tumor sites and the innovative strategies employed by NDDS to address them.
Bloodstream
Blood is the first biological barrier encountered by nanomedicines after intravenous injection into systemic circulation. To concentrate drugs at tumor sites and reduce toxicity to non-target organs, nano-drugs entering the bloodstream must evade protein adsorption, premature degradation by enzymes, and clearance by the reticuloendothelial system.44 NP properties such as size, morphology, and surface charge affect blood clearance to a certain extent. NP biomimetic coatings can significantly circumvent blood clearance by increasing the biocompatibility of the nano-drugs. Different characteristics of biomimetic membrane have different effects on blood clearance. Among these, the source of the biomimetic membrane is one factor that affects blood clearance. Immune rejection is predominant among allogeneic cells; however, this barrier is affected in unmodified autologous cells. Excessive modifications alter the structure of the CM and cause toxic reactions.45 Moreover, overexpression of the IgG receptor CD64 on the cell surface may prevent immune rejection by allogeneic cells.46
Tumor Tissue Infiltration
After being in blood circulation, NPs infiltrate tumor tissue from blood vessels with certain endothelial cell gaps, which are influenced by tumor vascular aberrations, TME, and NP properties.47 Biomimetic NPs can cross endothelial barriers using neutrophils (NE) or genetically modified CMs with endothelial crossing capabilities.48,49 In addition, dysfunctional blood vessels and high interstitial pressure in tumor tissue hinder further diffusion of NPs from blood vessels to tumor tissue.50 Anti-angiogenic drugs (AADs) can normalize tumor blood vessels within a certain time window while reducing tumor interstitial pressure to enhance drug penetration into the tumor.51,52
The dense three-dimensional network structure of the extracellular matrix (ECM) also poses a significant obstacle to the diffusion-dominated transportation of nanomedicines. Furthermore, the variability of the TME, the uneven distribution of substances in tumor tissue gaps, and the interaction between drugs and the matrix are factors that hinder further drug diffusion. Therefore, enhancing the efficiency of nanomedicine penetration across this complex transportation environment into the entire tumor tissue is crucial for improving the effectiveness of chemotherapy. Engineered biomimetic nanocarriers expressing ECM-degrading enzymes can improve the therapeutic effects by breaking the ECM barrier and improving cell infiltration.53
Cellular Membrane Traversal and Subsequent Endosomal Compartmentalization
Chemotherapeutic drugs usually must enter the interior of tumor cells to exert their efficacy. The steric hindrance of NPs, the dense cell matrix, and electrostatic interactions between the surface charge of NPs and tumor matrix proteins can reduce the diffusion rate.54 After contact with the CM, nanomedicines enter the cell through various pathways, such as endocytosis and lipid fusion. Additionally, to effectively exert anti-tumor effects, it is necessary to avoid degradation of endosomes/lysosomes.55 Effective intracellular delivery can be achieved by modifying BNDDS with tumor-penetrating peptides and tumor cell-specific recognition antibodies or ligands.56,57
Multidrug Resistance from Drug Efflux Pumps
Multidrug resistance (MDR) in tumor cells is the main obstacle to the failure of chemotherapy for cancer treatment. Both intrinsic and contact-acquired drug resistance involve the expulsion of drugs from cells, resulting in low intracellular concentrations that weaken the effectiveness of the treatment. MDR results from a multi-gene and multistep combination mechanism, which may include increased drug efflux or decreased uptake, enhanced drug metabolism, augmented DNA damage repair, changed drug-target caused by mutations, deregulation of microRNAs and mechanisms of drug resistance mediated by the TME.58 Among them, the overexpression of efflux transporters is the main cause of drug resistance to many chemotherapy drugs. In recent years, researchers have committed to developing nanotechnology-based delivery systems to overcome MDR in tumors.59
BNDDS Based on Natural CMs
The features of NPs control the behavior of loaded drugs in vivo. Therefore, modular design strategies aimed at improving the transport efficiency of NDDS, such as including antigens corresponding to the target ligands and including molecular triggers that respond to the target microenvironment, are constantly evolving. One of the most intriguing recent methods is the direct utilization of CM components to functionalize NPs. CM-coated NPs (CM-NPs) employ biomimetic and nanotechnology techniques to envelope NP surfaces through methods such as co-extrusion, extrusion/ultrasound, frozen-thaw/ultrasound, extrusion/ultrasound, and stirring. This imparts unique surface properties, mirroring primitive cells onto NPs. This novel intelligent nano-drug targeting platform can efficiently avoid recognition and clearance by the immune system, prolonging the circulation time of drugs in the body and achieving targeted delivery of nano-drugs under the action of specific CMs.11,60–62 Compared with traditional functionalization methods, this top-down membrane coating approach provides a simplified method for preparing multifunctional and multiantigen NPs. Based on these advantages, NPs with CMs as a camouflage material are widely designed to promote development in biomedical fields, including detoxification,63–65 tumor diagnosis and tumor therapy (tumor-targeted chemotherapy,66–69 photothermal therapy,70 immunotherapy,71 starvation-activated cancer therapy72).
A variety of natural CMs are used to camouflage NPs, including blood CM (platelet membrane, red CM), white CM (macrophages,73 lymphocytes, dendritic cells, spongy NE membrane),74,75 cancer CM (CCM), stem CM, and adipocyte membrane. Depending on the cellular membranes wrapped around the NP surfaces, the corresponding CM-NPs have different advantages. In this section, we discuss the crucial advances in CM-mediated NDDS according to cell type and their corresponding uses.
Red Blood CM-Camouflaged Nano-Drug Delivery System (RBCM-NDDS)
RBCs, the most abundant cell types in blood, have the longest blood circulation time (~120 d). Mature RBCs with hemoglobin as the main component lack nuclei and most organelles, making the extraction and purification of the red blood CM (RBCM) relatively simple. High-density self-biomarkers on the RBC surface, such as CD47, ensure cell stability and immune escape characteristics, promoting their application as coating materials in drug delivery systems.76 The introduction of RBCM-encapsulated poly (lactic-co-glycolic acid) (PLGA) biomimetic NPs marked the beginning of BNDDS based on the CM in 2011.77 Subsequently, RBCM has been widely used to encapsulate perfluorocarbons, polymers, MSNs, magnetic materials, and metal organic frameworks (MOF) for image-guided cancer radiotherapy and chemotherapy.78
RBCM-NDDS has been extensively developed for anticancer applications in preclinical models (Table 2), especially in drug delivery.79,80 Zhai et al81 used RBCM-derived vesicles to camouflage gelatin nanogel cores loaded with methylene blue and cisplatin for targeting combat metastatic breast cancer. The obtained nanocarrier possessed high tumor-targeting penetrability, effective lysosome escape, and a high lung metastasis inhibition rate without significant toxicity. To overcome the treatment limitations caused by hypoxia, Gao et al82 used the long-circulation properties of RBCM to prepare a BNDDS to improve hypoxia and enhance the effectiveness of tumor chemotherapy, which was superior to bare NPs in terms of circulation time, permeability, and safety. Bovine serum albumin-Chlorine e6 and glucose oxidase, loaded with MnO2 NPs encapsulated in RBCM, have been used to treat breast cancer. The results indicate this biomimetic hybrid nanoenzyme can enhance the starvation therapy and photodynamic therapy of hypoxic tumors by self-supplying H+ and accelerating O2 generation.83 Liang et al84 used RBCM to encapsulate NPs in which matrix metalloproteinase 2-cleavable peptides and miR-126-3p self-assemble. The obtained stealth BNDDS was used for efficient lung adenocarcinoma therapy by reversing DNA methylation-induced miRNA silencing (Figure 3A). Interestingly, Miao et al85 constructed a BNDDS using RBCM-camouflaging NPs that simulated different life stages of RBCs. Research demonstrates that particles closely emulating healthy RBCM exhibit superior anti-tumor effects, offering insights for tailored carriers and personalized treatment (Figure 3B). To overcome traditional immunotherapy limitations, Xu et al86 developed a BNDDS by encapsulating Mn-based MOFs loaded with polyphyllin I within RBCM. BNDDS demonstrated superior anti-tumor effects compared to traditional immunotherapy by activating the cGAS/STING pathway (Figure 3C).
 |
Figure 3 Preparation and application of RBCM-NPs in tumor treatment. (A) Schematic diagram of RBCM-NPs in gene therapy. (B) Design of RBC-NPs that mimic different stages of natural erythrocytes. (C) Schematic diagram of RBCM-NPs in immunotherapy. Notes: (A) Reprinted with permission from Liang L, Cen H, Huang J, et al. The reversion of DNA methylation-induced miRNA silence via biomimetic nanoparticles-mediated gene delivery for efficient lung adenocarcinoma therapy. Mol Cancer. 2022;21(1):186. Copyright (2022) BioMed Central, Open Access.84 (B) Reprinted with permission from Miao Y, Yang Y, Guo L, et al. Cell Membrane-Camouflaged Nanocarriers with Biomimetic Deformability of Erythrocytes for Ultralong Circulation and Enhanced Cancer Therapy. ACS Nano. 2022;16(4):6527–6540. Copyright © 2022, American Chemical Society.85 (C) Reprinted with permission from Xu M, Chang Y, Zhu G, Zhu X, Song X, Li J. Transforming Cold Tumors into Hot Ones with a Metal Organic Framework-Based Biomimetic Nanosystem for Enhanced Immunotherapy. ACS Appl Mater Interfaces. 2023;15(14):17,470–17,484. Copyright (2023) American Chemical Society.86 |
 |
Table 2 Examples of RBCM Coating NPs in Cancer Treatment |
However, the lack of RBCM targeting is a major barrier limiting the clinical translation of RBCM-NDDS.78 This defect can fine-tune the function of the BNDDS by further engineering the natural CM coating, which artificially endows the CM-NPs with a satisfactory tumor-targeting ability. Gao et al93 developed a nucleic acid nanogel, Vir Gel, which simulates the structure of a virus. It comprises a functional polypeptide-modified RBC membrane coating embedded with a nanocore of miR155. RBCM further protected and extended the circulation of miR155 in the blood based on the nanogel. Simultaneously, modification of the two targeted peptides increased drug targeting. More interestingly, peptide-modified CBM-encapsulated GC-rich DNA biomimetic pseudonuclear NPs have been developed to selectively target healthy tissues and serve as bait to prevent doxorubicin (DOX) from embedding into healthy tissues, improving the body’s tolerance to DOX and reducing toxic side effects.64 NPs with malarial RBCs for tumor-targeted drug delivery have been prepared recently and were inspired by the specific tropism of RBCs infected with malaria,91 providing conceptual proof for the use of this malaria biomimetic for tumor-targeted drug delivery (Figure 4). Modified nanovesicles (NVs) are a special type of membrane carrier. Wang et al94 modified erythrocyte-derived mimic vesicles (MVs) with AS1411 aptamer for tumor-targeting. The BNDDS prepared with these engineered MVs showed high loading rates and low drug resistance. Collectively, taking advantage of RBCM can improve the biocompatibility of NDDS, prolong the circulating half-life, and improve the pharmacokinetic behavior of drugs, and has been widely used to enhance drug delivery and the effectiveness of immunotherapy, photochemical therapy, and gene therapy in recent years.95,96
 |
Figure 4 Schematic diagram of modified RBCM-NPs for targeted drug delivery. Notes: Reprinted with permission from Pihl J, Clausen TM, Zhou J, et al. Malaria Biomimetic for Tumor-Targeted Drug Delivery. ACS Nano. 2023. Copyright (2023) American Chemical Society.91 |
Platelet Membrane-Camouflaged Nano-Drug Delivery System (PLTM-NDDS)
Platelets are another type of anucleated blood cell that have also been widely used as a source of CMs for the surface coating of NPs. Besides their central role in hemostasis,97 platelets have a variety of other functions, such as the ability to respond to inflammatory signals and target injured tissues, tumor sites, and areas of vascular inflammation. These disease-related functions are largely because of the expression of surface markers, such as CD47, CD55, CD59, and P-selectin glycoprotein ligand-1, which can be transferred to NPs through membrane coating. There is an intrinsic affinity between platelets and circulating tumor cells (CTCs). Considering the important physiological and pathological roles of platelets in the tumor environment, an increasing number of scientists have attempted to coat platelet membranes on the surface of NPs to endow nanocarriers with the functions of natural platelets.98 Compared to RBC, platelets have a better targeting ability, but a small proportion of blood and poor stability are the main obstacles limiting their clinical conversion.8
The PLTM-NDDS has also been widely studied for tumor treatment (Table 3). The first use of PLTM as a membrane source for encapsulating NPs was reported in 2015. Compared to bare NPs, PLTM-NDDS has better immune escape and fewer toxic side effects.99 Simultaneously, NP accurately targeted the target position due to the adhesion factor on the PLTM surface. Jiang et al100 used PLTM to encapsulate mesoporous magnetic NPs (Fe3O4) loaded with sulfasalazine for immunotherapy. The PLTM coating enabled effective evasion of the immune system by PLTM-NDDS, leading to sufficient tumor enrichment. To achieve the combination of vascular disrupting agent (VDA) and AAD in the same nanocarrier and to improve the targeting of this carrier, Li et al101 self-assembled BNDDS by encapsulating PLTM on the surface of MSNs loaded with VDA and AAD, using PLTM’s inflammatory tendency and interaction with CTCs to achieve precise tumor-targeting. Similarly, PLTM-NDDS has also been used for the efficient targeted delivery of small interfering RNAs (siRNAs) in vivo to achieve the silencing of target genes for anti-tumor gene therapy.102 Furthermore, immunotherapy, which modulates the TME, has become a popular topic in tumor therapy in recent years. Wang et al103 used PLTM coated MOF NPs loaded with lactate oxidase and oxaliplatin to enhance immunotherapy. This intelligent nanoplatform autonomously modulates the immunosuppressive TME and inhibits tumor growth by depleting lactate and amplifying immunogenic cell death (ICD)-induced immunotherapy. Ning et al104 designed PLTM camouflaged-cuproptosis sensitization system (PTC), a system core constructed by co-extrusion using cuprous oxide NPs and an AIE photosensitizer (TBP-2) for targeted induction of tumor cuproptosis to prevent lung metastasis of breast cancer (Figure 5). A platelet-covered platform has also been used for the targeted delivery of hirudin to eliminate thrombotic complications during tumor treatment.105 More interestingly, Zou et al106 developed a micro-sized cellular platelet “ghosts” (PGs) encapsulated biomimetic cascade delivery system. Compared to nanoscale PLTM encapsulated carriers, it exhibits enhanced internalization and cytotoxicity on 4T1 cells, as well as higher anti-tumor photothermal efficacy.
 |
Figure 5 Schematic diagram of PLTM-NPs for boosting cuproptosis to inhibit breast cancer metastasis and rechallenge. Notes: Reprinted with permission from Ning S, Lyu M, Zhu D, et al. Type-I AIE Photosensitizer Loaded Biomimetic System Boosting Cuproptosis to Inhibit Breast Cancer Metastasis and Rechallenge. ACS Nano. 2023;17(11):10,206–10,217. Copyright (2023) American Chemical Society.104 |
 |
Table 3 Examples of PLTM Coating NPs in Cancer Treatment |
Similarly, the functionalization modification of PLTM can further enhance the transport performance of BNDDS. Li et al110 used horseradish peroxidase and programmed death-ligand 1 (PD-L1)-modified PLTM to coat Prussian blue NPs, exhibiting excellent photothermal conversion efficiency for combined immunotherapy and photothermal therapy. The obtained BNDDS showed good tumor homing and enhanced anti-tumor effects. Exploiting the tumor homing and targeting capabilities of activated platelets in metastasis, Zhou et al111 functionalized activated PLTM with tumor-targeting VAR2CSA malaria protein, camouflaging docetaxel-loaded NPs. The personalized BNDDS can target carcinomas, especially metastatic cancer, and provide a reference drug delivery platform to treat metastatic cancers (Figure 6). Thus, PLTM-NDDS not only provides immune escape but also facilitates active or passive tumor cell targeting, showcasing its potential as a drug carrier.
 |
Figure 6 Schematic diagram the (A) preparation and (B) in vivo primary tumor and lung metastatic site targeted therapy of modified PLTM-NPs. Notes: (A) and (B) reprinted with permission from Zhou M, Lai W, Li G, et al. Platelet Membrane-Coated and VAR2CSA Malaria Protein-Functionalized Nanoparticles for Targeted Treatment of Primary and Metastatic Cancer. ACS Appl Mater Interfaces.2021;13(22):25,635–25,648. Copyright (2021) American Chemical Society.111 |
Cancer CM-Camouflaged Nano-Drug Delivery System (CCM-NDDS)
Compared with most other donor cells, cancer cells can be easily cultured in vitro to obtain ample membranes. CCMs inherit homologous targeting and antigenic library functions, allowing them to be targeted naturally, without complex chemical modifications. CCM-NDDS can evade the immune system and specifically enrich in tumors, reducing damage to normal cells.62
CCMs are widely used as a source of tumor-related antigens to construct CCM-NDDS for tumor therapy (Table 4). For example, Fang et al113 used the homologous adhesion of human breast cancer cells (MDA-MB-435) to prepare a core-shell nanostructured carrier with a complete CCM antigen array. They found that cancer CM-camouflaged NPs (CCM-NPs) were more effective for homologous targeting than traditional NPs and RBCM-NPs. Similarly, Chen et al114 prepared an NDDS using a PLGA nanocore loaded with indocyanine green, further coating the NPs with CCM to enhance drug targeting and immune compatibility. Xu et al115 reported the functionalization of osimertinib-loaded polymer NPs with tumor CMs to acquire BNDDS with dual-targeted anti-tumor therapy in vivo. Animal experiments have shown that CM-NDDS has an extended circulating half-life in vivo, and homologous targeting is mediated by cancer cell surface adhesion molecules and tumor-specific binding proteins, including Galectin-3, E-cadherin, and CD44. Similar studies incorporated CCM-NPs loaded with dacarbazine and anti-programmed cell death protein 1 antibody (aPD1) for melanoma treatment via combined chemotherapy and immune checkpoint blockade.116 Recently, Zheng et al117 developed a nanocarrier coated with triple-negative breast cancer membranes to overcome the short half-life of artesunate. These biomimetic particles can reduce shear-wave elasticity (SWE) stiffness by inhibiting the functional state of tumor-associated fibroblasts, providing a theoretical basis for predicting the efficacy of clinical neoadjuvant therapy through SWE imaging (Figure 7).
 |
Figure 7 Schematic of the (A) preparation of CCM-NP and (B and C) discussion on the mechanism of predicting TNBC tumor treatment efficacy using shear wave elastography. Notes: (A–C) Reprinted with permission from Zheng D, Zhou J, Qian L, et al. Biomimetic nanoparticles drive the mechanism understanding of shear-wave elasticity stiffness in triple-negative breast cancers to predict clinical treatment. Bioact Mater. 2023;22:567–587. Copyright © 2022 The Authors. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).117 |
 |
Table 4 Examples of CCM Coating NPs in Cancer Treatment |
CMM-NPs are not only used for the targeted delivery of drugs to tumors, but also for multifunctional combination therapy. Li et al118 designed a personalized cancer vaccine by camouflaging surgically derived CCMs efficiently loaded with imiquimod (R837) for prostate cancer immunotherapy. Another NDDS with satisfactory performance was fabricated using MSNs incorporated with propranolol and CpG. The nanoscale platform was further encapsulated by CM for incorporating multifunctional biomimetic nanovaccines and the blockade of β-adrenergic signaling, which provides a potential superior candidate for enhancing cancer immunotherapy.119 Shen et al120 functionalized black titanium NPs with iridium complexes and encapsulated them in the CCM for multimodal imaging and targeted synergistic NIR-II photothermal and sonodynamic therapy. Compared to non-functionalized NPs, these particles selectively accumulate in mitochondria, enhancing selectivity toward cancer cells, presenting a dual targeting concept. In another study using Au nanocores (AuNCs) with good loading capacity and photothermal performance, Zhang et al121 prepared a multifunctional biomimetic nanoplatform loaded with the chemotherapy drugs GEM and the NO donor L-Arg, achieving a combination of GEM chemotherapy, NO gas therapy, and photothermal therapy. The pancreatic ductal adenocarcinoma (PDAC) CM coating further enhanced the tumor-targeted accumulation and deep penetration of GEM, reduced the blood clearance rate of this mixed nano-drug, and reversed the resistance to traditional PDAC chemotherapy. To improve intracellular penetration efficiency, LN1 cell-penetrating peptide was added to the coating components to functionalize the NPs with optimal magnetothermal efficiency (ie, Fe3O4@Mn0.5Zn0.5Fe2O4 @CoFe2O4). The obtained novel magnetic NPs with a core-shell-shell structure (TMNP) improved the magnetocaloric conversion efficiency and reduced the cancer cell migration rate (Figure 8).122 Anti-CD28 antibodies-conjugated CCM-NPs have also been developed as anti-cancer nanovaccines, aiming to enhance the T-cell response and efficacy. This was achieved through dual interactions with dendritic cells and T cells.129 Li et al123 constructed a self-amplifying biomimetic nanosystem, mEHGZ, which uses calreticulin-overexpressed CCM to generate NDDS. mEHGZ promotes ICD induction at the tumor site, significantly enhancing the sensitivity of the tumor immune microenvironment (TIME) to PD-L1 antibodies. Similarly, genetically engineered NSCLC cell lines that overexpress programmed death-1 (PD-1) developed biomimetic NVs for efficient cargo transport targeting non-small cell lung cancer.130 Qi et al131 developed a generic cell-friendly supramolecular strategy based on noncovalent interactions to engineer supramolecular CM vesicles for tumor photodynamic therapy and immunotherapy, providing a new approach for the surface modification of bionic NDDS.
 |
Figure 8 Application in targeted magnetic hyperthermia of the novel shape-anisotropic magnetic core−shell−shell NPs. Notes: Reprinted with permission from Nica V, Marino A, Pucci C, et al. Cell Membrane Coated and Cell-Penetrating Peptide-Conjugated Trimagnetic Nanoparticles for Targeted Magnetic Hyperthermia of Prostate Cancer Cells. ACS Appl Mater Interfaces. 2023;15(25):30,008–30,028. Copyright © 2023 The Authors. Published by American Chemical Society. This publication is licensed under CC-BY 4.0.122 |
Stem CM-Camouflaged Nano-Drug Delivery System (SCMs-NDDS)
Stem cells (SCs) are a class of cells with an unlimited or immortal capacity for self-renewal that produces at least one type of highly differentiated daughter cells. Mesenchymal SCs (MSCs) are important members of the stem cell family, are easy to acquire, and have strong value-added abilities.133 MSCs can interact with tumor cells, different components of the TME, and the immune system through complex signaling networks, showing an inflammatory tendency and tumor tropism. Simultaneously, MSCs lacking co-stimulatory factors (such as B7-1, B7-2, and CD40-L) and low expression of human leukocyte antigen and major histocompatibility complex class I can bypass the host immune response and protect themselves from immune attacks by producing special immunosuppressive factors.134 Therefore, wrapping NPs with MSC membranes not only enhances biocompatibility but also maximizes the therapeutic effect of NPs by mimicking the targeting capabilities of MSCs.135 Thus far, SCM-NDDS has been widely used in research on breast cancer, glioblastoma, cervical cancer, bone tumors, acute myeloid leukemia, oral squamous cell carcinoma, and other tumor models (Table 5). Different sources of MSCs have varying therapeutic potentials for different diseases because of disparities in accessibility, content, proliferation, immunoregulation, and cytokine profiles.136
 |
Table 5 Examples of SCM Coating NPs in Cancer Treatment |
Because of their inherent homing ability and immune compatibility, MSCs show great potential in the field of tumor-targeting drug therapy.133 Animal experiments have shown that SCM-encapsulated nanocomposites loaded with mitoxantrone are enriched at tumor sites and effectively inhibit tumor growth. Compared to single nanocomposites, MSCs/NP systems exhibited a swifter migration rate toward malignant glioma cells (U87).137 Another study showed that tumor cell uptake of PLGA NPs coated with a human umbilical MSC membrane was three times more efficient than that of uncoated identical NPs, and biomimetic NPs exhibited greater tumor toxicity.138 Similarly, Li et al149 prepared MSC membrane-encapsulated mesoporous silica NPs (MSN @ M) for treating malignant liver cancer. Compared with MSN, MSN @ M have a lower phagocyte clearance rate and stronger tumor-targeting and penetration ability in vivo. Moreover, the MSC membranes significantly enhance the drug loading capacity of MSN @ M and contribute to the sustained release of DOX.
SCM-encapsulated NPs are widely used in gene therapy, tumor imaging, photothermal therapy, and photodynamic therapy (Table 5). For example, Zhang et al139 designed polydopamine NPs disguised by stem CMs for synergistic chemotherapy and photothermal therapy of malignant bone tumors. Gao et al140 developed MSNs coated with mesenchymal stem cell (BMSC) membranes for photodynamic therapy (PDT) of tumors. Mu et al141 prepared a membrane nanocomposite for delivering siRNA using magnetic NPs coated with SCM. Besides targeting solid tumors, Ho et al142 developed a local CRISPR-Cas9 delivery system that targeted leukemia SCs (LSCs) to enhance gene editing efficiency and acute myeloid leukemia therapeutic efficacy. To enhance the targeting and bone marrow luminal residence time, MSC membrane-coated nanofiber (MSCM-NF) scaffolds were loaded with LNP-Cas9 RNP and the chemokine CXCL12. Furthermore, the release of CXCL12 induced in vitro migration of LSCs onto the MSCM-NF scaffolds, resulting in knockout of the interleukin-1 receptor accessory protein (IL1RAP) gene. In turn, IL1RAP knockout reduced the colony-forming ability and leukemic load of LSC. Satisfactory targeting can also be achieved by further engineering modifications of the SCM-NDDS to fine-tune its functionality. Xie et al146 developed hollow manganese dioxide (HMnO2) NPs disguised by TAT peptide-modified human umbilical cord MSCs for targeted drug delivery. The newly obtained BNDDS showed excellent nuclear targeting ability. Genetic engineering modification of CM protein expression is another powerful method that has been used to produce CM-camouflaged NPs (CM-NPs) with higher functions. In a study by Chang et al,150 human pluripotent SCs were genetically engineered through CRISPR/Cas9 mediated gene knockout to express anti-glioblastoma (GBM)-chimeric antigen receptor (CAR) constructs with T-specific CD3ζ or NE-specific γ-signaling domains. CAR NEs with optimal anti-tumor activity were produced to deliver and release TME-responsive NPs specifically and noninvasively to target GBM. This combination of chemotherapy and immunotherapy exhibited excellent and specific anti-GBM activity, enhancing drug delivery precision and prolonging the life of female tumor-bearing mice (Figure 9).
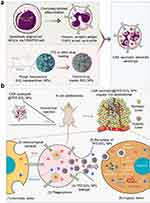 |
Figure 9 Schematic diagram of (a) the preparation of chimeric antigen receptor (CAR)-neutrophil-NPs using genetically engineered human pluripotent stem cells and (b) the mechanism of its roles for glioblastoma chemo-immunotherapy. Notes: (a) and (b) Reprinted with permission from Chang Y, Cai X, Syahirah R, et al. CAR-neutrophil mediated delivery of TME-responsive nano-drugs for glioblastoma chemo-immunotherapy. Nat Commun. 2023;14(1):2266. Copyright © The Author(s) 2023, http://creativecommons.org/licenses/by/4.0/ License.150 |
White Blood CM-Camouflaged Nano-Drug Delivery System (WBCMs-NDDS)
Leukocytes, including monocytes, macrophages, lymphocytes, natural killer (NK) cells, and dendritic cells, are blood cells widely distributed in the bloodstream and lymphatic system. They can migrate to sites of inflammation outside blood vessels, actively deforming and eliminating pathogens. White blood cells (WBCs), play a crucial role in various stages of cancer development through specific targeting and immune monitoring.151 In contrast, the reticuloendothelial system plays an important role in the circulation duration and clearance of NPs from the blood.152 Based on these functions, researchers have coated the surfaces of NPs with WBCMs to endow them with a wide range of activities, including prolonging blood circulation, recognizing and presenting antigens, enhancing the targeting ability and biocompatibility, slowly controlling drug release, and lowering in vivo toxicity (Table 6). However, the characteristics of some WBCs that promote tumor progression are key issues that deserve further attention.8 The WBCMs currently used for BNDDS construction are macrophage (Mø), dendritic cell (DC), neutrophils (NE), T-lymphocyte, and NK CMs. Among these, most research has focused on BNDDS using Mø membranes.
 |
Table 6 Examples of WBCMs-NDDS in Cancer Treatment |
Mø Membranes
Mø are the most abundant xenogeneic innate immune cells in the TME.153 Mø possess surface markers like CD11b or CD49d that interact with chemokines and inflammatory factors expressed in tumor tissue, allowing them to specifically target certain tumor cells.154 Simultaneously, according to the polarization state, tumor type, and disease stage, tumor-associated macrophages can promote and prevent cancer growth. Therefore, Mø serve as both therapeutic targets and drug carriers.155 In addition, M1 macrophage-derived NVs can act as immunomodulators, inducing the conversion of tumor-related macrophages to the M1 type and promoting the therapeutic effect of anti-PD-L1 antibodies.156 Currently, various delivery vectors, including gold based nanoplatforms, up-conversion NPs, liposomes with emtansine, and MSNs, have been integrated into macrophage membranes and have shown high targeting, low immunogenicity, and good systemic circulation stability to treat malignant tumors, including breast cancer, colorectal cancer, melanoma, and glioma.
Based on Mø’s inherent tumor-targeting and immune affinity, Mø membrane-NDDS has been widely used in effective cancer imaging, enhanced cancer photothermal therapy, tumor-targeted chemotherapy, and other tumor treatment strategies.8 Zhang et al157 developed a pH-sensitive NP delivery system (Møm-NPs) for human breast cancer cell lines. These pH-sensitive NPs respond to pH differences in the TME and are controlled to release anti-tumor drugs. As a homing navigator, surface modification of NPs with Mø membranes promotes their accumulation at tumor sites, enhances their anti-tumor effect, and decreases their cytotoxicity. Another study loaded sub-5 nm PEGylated gold (Au) NPs onto the shuttle machine Mø, achieving active or passive delivery of tumors. Although the delivery efficiency of the obtained NPs is lower than free NPs, this safe delivery platform can prevent ultrasmall NPs from translocating across biological barriers during tumor-targeting while overcoming the specificity limitations of passive and active tumor-targeting delivery NP models.158 Liang et al159 used Mø membrane to wrap liposome cores loaded with the anti-cancer drug DOX and the imaging agent quaternary quantum dots (QDs) for improving tumor imaging and anti-metastasis therapy. The Mø membranes in this biomimetic platform not only promote NPs in evading immune clearance, prolonging circulation in the bloodstream, and effectively targeting tumors, but also stabilize the structure of artificial liposomes, preventing substance leakage. Another study demonstrated that Mø membrane-decorated DSPE-PEG loaded with near-infrared Ib (NIR-Ib) fluorescent dye IR-792 can penetrate the blood-brain barrier and selectively aggregate at tumor site, achieving a combination of NIR-Ib imaging and NIR-Ib imaging guided photothermal therapy, significantly inhibiting the growth of glioma.160
Biomimetic nanoplatforms based on monocytes/macrophages have been used for multifunctional co-occurrences to improve the therapeutic effects on tumors. For example, an ultrasound-driven white blood CM-coated gallium nanoswimmer loaded with DOX was developed for enhanced photothermal and chemical cancer treatments. This nanoswimmer exhibits multifunctionality, including active movement, anti-biofouling properties, cancer cell recognition, imaging, drug delivery, and photothermal cancer treatment, providing a new multifunctional platform for medical applications.161 Ding et al162 modified the Mø membrane with folic acid ligands and luciferase NanoLuc and encapsulated the photoreceptor CRY2 and its interacting partner CIB1 plasmid. In a mouse model of retinoblastoma, the camouflaged NP-based optogenetic system demonstrated a strong ability to target tumors and effectively load plasmids (Figure 10). Tumor-associated macrophage membrane-loaded NPs prepared by Chen et al163 selectively accumulated in the TME, which eliminated the growth of primary tumors and had a significant inhibitory effect on tumor metastasis. Recently, a novel core/shell structure nanoplatform has been developed, comprised MSNs coated with macrophage CM and simultaneously loaded with catalase, DOX and resiquimod (R848). The multifunctional platform actively targets tumor sites through ligand binding, facilitating hypoxia reversal and improving the immunosuppressive TME through various pathways. These include supplying oxygen in situ via catalase, inhibiting the adenosine A2AR pathway in regulatory T (Treg) cells, inducing ICD in tumor cells with DOX, and enhancing CD8+ T-cell activation with R848. The results showed that the expression of regulatory T cells in the tumor tissue decreased to 9.79%, and the tumor growth inhibition rate reached 73.58%, considerably enhancing the effectiveness of anti-tumor immunotherapy.164
 |
Figure 10 Schematic diagram of MM-NPs in situ bioluminescence-driven optogenetic therapy. Notes: Reprinted with permission from Ding J, Lu J, Zhang Q, et al. Camouflage Nanoparticles Enable in Situ Bioluminescence-Driven Optogenetic Therapy of Retinoblastoma. ACS Nano. 2023;17(8):7750–7764. Copyright (2023) American Chemical Society.162 |
NE Membranes
NEs are the most abundant circulating leukocytes in the human circulatory system, accounting for 40–70% of all human leukocytes. They also participate in proinflammatory and immune responses against pathogens, but when faced with microbial challenges or tissue injury, some reactions are nonspecific, causing damage to normal tissues. NEs can inhibit or promote tumor growth and play important roles in tumorigenesis, development, and treatment.167,168 NEs contribute to malignant tumor metastasis by targeting CTCs through adhesion factors and ligands, mediating the formation of an ecological niche before cancer metastasis.169 Based on these characteristics, nanocarriers simulating NEs have been widely studied to identify and inhibit tumors.
Animal experiments have shown that NE-encapsulated PLGA NPs loaded with carfilzomib, a proteasome inhibitor, can target and capture CTCs and effectively inhibit cancer metastasis by enhancing homing to the pre-metastasis niche.170 Similarly, Zhang et al171 constructed BNDDS using a zeolitic imidazolate framework-8 (ZIF-8) coated with an NE membrane, which was co-loaded with glucose oxidase (GOx) and chloroperoxidase (CPO). The obtained biomimetic system demonstrated good immune compatibility and inflammatory targeting by simulating natural NEs, and produced seven times more reactive hypochlorous acid than natural NEs through enzymatic cascade reactions. NEs membranes also allow carriers to cross biological barriers (blood-pancreatic and blood-brain barriers). Cao et al172 designed a formulation of NE membrane-encapsulated NPs (NNPs) loaded with celastrol and demonstrated in animal models that the formulation could overcome the blood-pancreatic barrier for in vivo pancreas-specific drug delivery. Similar experiments demonstrated that NE-mediated delivery of anti-cancer drugs inhibits the recurrence of postoperative malignant gliomas.173 Xia et al174 introduced an innovative approach by creating a sponge-like NE CM-wrapped nanosystem (NM/PPcDG/D), merging sponge NPs and anti-inflammatory agents. This BNDDS exhibits a natural affinity for postoperative inflammatory sites, reduces the recruitment and function of myelogenous Sexual inhibition cells (MDSCs), and thus alleviates immunosuppression in 4T1 tumor resection models. Combined with the ICD effect of DOX, it effectively inhibits lung metastasis and reduces vascular permeability, mitigating the implantation of CTCs in the lungs. This provides a new treatment plan for improving the effectiveness of postoperative tumor treatment. In another study, researchers designed bovine serum albumin NPs conjugated with anti-CD11b and IR-820, loaded with decitabine (DAC), to enable precise drug delivery and photothermal-induced tumor immunotherapy. The NDDS uses anti-CD11b to target activated NEs in the bloodstream, achieving precise targeting of tumor areas in the postoperative inflammatory microenvironment. Incorporating IR820 provides a photothermal control system, allowing the NDDS to escape from the cell carrier. Simultaneously, the released DAC upregulates gasdermin E, and laser irradiation activates caspase-3, which causes cell pyroptosis and regulates the immunosuppressive microenvironment while inducing persistent and powerful immune memory, preventing lung metastasis (Figure 11).175
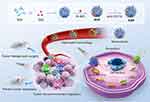 |
Figure 11 Schematic diagram of NEM-NPs for photothermal-induced tumor immunotherapy by triggering pyroptosis. Notes: Reprinted with permission from Yu X, Xing G, Sheng S, et al. Neutrophil Camouflaged Stealth Nanovehicle for Photothermal-Induced Tumor Immunotherapy by Triggering Pyroptosis. Adv Sci. 2023;10(15):e2207456. Copyright © 2023 The Authors. Advanced Science published by Wiley‐VCH GmbH. This is an open access article under the terms of the http://creativecommons.org/licenses/by/4.0/ License.175 |
DC Membranes
DCs, originating from pluripotent hematopoietic SCs in the bone marrow, are the most effective antigen-presenting cells that can proficiently ingest, process, and present antigens.176,177 These cells play a crucial role not only in the intrinsic immune response but also in initiating adaptive immune responses and influencing the type of immune response, which ultimately assists in immune responses.178 DC vaccine is a safe and effective therapeutic strategy that has made considerable progress in basic science research and clinical therapeutic areas.179
Animal studies have demonstrated that DC-coated PLGA NPs loaded with IL-2 inherit DC antigen presentation and T-cell stimulation abilities, enhancing T-cell activation. Compared with traditional DC vaccines, mini-DC-NP nanovaccines exhibit more potent tumor treatment and prevention capabilities.180 Remarkably, Liu et al181 proposed a novel personalized nanovaccine formulation that can directly activate both naive T cells and exhausted T cells. The formulation was developed utilizing recombinant adenovirus-engineered mature DCs, with major histocompatibility complex class I (MHC-I) molecules, anti-PD1 antibodies, and B7 co-stimulatory molecules simultaneously anchored on the membrane of mature DC through cell reprogramming. This approach effectively inhibits tumor cell growth through antigen self-presentation and immunosuppression reversal, leading to optimal therapeutic outcomes. NDDS based on DC membranes can also efficiently cross biological barriers and target the TIME. Ma et al182 used activated mature dendritic cell membrane (aDCM) to encapsulate a PLGA copolymer loaded with rapamycin (RAPA) to prepare aDCM @PLGA /RAPA. In vivo and in vitro results show these NPs effectively penetrate the blood-brain barrier and activate CD8+T cells, reshaping the glioma TIME and significantly inhibiting tumor growth, providing a simple and effective platform for personalized immunotherapy (Figure 12). In another study, DC membranes were encapsulated on nanoaggregates of near-infrared aggregation-induced emission emitters (NIR-AIEgens) to form biomimetic AIE photosensitizers (DC@BPBBT dots) with antigen presentation and “free-riding” capabilities. Extracellular membrane allows DC@BPBBT dots to target endogenous T cells, selectively aggregating AIE molecules onto the lipid droplets of tumor cells, ultimately increasing the tumor delivery rate approximately 1.6 times.183
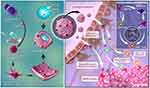 |
Figure 12 Schematic diagram of DCM-NPs activate immunotherapy through direct or indirect pathways. Notes: Reprinted with permission from Ma X, Kuang L, Yin Y, et al. Tumor-Antigen Activated Dendritic Cell Membrane-Coated Biomimetic Nanoparticles with Orchestrating Immune Responses Promote Therapeutic Efficacy against glioma. ACS Nano. 2023;17(3):2341–2355. Copyright (2023) American Chemical Society.182 |
T-Lymphocyte CMs
T lymphocytes, derived from bone marrow hematopoietic SCs (BMSCs), are widely present in the human body and mainly participate in cellular immune processes. As the main immune cells in the TIME, T lymphocytes play an important role in controlling the occurrence and development of tumors. High-density cytotoxic CD8+T cells and CD4+T helper cells are associated with favorable prognosis of various cancers. In contrast, Treg cells suppress anti-tumor immunity by influencing the proliferation and activation of cytotoxic CD8+T cells.184 Because of the abundance of proteins that bind to tumor cells, such as adhesins LFA-1 and PD-1, and inflammatory tendencies on T-CMs, many studies have used T-CMs to disguise NPs for cancer treatment by “active targeting” or “immune checkpoint”.
To address the challenge of cancer immunotherapy, Kang et al185 coated T-CMs from the EL4 cell line on PLGA NPs to prepare a biomimetic carrier-TCMNP for melanoma immunotherapy. Dacarbazine-loaded BNDDS TCM-NPs target tumors through proteins originating from T-CMs and kill cancer cells by releasing anti-cancer molecules and inducing Fas ligand-mediated apoptosis. This strategy is more cost-effective and less time-consuming than adoptive T-cell transfer therapy. Compared to immune checkpoint blockades, TCM-NPs exhibit lower systemic toxicity owing to their higher tumor-targeting efficiency and multiple treatment mechanisms. Zhang et al186 camouflaged the paclitaxel-carrying PLGA nanocore with a human cytotoxic T-lymphocyte membrane, demonstrating excellent tumor-specific recognition (Figure 13). Similarly, Zhai et al187 created a cytotoxic T-lymphocyte membrane-decorated epigenetic nanoinducer (OPEN) loaded with ory-1001 for cancer immunotherapy. The outer shell of the vector was engineered using PD-1. The T-lymphocyte membrane endows OPEN with the ability to specifically recognize and block a variety of immune checkpoint ligands. This strategy supplements interferon in tumors and blocks interferon-induced immune checkpoint upregulation, increasing the recruitment, proliferation, and activity of tumor-infiltrating lymphocytes. OPEN shows significant anti-tumor effects in various tumor models.
 |
Figure 13 Preparation and application of T-CM-camouflaged NPs (TCM-NPs) in tumor treatment. Notes: Reprinted with permission from Zhang L, Li R, Chen H, et al. Human cytotoxic T-lymphocyte membrane-camouflaged nanoparticles combined with low-dose irradiation: a new approach to enhance drug targeting in gastric cancer. Int J Nanomedicine. 2017;12:2129–2142. Copyright (2017) Dove Medical Press Ltd, Open Access.186 |
NK CMs
NK cells serve as the primary defense against tumors in the innate immune system.189 Unlike T and B lymphocytes, they can identify and destroy tumor cells directly via the perforin/granzyme and Fas/Fasl pathways without the need for antigen pre-sensitization.190 Surface-activated and inhibitory receptors are responsible for coordinating NK cell activity. This ongoing balance of activation and inhibition enables NK cells to maintain self-tolerance and efficiently target tumors.191 NK cells hold great potential as effector cells for adoptive immune therapy in cancer treatment.192 However, the therapeutic effect is compromised by immunosuppression in the TME. Therefore, several studies are underway to create new nanomedicines that regulate the interactions between immune and tumor cells.193,194
Based on the efficient recognition of tumors by NK CMs, NK CM-encapsulated NPs (NKCM-NPs) have shown great potential for enhancing the tumor-targeting homing ability of NDDS.195 For example, Deng et al196 developed NK CM-camouflaged NPs to enhance anti-tumor immune efficiency and photodynamic therapy. The obtained BNDDS inherited the antigen spectrum of natural NK cells and induced or enhanced the polarization of proinflammatory M1 macrophages. In animal experiments, this biomimetic nanostrategy demonstrated good tumor elimination and inhibition ability against ovarian cancer (Figure 14). Du et al197 created self-assembled NKCM-NPs for chemoimmunotherapy of breast cancer. The NK CM-camouflaged modification of NDDS enhances the highly effective targeting ability of breast cancer cells. It can also trigger tumor-specific immune responses by inducing macrophage polarization toward the tumor-suppressive M1 phenotype. Another NEM-NDDS prepared for targeted tumor therapy showed good biocompatibility and extended drug circulation time.198 Furthermore, in recent years, there has been significant focus on NK cells as potential targets for immunotherapy.
 |
Figure 14 Schematic diagram of NKCM-NPs in photodynamic therapy combined with chemoimmunotherapy. Notes: Reprinted with permission from Deng G, Sun Z, Li S, et al. Cell Membrane Immunotherapy Based on Natural Killer Cell Membrane Coated Nanoparticles for the Effective Inhibition of Primary and Abscopal Tumor Growth. ACS Nano. 2018;12(12):12,096–12,108. Copyright (2018) American Chemistry Society.196 |
Bacterial Membrane-Camouflaged NDDS (BM-NDDS)
Bacterial membranes (BMs) have been proposed as attractive vaccine materials because of their immunogenic antigens with intrinsic surface adjuvant properties.199 BMs play an important role in immune responses by stimulating the innate immune system and promoting adaptive immune responses. Scientists wrap BMs around synthetic nanocores, endowing NPs with complex biological characteristics similar to BMs and simulating the process of bacteria presenting natural antigens to the immune system.200 Patel et al201 used BN-NPs comprising BMs and imine groups enveloping the core of PC7A/CpG peptides to treat syngeneic melanoma and neuroblastoma in situ. The experimental results showed that the combination of radiation and BN-NPs enhanced the local antigen capture and immune stimulation. However, toxicity is a problem that needs to be addressed in the clinical transformation of vaccine platforms based on BMs.
Bacterial outer membrane vesicles (OMV), spherical bilayer NPs released from the bacterial outer membrane, are a unique form of BM. These vesicles possess immunogenic antigens similar to those of the parental bacteria, making them valuable for enhancing the stability and targeting capabilities of NPs when used as CM coating materials.202 Gao et al203 separately wrapped β-Cyclodextrin (β-CD) and Adamantane (ADA)-modified gold NPs with Escherichia coli OMVs. Upon intravenous injection, these NPs were selectively engulfed by phagocytic immune cells and then self-assembled into considerable intracellular aggregates through host–guest interactions, demonstrating enhanced targeting efficiency and therapeutic effects in melanoma tissues. Chen et al204 developed mesoporous polydopamine (MPD) nanocore mimetic NPs encapsulated in bilayer membrane vesicles (DMVs) derived from VNP20009, combining biocompatibility and immune activation. This synergy between MPD-mediated photothermal therapy and immunotherapy through DMV amplification proved effective. This study showed that the intravenous injection of MPD@DMV was superior to the intratumoral injection in terms of its long-term immune effects (Figure 15).
 |
Figure 15 Schematic diagram of BM-NPs in photothermal therapy collaborative immunotherapy. Reproduced, with permission. Notes: Reprinted with permission from Chen W, Song Y, Bai S, et al. Cloaking Mesoporous Polydopamine with Bacterial Membrane Vesicles to Amplify Local and Systemic Anti-tumor Immunity. ACS Nano. 2023;17(8):7733–7749. Copyright (2023) American Chemical Society.204 |
Extracellular Nanovesicle-Camouflaged NDDS (EV-NDDS)
Extracellular nanovesicles (EVs), comprising exosomes and microbubbles, act as significant mediators in cell communication, migration, signal transduction, and disease pathogenesis.205,206 EVs inherit antigens and properties of the source cells, and are released by natural cells.207,208 It is recognized as a potentially favorable lipid bilayer nanocarrier for treating cancer due to its high capacity for intracellular uptake, excellent ability to be compatible with living organisms, low toxicity, ability to hold high amounts of drugs, easy modifiability, and potential for large-scale manufacturing.209,210 Nonetheless, drug delivery systems using EVs remain restricted in industrial production due to their insufficient quantities.211
Recently, EVs have been widely used to design actively targeted biomimetic nanomedicines. Zhao et al212 constructed biomimetic NPs by wrapping an exosome membrane derived from autologous breast cancer cells onto cationic bovine serum albumin coupled with siS100A4. This biomimetic carrier possesses selective targeting of the pre-lung metastasis niche and good biocompatibility while protecting siS100A4 from degradation. Similarly, extracellular vesicle membranes derived from M2 macrophages have been used for preparing biomimetic NPs to achieve effective drug accumulation in the lungs.213 Irradiated tumor cell-derived microparticles have also been shown to enhance drug particle targeting.214 Surface modification methods, similar to natural CMs, also apply to EVs to enhance their precise targeting and tumor penetration ability. A specific anti-glioma-targeted delivery system, using angiopep-2 and TAT peptide-functionalized small extracellular vesicles, efficiently crosses the blood-brain barrier, targets gliomas, penetrates tumors, and achieves precise glioma chemotherapy, minimizing drug-related toxicities.215
Bacterial extracellular vesicles (BEVs), which are promising natural nanocarriers,216 have shown great potential in various treatments, including drug delivery,217 tumor immunotherapy,218 and the treatment of infectious diseases.219 Particularly, preparing BEV-based nanovaccines has become a research hotspot in recent years.220,221 For example, Li et al222 proposed an in situ vaccine based on OMVs from E. coli BL21-CodonPlus (DE3) to promote immune-mediated tumor clearance after photothermal therapy by orchestrating antigen capture and immune regulation. Similar to functionalized mammalian CMs, bacterial OMVs can also enhance the anti-tumor effect of biomimetic NPs through OMV genetic engineering or surface modification.223 In another interesting study, a universal modular platform involving avidin-based vaccine antigen cross-linking (AvidVax) was developed for the rapid assembly of antigens of interest on OMV surfaces. Unlike previous covalent binding strategies, which were mainly limited to protein and peptide antigens, the AvidVax method can accurately and uniformly load multiple subunit antigen molecular arrays. The development of this platform has significantly affected the vaccine industry (Figure 16).224
 |
Figure 16 Schematic diagram of the modular nanovaccine based on rapidly self-assembling OMVs. (a) Schematic of Avidin-based vaccine antigen crosslinking (AvidVax) technology. (b) Genetic architecture of synthetic antigen-binding protein (SNAP) constructs tested. Notes: (a) and (b) reprinted with permission from Weyant KB, Oloyede A, Pal S, et al. A modular vaccine platform enabled by decoration of bacterial outer membrane vesicles with biotinylated antigens. Nat Commun. 2023;14(1):464. Copyright © 2023, The Author(s), Creative Commons CC BY license.224 |
Other CM-Camouflaged NDDS
In addition, some special cell types have been designed for drug delivery. For example, tumor-associated fibroblasts (TAFs), an important component of the TME, have multiple functions, such as secreting various inflammatory and growth factors, reshaping the extracellular matrix, and developing resistance to various treatments. TAFs play a dual role in promoting and inhibiting tumor occurrence, development, and metastasis.225 In addition, TAFs can crosstalk with both cancer cells and infiltrating immune cells in the TME.226 Li et al227 camouflaged near-infrared (NIR)-absorbing semiconducting polymer NPs with activated fibroblasts (AF) for combined photodynamic and photothermal therapy. The CM coating provides homologous targeting of cancer-related fibroblasts, facilitating the accumulation of NPs in tumors and enhancing the effectiveness of photo-diagnosis and phototherapy. In another example, Zhang et al228 used a PD-1-expressing hek293T CM to encapsulate a sinophoryrin sodium-binding human serum albumin-perfluorotribulamine nanoemulsion, which was used for synergistic photodynamic immunotherapy of hypoxic 4T1 breast tumors and distant metastases. Interestingly, unlike traditional membrane encapsulation strategies, Niu et al229 patched heparin-based NPs (DNs) loaded with DOX onto the surface of natural grapefruit extracellular vesicles (EVs) to achieve efficient drug delivery with 4-fold drug loading.
Hybrid Membrane-Camouflaged NDDS (HM-NDDS)
NPs disguised by single-CMs exhibit various characteristics. However, owing to differences in cellular functions, different natural CM-encapsulated NDDS have deficiencies in certain functions, such as poor targeting of the RBCM. Therefore, to further enhance the advantages of CMs while improving tumor homing and tissue penetration, reducing toxic side effects, and obtaining acceptable biodistribution and therapeutic indices for drugs and NPs, researchers have used specific synthesis techniques to fuse several CMs to form hybrid membranes.230 The resulting formulations inherit the specific functions of the respective membranes, and the newly synthesized hybrid membranes are endowed with the corresponding functions by adjusting the ratio of the source membranes to achieve the organic integration of multiple functions into the same NPs. Successfully developed hybrid CM-NPs include RBC-PLT hybrid CM-coated NPs, RBC-cancer hybrid CM-coated NPs, PLT-WBC hybrid CM-coated NPs, DC-cancer hybrid CM-coated NPs, PLT-cancer stem cell hybrid CM-coated NPs, PLT-cancer hybrid CM-coated NPs, Mø-cancer hybrid CM-coated NPs, and bacterial vesicle-cancer hybrid CM-coated NPs (Table 7).
 |
Table 7 Example of NPs Encapsulated Within Hybridized Cells in Cancer Treatment |
In 2017, RCM and PLT hybrid CMs were developed as the first hybrid membrane sources for encapsulating NDDS.231 Subsequently, an increasing number of studies have used mixed CMs as envelopes to disguise NPs. Targeting cancer cells in the TME is an important area of oncology research. Wang et al232 coated hollow copper sulfide NPs (CuS NPs) loaded with DOX with a hybrid CM of RBCs and melanoma cells (B16-F10 cells) for combined photothermal/chemotherapy in melanoma. Compared with bare CuS NPs, this multifunctional platform showed highly specific self-recognition of the source cell line in vitro and a significantly prolonged circulation lifetime in vivo. This enhances the homogeneous targeting ability inherited from source cytogenetics. Likewise, Rezaei et al233 established a different NDDS using 4T1 CCMs, RBCMs, and hybrid erythrocyte cancer membranes to disguise reduction-sensitive chitosan NPs loaded with DOX to enhance the efficacy of chemotherapy in breast cancer. The results showed that compared with free nanocarriers, membrane camouflage carriers exhibited prolonged circulating life and enhanced tumor homotype-targeting chemotherapy drug delivery both in vitro and in vivo. Interestingly, the homotypic targeting characteristics of breast cancer cells were increased by optimizing the RBCM:4T1CM ratio. Ma et al239 evaluated the ability of PLGA NPs wrapped with glioma-associated stromal cell (GASC)-glioma cell fusion CMs to target gliomas in the TME. The hybrid CM-encapsulated NPs, inheriting the membrane proteins of the glioma and GASC membranes, exhibited slower blood clearance and higher tumor-targeting efficiency and binding affinity compared to the nanoformulations coated with CCMs alone. Another novel NDDS with a hybrid membrane was developed, which was constructed using NE and macrophage membrane-camouflaged PLGA NPs loaded with RAPA. This nanoplatform efficiently coordinated and promoted the transport through the blood-brain barrier and the targeting efficiency of gliomas.246 Interestingly, a hybrid membrane of exosomes and Golgi membranes was modified on the surface of PLGA NPs embedded with retinoic acid (RA) to prepare GENPs. This hybrid membrane enables efficient penetration of tumor cells, delivery of RA, disruption of the Golgi apparatus, interference with PD-L1 synthesis, and inhibition of extracellular vesicle secretion carrying PD-L1. A significantly decreased recurrence rate and prolonged survival were observed in a mouse model of incomplete metastatic melanoma resection. This strategy is a promising treatment for patients insensitive to immune checkpoint inhibitors (Figure 17).250
 |
Figure 17 Schematic diagram of exosomes-Golgi apparatus HCM-NPs in tumor treatment. Notes: Reprinted with permission from Ye H, Wang K, Zhao J, et al. In Situ Sprayed Nanovaccine Suppressing Exosomal PD-L1 by Golgi Apparatus Disorganization for Postsurgical Melanoma Immunotherapy. ACS Nano. 2023;17(11):10,637–10,650. Copyright (2023) American Chemistry Society.250 |
Besides fusing two types of natural CMs, researchers have used a mixture of natural CMs and engineered membranes as coating materials to enhance the function of BNDDS by artificially imparting satisfactory biological properties to NPs. Zhang et al251 developed a comprehensive treatment platform for TME reprogramming and immune cell activation. A mixed membrane composed of a mesenchymal stem CM (MSCm) and a pH-sensitive liposome (pSL) was used to encapsulate manganese oxide (MnO2) NPs loaded with BPTES, a glutamine metabolism inhibitor. This hybrid synthetic CM endowed the NPs with targeting capability for the TME, as well as the characteristics of internal/lysosomal escape and sensitive release. The combination of red blood CMs with pH-responsive liposomes not only optimized the pharmacokinetics and tumor accumulation of nanomedicine but also addressed the limitations of DNA carriers in terms of packaging capacity and interception by the reticuloendothelial system. Mouse experiments demonstrated that a mixed membrane-camouflaged HER2-targeted DNA-aptamer-modified DNA tetrahedron combined with maytansine (DM1) was potent in the treatment of HER2 positive breast cancer.252 Hybrid membrane vesicles have also been studied and showed enhanced anti-tumor effects. Chen et al247 designed and constructed a eukaryotic-prokaryotic vesicle nanoplatform with strong immunotherapeutic effects by fusing melanoma CM vesicles and attenuated Salmonella OMVs. In another study, Rao et al253 developed a ternary hybrid NV (hNV) integrating the functions of platelet-derived NVs (P-NV), cancer cell-derived NVs overexpressing SIRPα variants (Sα V-C-NV), and M1-type macrophage-derived NVs (M1-NV). These findings provide valuable insights into the development of novel anti-tumor immune response strategies (Figure 18).
 |
Figure 18 Schematic diagram of (a) the composition of hybrid cell membrane nanovesicles (hNVs) and (b) the mechanism by which hnv effectively enhances the immune response of macrophages. Notes: (a and b) reprinted with permission from Rao L, Wu L, Liu Z, et al. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat Commun. 2020;11(1):4909. Copyright © 2020, This is a US government work and not under copyright protection in the US; foreign copyright protection may apply, http://creativecommons.org/licenses/by/4.0/ License.253 |
Novel NDDS Based on Simulated Viruses or CMs
Virus-based immunotherapy has shown potential for overcoming resistance to immune checkpoint blockade, which is a promising method for cancer treatment.254,255 Recently, simulating the infection mechanism of natural viruses within cells has become an attractive concept for anti-tumor immunotherapy. For example, Zhao et al256 used the herpes simplex virus (HSV) to trigger a natural immune response as an entry point to construct novel mimics of HSV-NPs applied in cancer-targeted therapy. In this system, DNAzyme-loaded Mn-ZIF-90 NPs (ZM@TD) were designed to mimic the viral nucleocapsid containing the genome, whereas the erythrocyte membrane was modified with RGD and HA2 functional peptides to simulate the viral envelope. This biomimetic platform not only effectively evades rapid elimination in blood circulation but also mimics the various infection processes of HSV. It successfully activated innate immunity through the cGAS-STING pathway, resulting in 68% regression of primary tumors and a 32-day increase in the median survival time of 4T1 tumor-bearing mice.
Coating particles with engineered simulated CMs is another way to achieve a functionalized NDDS. Han et al257 prepared CpG-OD-adsorbed PLGA NPs loaded with berberine and melanoma antigen-Hgp peptide fragments. The NP surfaces were then coupled with M2pep (an m2-like macrophage-binding peptide) and a receptor-scavenging B-type 1 targeted peptide to prepare m2-like tumor-associated macrophage (TAM)-targeted nanocomposites. These nanocomposites promote the delivery of baicalin, antigens, and immune stimulators to TAMs, polarizing and reversing M2-like TAM phenotypes. This dual action successfully transforms the TME while eliminating tumor cells. Formulations featuring core-shell structures similar to those of CM-NPs have also been studied for constructing intelligent delivery systems. Xue et al258 programmatically attached an anti-degradable Y-shaped skeleton rigid triangular DNA brick (sticky YTDB) with a sticky end onto Au-NPs loaded with siRNA, forming a multifunctional three-dimensional DNA shell with active tumor-targeting capabilities. This shell protects the siRNA from enzymatic degradation, provides serum stability, and enables cancer-specific cell internalization of the siRNA-encapsulated formula.
Conclusion
This review summarizes BNDDS and the recent advancements in cancer treatment. As a nature-inspired drug delivery system, BNDDS is expected to be a promising platform for tumor therapy. Compared to synthetic nano-drug delivery system, the innovative composite platform can achieve targeted drug delivery and overcome biological barriers by mimicking natural cells. Firstly, the biomimetic membrane shell can avoid the drug being cleared by the immune system, thus improving the stability and durability of the drug. Second, the bionic membrane shell-encapsulated nanocarriers can preferentially target the therapeutic dose to tumor tissues, reducing damage to healthy tissues. In addition, by utilizing the supportive role of the nanoparticle core, the bionic membrane shell can maintain a stable morphological structure, ensuring the accuracy and controllability of drug delivery. Most importantly, BNDDS possesses the ability to load multiple types of therapeutic drugs, which is significant in multi-drug combination therapy. Researchers can select or synthesize specific biomimetic membrane shells tailored to their intended applications, allowing for customized formulations. Based on these advantages, BNDDS have been extensively studied in preclinical models to evaluate their potential for drug delivery, photothermal therapy, and immunotherapy, as well as multimodal theranostic treatment.
Despite progress made by BNDDS, challenges and security issues still need to be addressed. Currently, research is primarily conducted in laboratory settings; however, it is important to consider the species differences between animal models and humans, as they have a significant impact on CM-NPs. In addition, research on the impact of tumor behavior and function is limited. Given the high heterogeneity of the physiological and pathological characteristics among different tumors, it is crucial to develop models that can better simulate tumors and formulate treatment strategies tailored to the specific heterogeneity of each tumor. However, CM-NPs have several limitations that hinder their clinical application. First, research on CM-NPs is still in its early stages and the production technology is not fully developed, making it challenging to achieve large-scale preparation. Second, the characterization methods for CM-NPs are limited, and the effectiveness of the membrane coating can be confirmed only through particle size detection and morphological observation. Third, CM-NPs still pose potential safety risks, such as uncontrolled differentiation and proliferation, cell embolism, autoimmune reactions, and specific storage requirements. Therefore, extensive fine-tuning is necessary to ensure a successful clinical translation. Furthermore, most current studies have primarily focused on the accumulation of CM-NPs at the tumor site, neglecting how the physical and chemical properties of CM-NPs, including size, surface charge, shape, and elasticity, influence the interactions between biomimetic nanocarriers and organisms.259 In addition, limited attention has been paid to the overall lifespan and quality of life.
The use of NDDS based on CMs or engineered CM encapsulation has demonstrated notable advantages in tumor treatment and diagnosis. As biomimetic nanotechnology continues to be researched, the development of membrane-biomimetically modified NPs is anticipated to become a crucial approach in tumor treatment, with the potential to greatly affect human cancer treatment and enhance patient prognosis.
Abbreviations
AAD, anti-angiogenic drug; AF, activated fibroblast; BEV, bacterial extracellular vesicle; BM, bacterial membrane; BMSC, bone marrow hematopoietic stem cell; BNDDS, biomimetic nano-drug delivery system; CAR, chimeric antigen receptor; CCM, cancer cell membrane; CM, cell membrane; EV, extracellular vesicle; GASC, glioma-associated stromal cell; HSV, herpes simplex virus; ICD, immunogenic cell death; LSC, leukemia stem cell; MOF, metal organic framework; MSC, mesenchymal stem cell; MSN, mesoporous silica nanoparticle; NK, natural killer; OMV, outer membrane vesicle; RA, retinoic acid; RBC, red blood cell; RBCM, red blood cell membrane; SC, stem cell; SWE, shear-wave elasticity; TAM, tumor-associated macrophage; VDA, vascular disrupting agent; WBC, white blood cell.
Acknowledgments
This study was supported by the Shanghai Natural Science Foundation (23ZR1453400).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Wei W, Zeng H, Zheng R, et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. 2020;21(7):e342–e349. doi:10.1016/S1470-2045(20)30073-5
3. Chen HY, Deng J, Wang Y, Wu CQ, Li X, Dai HW. Hybrid cell membrane-coated nanoparticles: a multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020;112:1–13. doi:10.1016/j.actbio.2020.05.028
4. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124.doi:10.1038/s41573-020-0090-8
5. Su H, Wang Y, Liu S, et al. Emerging transporter-targeted nanoparticulate drug delivery systems. Acta Pharm Sin B. 2019;9(1):49–58. doi:10.1016/j.apsb.2018.10.005
6. Hettiarachchi SD, Zhou Y, Seven E, et al. Nanoparticle-mediated approaches for Alzheimer’s disease pathogenesis, diagnosis, and therapeutics. J Control Release. 2019;314:125–140. doi:10.1016/j.jconrel.2019.10.034
7. Lee SY, Cho HJ. An α-tocopheryl succinate enzyme-based nanoassembly for cancer imaging and therapy. Drug Deliv. 2018;25(1):738–749. doi:10.1080/10717544.2018.1446476
8. Oroojalian F, Beygi M, Baradaran B, Mokhtarzadeh A, Shahbazi MA. Immune cell membrane-coated biomimetic nanoparticles for targeted cancer therapy. Small. 2021;17(12):e2006484. doi:10.1002/smll.202006484
9. Kim W, Ly NK, He Y, Li Y, Yuan Z, Yeo, Y. Protein corona: friend or foe? Co-opting serum proteins for nanoparticle delivery. Adv Drug Deliv Rev. 2023;192:114635. doi:10.1016/j.addr.2022.114635
10. Valkenburg KC, de Groot AE, Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol. 2018;15(6):366–381. doi:10.1038/s41571-018-0007-1
11. Dhas N, García MC, Kudarha R, et al. Advancements in cell membrane camouflaged nanoparticles: a bioinspired platform for cancer therapy. J Control Release. 2022;346:71–97. doi:10.1016/j.jconrel.2022.04.019
12. Muhamad N, Plengsuriyakarn T, Na-Bangchang K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: a systematic review. Int J Nanomed. 2018;13:3921–3935. doi:10.2147/IJN.S165210
13. Ding Z, Chen W, Jiang F, Mo M, Bi Y, Kong F. Synthesis, characterization and in vitro digestion of folate conjugated chitosan-loaded proanthocyanidins nanoparticles. Food Res Int. 2023;163:112141. doi:10.1016/j.foodres.2022.112141
14. Salehiabar M, Ghaffarlou M, Mohammadi A, et al. Targeted CuFe2O4 hybrid nanoradiosensitizers for synchronous chemoradiotherapy. J Control Release. 2023;353:850–863. doi:10.1016/j.jconrel.2022.12.004
15. Treuel L, Brandholt S, Maffre P, Wiegele S, Shang L, Nienhaus GU. Impact of protein modification on the protein corona on nanoparticles and nanoparticle-cell interactions. ACS Nano. 2014;8(1):503–513. doi:10.1021/nn405019v
16. Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010;49(36):6288–6308. doi:10.1002/anie.200902672
17. Abu Lila AS, Kiwada H, Ishida T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J Control Release. 2013;172(1):38–47. doi:10.1016/j.jconrel.2013.07.026
18. Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10(7):521–535. doi:10.1038/nrd3499
19. Wang X, Xia Z, Wang H, et al. Cell-membrane-coated nanoparticles for the fight against pathogenic bacteria, toxins, and inflammatory cytokines associated with sepsis. Theranostics. 2023;13(10):3224–3244. doi:10.7150/thno.81520
20. Fang RH, Kroll AV, Gao W, Zhang L. Cell membrane coating nanotechnology. Adv Mater. 2018;30(23):e1706759. doi:10.1002/adma.201706759
21. Parodi A, Molinaro R, Sushnitha M, et al. Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials. 2017;147:155–168. doi:10.1016/j.biomaterials.2017.09.020
22. Craparo EF, Cabibbo M, Scialabba C, Giammona G, Cavallaro G. Inhalable formulation based on lipid-polymer hybrid nanoparticles for the macrophage targeted delivery of roflumilast. Biomacromolecules. 2022;23(8):3439–3451. doi:10.1021/acs.biomac.2c00576
23. Farjadian F, Ghasemi A, Gohari O, Roointan A, Karimi M, Hamblin MR. Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine (Lond). 2019;14(1):93–126. doi:10.2217/nnm-2018-0120
24. Zeng S, Tang Q, Xiao M, et al. Cell membrane-coated nanomaterials for cancer therapy. Mater Today Bio. 2023;20:100633. doi:10.1016/j.mtbio.2023.100633
25. Bogart LK, Pourroy G, Murphy CJ, et al. Nanoparticles for imaging, sensing, and therapeutic intervention. ACS Nano. 2014;8(4):3107–3122. doi:10.1021/nn500962q
26. Li N, Cui W, Cong P, et al. Biomimetic inorganic-organic hybrid nanoparticles from magnesium-substituted amorphous calcium phosphate clusters and polyacrylic acid molecules. Bioact Mater. 2021;6(8):2303–2314. doi:10.1016/j.bioactmat.2021.01.005
27. Furtado D, Björnmalm M, Ayton S, Bush AI, Kempe K, Caruso F. Overcoming the blood-brain barrier: the role of nanomaterials in treating neurological diseases. Adv Mater. 2018;30(46):e1801362. doi:10.1002/adma.201801362
28. Aryal S, Hu CM, Fang RH, et al. Erythrocyte membrane-cloaked polymeric nanoparticles for controlled drug loading and release. Nanomedicine (Lond). 2013;8(8):1271–1280. doi:10.2217/nnm.12.153
29. Rao L, Cai B, Bu LL, et al. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano. 2017;11(4):3496–3505. doi:10.1021/acsnano.7b00133
30. Large DE, Abdelmessih RG, Fink EA, Auguste DT. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176:113851. doi:10.1016/j.addr.2021.113851
31. Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6(12):1078–1094. doi:10.1038/s41578-021-00358-0
32. Su Y, Zhang B, Sun R, et al. PLGA-based biodegradable microspheres in drug delivery: recent advances in research and application. Drug Deliv. 2021;28(1):1397–1418. doi:10.1080/10717544.2021.1938756
33. Afsharzadeh M, Hashemi M, Mokhtarzadeh A, Abnous K, Ramezani M. Recent advances in co-delivery systems based on polymeric nanoparticle for cancer treatment. Artif Cells Nanomed Biotechnol. 2018;46(6):1095–1110. doi:10.1080/21691401.2017.1376675
34. Neek M, Kim TI, Wang SW. Protein-based nanoparticles in cancer vaccine development. Nanomedicine. 2019;15(1):164–174. doi:10.1016/j.nano.2018.09.004
35. Iqbal H, Yang T, Li T, et al. Serum protein-based nanoparticles for cancer diagnosis and treatment. J Control Release. 2021;329:997–1022. doi:10.1016/j.jconrel.2020.10.030
36. Jia Z, Dai R, Zheng Z, et al. Hollow carbon-based nanosystem for photoacoustic imaging-guided hydrogenothermal therapy in the second near-infrared window. RSC Adv. 2021;11(20):12022–12029. doi:10.1039/D1RA00093D
37. Shang L, Zhou X, Zhang J, Shi Y, Zhong L. Metal nanoparticles for photodynamic therapy: a potential treatment for breast cancer. Molecules. 2021;26(21):6532. doi:10.3390/molecules26216532
38. Yen SK, Padmanabhan P, Selvan ST. Multifunctional iron oxide nanoparticles for diagnostics, therapy and macromolecule delivery. Theranostics. 2013;3(12):986–1003. doi:10.7150/thno.4827
39. Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24(12):1504–1534. doi:10.1002/adma.201104763
40. Wang S, McGuirk CM, d’Aquino A, Mason JA, Mirkin CA. Metal-organic framework nanoparticles. Adv Mater. 2018;30(37):e1800202. doi:10.1002/adma.201800202
41. Ettlinger R, Lächelt U, Gref R, et al. Toxicity of metal-organic framework nanoparticles: from essential analyses to potential applications. Chem Soc Rev. 2022;51(2):464–484. doi:10.1039/D1CS00918D
42. Wu MX, Yang YW. Metal-Organic Framework (MOF)-based drug/cargo delivery and cancer therapy. Adv Mater. 2017;29(23). doi:10.1002/adma.201606134
43. Zhang W, Huang X. Stem cell membrane-camouflaged targeted delivery system in tumor. Mater Today Bio. 2022;16:100377. doi:10.1016/j.mtbio.2022.100377
44. Yang L, Yang Y, Chen Y, Xu Y, Peng, J. Cell-based drug delivery systems and their in vivo fate. Adv Drug Deliv Rev. 2022;187:114394. doi:10.1016/j.addr.2022.114394
45. Brenner JS, Mitragotri S, Muzykantov VR. Red blood cell hitchhiking: a novel approach for vascular delivery of nanocarriers. Annu Rev Biomed Eng. 2021;23:225–248. doi:10.1146/annurev-bioeng-121219-024239
46. Gravina A, Tediashvili G, Rajalingam R, et al. Protection of cell therapeutics from antibody-mediated killing by CD64 overexpression. Nat Biotechnol. 2023;41(5):717–727. doi:10.1038/s41587-022-01540-7
47. Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi:10.1038/nrc.2016.108
48. Wang H, Alarcón CN, Liu B, et al. Genetically engineered and enucleated human mesenchymal stromal cells for the targeted delivery of therapeutics to diseased tissue. Nat Biomed Eng. 2022;6(7):882–897. doi:10.1038/s41551-021-00815-9
49. Lesch S, Blumenberg V, Stoiber S, et al. T cells armed with C-X-C chemokine receptor type 6 enhance adoptive cell therapy for pancreatic tumours. Nat Biomed Eng. 2021;5(11):1246–1260. doi:10.1038/s41551-021-00737-6
50. Khawar IA, Kim JH, Kuh HJ. Improving drug delivery to solid tumors: priming the tumor microenvironment. J Control Release. 2015;201:78–89. doi:10.1016/j.jconrel.2014.12.018
51. Goel S, Duda DG, Xu L, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071–1121. doi:10.1152/physrev.00038.2010
52. Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409–426. doi:10.1007/s10456-017-9562-9
53. Parodi A, Haddix SG, Taghipour N, et al. Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS Nano. 2014;8(10):9874–9883. doi:10.1021/nn502807n
54. Harper RA, Carpenter GH, Proctor GB, et al. Diminishing biofilm resistance to antimicrobial nanomaterials through electrolyte screening of electrostatic interactions. Colloids Surf B Biointerfaces. 2019;173:392–399. doi:10.1016/j.colsurfb.2018.09.018
55. Wang J, Lu Z, Wientjes MG, Au JL. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010;12(4):492–503. doi:10.1208/s12248-010-9210-4
56. Cuellar M, Cifuentes J, Perez J, et al. Novel BUF2-magnetite nanobioconjugates with cell-penetrating abilities. Int J Nanomed. 2018;13:8087–8094. doi:10.2147/IJN.S188074
57. Gessner I, Neundorf I. Nanoparticles modified with cell-penetrating peptides: conjugation mechanisms, physicochemical properties, and application in cancer diagnosis and therapy. Int J Mol Sci. 2020;21(7):2536. doi:10.3390/ijms21072536
58. Assaraf YG, Brozovic A, Gonçalves AC, et al. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist Updates. 2019;46:100645. doi:10.1016/j.drup.2019.100645
59. Hu T, Gong H, Xu J, Huang Y, Wu F, He Z. Nanomedicines for overcoming cancer drug resistance. Pharmaceutics. 2022;14(8):1606. doi:10.3390/pharmaceutics14081606
60. Jiang Y, Chekuri S, Fang RH, Zhang L. Engineering biological interactions on the nanoscale. Curr Opin Biotechnol. 2019;58:1–8. doi:10.1016/j.copbio.2018.10.005
61. Song W, Jia P, Zhang T, et al. Cell membrane-camouflaged inorganic nanoparticles for cancer therapy. J Nanobiotechnology. 2022;20(1):289. doi:10.1186/s12951-022-01475-w
62. Dash P, Piras AM, Dash M. Cell membrane coated nanocarriers - an efficient biomimetic platform for targeted therapy. J Control Release. 2020;327:546–570. doi:10.1016/j.jconrel.2020.09.012
63. Ben-Akiva E, Meyer RA, Yu H, Smith JT, Pardoll DM, Green JJ. Biomimetic anisotropic polymeric nanoparticles coated with red blood cell membranes for enhanced circulation and toxin removal. Sci Adv. 2020;6(16):eaay9035. doi:10.1126/sciadv.aay9035
64. Jiang Q, Chen M, Yang X, et al. Doxorubicin detoxification in healthy organs improves tolerability to high drug doses for enhanced antitumor therapy. ACS nano. 2023;17(8):7705–7720. doi:10.1021/acsnano.3c00195
65. Hu CM, Fang RH, Copp J, Luk BT, Zhang L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat Nanotechnol. 2013;8(5):336–340. doi:10.1038/nnano.2013.54
66. Liu JM, Zhang DD, Fang GZ, Wang S. Erythrocyte membrane bioinspired near-infrared persistent luminescence nanocarriers for in vivo long-circulating bioimaging and drug delivery. Biomaterials. 2018;165:39–47. doi:10.1016/j.biomaterials.2018.02.042
67. Liu W, Ruan M, Wang Y, et al. Light-triggered biomimetic nanoerythrocyte for tumor-targeted lung metastatic combination therapy of malignant melanoma. Small. 2018;14(38):e1801754. doi:10.1002/smll.201801754
68. Guo J, Agola JO, Serda R, et al. Biomimetic rebuilding of multifunctional red blood cells: modular design using functional components. ACS Nano. 2020;14(7):7847–7859. doi:10.1021/acsnano.9b08714
69. Chai Z, Ran D, Lu L, et al. Ligand-modified cell membrane enables the targeted delivery of drug nanocrystals to glioma. ACS Nano. 2019;13(5):5591–5601.
70. Jiang Q, Liu Y, Guo R, et al. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials. Biomaterials. 2019;192:292–308. doi:10.1016/j.biomaterials.2018.11.021
71. Feng Q, Li Y, Wang N, et al. A biomimetic nanogenerator of reactive nitrogen species based on battlefield transfer strategy for enhanced immunotherapy. Small. 2020;16(25):e2002138. doi:10.1002/smll.202002138
72. Zhang L, Wang Z, Zhang Y, et al. Erythrocyte membrane cloaked metal-organic framework nanoparticle as biomimetic nanoreactor for starvation-activated colon cancer therapy. ACS nano. 2018;12(10):10201–10211. doi:10.1021/acsnano.8b05200
73. Song X, Qian R, Li T, et al. Imaging-guided biomimetic M1 macrophage membrane-camouflaged magnetic nanorobots for photothermal immunotargeting cancer therapy. ACS Appl Mater Interfaces. 2022;14(51):56548–56559. doi:10.1021/acsami.2c16457
74. Jain N, Shahrukh S, Famta P, et al. Immune cell-camouflaged surface-engineered nanotherapeutics for cancer management. Acta Biomater. 2023;155:57–79. doi:10.1016/j.actbio.2022.11.001
75. Chen Y, Qin D, Zou J, et al. Living leukocyte-based drug delivery systems. Adv Mater. 2022;35(17):e2207787. doi:10.1002/adma.202207787
76. Glassman PM, Hood ED, Ferguson LT, et al. Red blood cells: the metamorphosis of a neglected carrier into the natural mothership for artificial nanocarriers. Adv Drug Deliv Rev. 2021;178:113992. doi:10.1016/j.addr.2021.113992
77. Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108(27):10980–10985. doi:10.1073/pnas.1106634108
78. Xia Q, Zhang Y, Li Z, Hou X, Feng N. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm Sin B. 2019;9(4):675–689. doi:10.1016/j.apsb.2019.01.011
79. Rao L, Bu LL, Xu JH, et al. Red blood cell membrane as a biomimetic nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small. 2015;11(46):6225–6236. doi:10.1002/smll.201502388
80. Luk BT, Fang RH, Hu CM, et al. Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors. Theranostics. 2016;6(7):1004–1011. doi:10.7150/thno.14471
81. Zhai Y, Ran W, Su J, et al. Traceable bioinspired nanoparticle for the treatment of metastatic breast cancer via NIR-trigged intracellular delivery of methylene blue and cisplatin. Adv Mater. 2018;30(34):e1802378. doi:10.1002/adma.201802378
82. Gao M, Liang C, Song X, et al. Erythrocyte-membrane-enveloped perfluorocarbon as nanoscale artificial red blood cells to relieve tumor hypoxia and enhance cancer radiotherapy. Adv Mater. 2017;29(35). doi:10.1002/adma.201701429
83. Yang X, Yang Y, Gao F, Wei JJ, Qian CG, Sun MJ. Biomimetic hybrid nanozymes with self-supplied H+ and accelerated O2 generation for enhanced starvation and photodynamic therapy against hypoxic tumors. Nano Lett. 2019;19(7):4334–4342. doi:10.1021/acs.nanolett.9b00934
84. Liang L, Cen H, Huang J, et al. The reversion of DNA methylation-induced miRNA silence via biomimetic nanoparticles-mediated gene delivery for efficient lung adenocarcinoma therapy. Mol Cancer. 2022;21(1):186. doi:10.1186/s12943-022-01651-4
85. Miao Y, Yang Y, Guo L, et al. Cell membrane-camouflaged nanocarriers with biomimetic deformability of erythrocytes for ultralong circulation and enhanced cancer therapy. ACS Nano. 2022;16(4):6527–6540. doi:10.1021/acsnano.2c00893
86. Xu M, Chang Y, Zhu G, Zhu X, Song X, Li J. Transforming cold tumors into hot ones with a metal-organic framework-based biomimetic nanosystem for enhanced immunotherapy. ACS Appl Mater Interfaces. 2023;15(14):17470–17484. doi:10.1021/acsami.2c21005
87. Xu J, Chen T, Sun T, Yu C, Yan D, Zhu L. Erythrocyte membrane camouflaged siRNA/chemodrug nanoassemblies for cancer combination therapy. Biomater Sci. 2022;10(22):6601–6613. doi:10.1039/D2BM01478E
88. Li JQ, Zhao RX, Yang FM, Qi XT, Ye PK, Xie M. An erythrocyte membrane-camouflaged biomimetic nanoplatform for enhanced chemo-photothermal therapy of breast cancer. J Mater Chem B. 2022;10(12):2047–2056. doi:10.1039/D1TB02522H
89. Song Q, Yin Y, Shang L, et al. Tumor microenvironment responsive nanogel for the combinatorial antitumor effect of chemotherapy and immunotherapy. nano Lett. 2017;17(10):6366–6375. doi:10.1021/acs.nanolett.7b03186
90. Daniyal M, Jian Y, Xiao F, et al. Development of a nanodrug-delivery system camouflaged by erythrocyte membranes for the chemo/phototherapy of cancer. Nanomedicine. 2020;15(7):691–709. doi:10.2217/nnm-2019-0454
91. Pihl J, Clausen TM, Zhou J, et al. Malaria biomimetic for tumor targeted drug delivery. acs Nano. 2023;17(14):13500–13509. doi:10.1021/acsnano.3c01910
92. Zhang Z, Qian H, Huang J, et al. Anti-EGFR-iRGD recombinant protein modified biomimetic nanoparticles loaded with gambogic acid to enhance targeting and antitumor ability in colorectal cancer treatment. Int J Nanomed. 2018;13:4961–4975. doi:10.2147/IJN.S170148
93. Gao X, Li S, Ding F, et al. A virus-mimicking nucleic acid nanogel reprograms microglia and macrophages for glioblastoma therapy. Adv Mater. 2021;33(9):e2006116. doi:10.1002/adma.202006116
94. Wang T, Luo Y, Lv H, Wang J, Zhang Y, Pei R. Aptamer-based erythrocyte-derived mimic vesicles loaded with siRNA and doxorubicin for the targeted treatment of multidrug-resistant tumors. ACS Appl Mater Interfaces. 2019;11(49):45455–45466. doi:10.1021/acsami.9b16637
95. Zhao Z, Kim J, Suja VC, et al. Red blood cell anchoring enables targeted transduction and re-administration of AAV-mediated gene therapy. Adv Sci. 2022;9(24):e2201293. doi:10.1002/advs.202201293
96. Castro F, Martins C, Silveira MJ, Moura RP, Pereira CL, Sarmento B. Advances on erythrocyte-mimicking nanovehicles to overcome barriers in biological microenvironments. Adv Drug Deliv Rev. 2021;170:312–339. doi:10.1016/j.addr.2020.09.001
97. Paul DS, Bergmeier W. Novel mouse model for studying hemostatic function of human platelets. Arterioscler Thromb Vasc Biol. 2020;40(8):1891–1904. doi:10.1161/atvbaha.120.314304
98. Li S, Li L, Lin X, Chen C, Luo C, Huang Y. Targeted inhibition of tumor inflammation and tumor-platelet crosstalk by nanoparticle-mediated drug delivery mitigates cancer metastasis. ACS Nano. 2022;16(1):50–67. doi:10.1021/acsnano.1c06022
99. Hu CM, Fang RH, Wang KC, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–121. doi:10.1038/nature15373
100. Jiang Q, Wang K, Zhang X, et al. Platelet membrane-camouflaged magnetic nanoparticles for ferroptosis-enhanced cancer immunotherapy. Small. 2020;16(22):e2001704. doi:10.1002/smll.202001704
101. Li B, Chu T, Wei J, et al. Platelet-membrane-coated nanoparticles enable vascular disrupting agent combining anti-angiogenic drug for improved tumor vessel impairment. Nano Lett. 2021;21(6):2588–2595. doi:10.1021/acs.nanolett.1c00168
102. Zhuang J, Gong H, Zhou J, et al. Targeted gene silencing in vivo by platelet membrane-coated metal-organic framework nanoparticles. Sci Adv. 2020;6(13):eaaz6108. doi:10.1126/sciadv.aaz6108
103. Wang H, Wu C, Tong X, Chen S. A biomimetic metal-organic framework nanosystem modulates immunosuppressive tumor microenvironment metabolism to amplify immunotherapy. J Control Release. 2023;353:727–737. doi:10.1016/j.jconrel.2022.11.054
104. Ning S, Lyu M, Zhu D, et al. Type-I AIE photosensitizer loaded biomimetic system boosting cuproptosis to inhibit breast cancer metastasis and rechallenge. ACS Nano. 2023;17(11):10206–10217. doi:10.1021/acsnano.3c00326
105. Zhang K, Ma Z, Li S, et al. Platelet-covered nanocarriers for targeted delivery of hirudin to eliminate thrombotic complication in tumor therapy. ACS Nano. 2022;16(11):18483–18496. doi:10.1021/acsnano.2c06666
106. Zou J, He J, Wang X, et al. Glycoprotein Ib-regulated micro platelet ghost for biosafe distribution and photothermal oncotherapy. J Control Release. 2022;351:341–360. doi:10.1016/j.jconrel.2022.09.036
107. Bahmani B, Gong H, Luk BT, et al. Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors. Nature Commun. 2021;12(1):1999. doi:10.1038/s41467-021-22311-z
108. Pei W, Huang B, Chen S, Wang L, Xu Y, Niu C. Platelet-mimicking drug delivery nanoparticles for enhanced chemo-photothermal therapy of breast cancer. Int J Nanomed. 2020;15:10151–10167. doi:10.2147/IJN.S285952
109. Liu G, Zhao X, Zhang Y, et al. Engineering biomimetic platesomes for pH-responsive drug delivery and enhanced antitumor activity. Adv Mater. 2019;31(32):e1900795. doi:10.1002/adma.201900795
110. Li W, Li F, Li T, et al. Self-actuated biomimetic nanocomposites for photothermal therapy and PD-L1 immunosuppression. Front Chem. 2023;11:1167586. doi:10.3389/fchem.2023.1167586
111. Zhou M, Lai W, Li G, et al. Platelet membrane-coated and VAR2CSA malaria protein-functionalized nanoparticles for targeted treatment of primary and metastatic cancer. ACS Appl Mater Interfaces. 2021;13(22):25635–25648. doi:10.1021/acsami.1c02581
112. Han X, Chen J, Chu J, et al. Platelets as platforms for inhibition of tumor recurrence post-physical therapy by delivery of anti-PD-L1 checkpoint antibody. J Control Release. 2019;304:233–241. doi:10.1016/j.jconrel.2019.05.008
113. Fang RH, Hu CM, Luk BT, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14(4):2181–8. doi:10.1021/nl500618u
114. Chen Z, Zhao P, Luo Z, et al. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10(11):10049–10057. doi:10.1021/acsnano.6b04695
115. Xu B, Zeng F, Deng J, et al. A homologous and molecular dual-targeted biomimetic nanocarrier for EGFR-related non-small cell lung cancer therapy. Bioact Mater. 2023;27:337–347. doi:10.1016/j.bioactmat
116. Zhao P, Qiu L, Zhou S, Li L, Qian Z, Zhang H. Cancer cell membrane camouflaged mesoporous silica nanoparticles combined with immune checkpoint blockade for regulating tumor microenvironment and enhancing antitumor therapy. Int J Nanomed. 2021;16:2107–2121. doi:10.2147/IJN.S295565
117. Zheng D, Zhou J, Qian L, et al. Biomimetic nanoparticles drive the mechanism understanding of shear-wave elasticity stiffness in triple negative breast cancers to predict clinical treatment. Bioact Mater. 2023;22:567–587. doi:10.1016/j.bioactmat.2022.10.025
118. Li S, Dong S, Wu J, et al. Surgically derived cancer cell membrane-coated R837-loaded Poly(2-Oxazoline) nanoparticles for prostate cancer immunotherapy. ACS Appl Mater Interfaces. 2023;15(6):7878–7886. doi:10.1021/acsami.2c22363
119. Yang C, He Y, Chen F, Zhang F, Shao D, Wang Z. Leveraging β-adrenergic receptor signaling blockade for improved cancer immunotherapy through biomimetic nanovaccine. Small. 2023;19(14):e2207029. doi:10.1002/smll.202207029
120. Shen J, Karges J, Xiong K, Chen Y, Ji L, Chao H. Cancer cell membrane camouflaged iridium complexes functionalized black-titanium nanoparticles for hierarchical-targeted synergistic NIR-II photothermal and sonodynamic therapy. Biomaterials. 2021;275:120979. doi:10.1016/j.biomaterials.2021.120979
121. Zhang F, Hu Q, Li B, et al. A biomimetic nanodrug for enhanced chemotherapy of pancreatic tumors. J Control Release. 2023;354:835–850. doi:10.1016/j.jconrel.2023.01.007
122. Nica V, Marino A, Pucci C, et al. Cell-membrane-coated and cell-penetrating peptide-conjugated trimagnetic nanoparticles for targeted magnetic hyperthermia of prostate cancer cells. ACS Appl Mater Interfaces. 2023;15(25):30008–30028. doi:10.1021/acsami.3c07248
123. Li Z, Cai H, Li Z, et al. A tumor cell membrane-coated self-amplified nanosystem as a nanovaccine to boost the therapeutic effect of anti-PD-L1 antibody. Bioact Mater. 2022;21:299–312. doi:10.1016/j.bioactmat.2022.08.028
124. Jin F, Qi J, Liu D, et al. Cancer-cell-biomimetic upconversion nanoparticles combining chemo-photodynamic therapy and cd73 blockade for metastatic triple-negative breast cancer. J Control Release. 2021;337:90–104. Doi:10.1016/j.jconrel.2021.07.021
125. Li SY, Cheng H, Xie BR, et al. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy. ACS Nano. 2017;11(7):7006–7018. doi:10.1021/acsnano.7b02533
126. Xie W, Deng WW, Zan M, et al. Cancer cell membrane camouflaged nanoparticles to realize starvation therapy together with checkpoint blockades for enhancing cancer therapy. ACS Nano. 2019;13(3):2849–2857. doi:10.1021/acsnano.8b03788
127. Yang R, Xu J, Xu L, et al. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 2018;12(6):5121–5129. doi:10.1021/acsnano.7b09041
128. Meng X, Wang J, Zhou J, et al. Tumor cell membrane-based peptide delivery system targeting the tumor microenvironment for cancer immunotherapy and diagnosis. Acta Biomater. 2021;127:266–275. doi:10.1016/j.actbio.2021.03.056
129. Go S, Jung M, Lee S, et al. A personalized cancer nanovaccine that enhances T-cell responses and efficacy through dual interactions with dendritic cells and T cells. Adv Mater. 2023;35(49):e2303979. doi:10.1002/adma.202303979
130. Li B, Yang T, Liu J, et al. Genetically engineered PD-1 displaying nanovesicles for synergistic checkpoint blockades and chemo-metabolic therapy against non-small cell lung cancer. Acta Biomater. 2023;161:184–200. doi:10.1016/j.actbio.2023.03.002
131. Qi S, Zhang H, Zhang X, et al. Supramolecular engineering of cell membrane vesicles for cancer immunotherapy. Sci Bull. 2022;67(18):1898–1909. doi:10.1016/j.scib.2022.08.030
132. Li Y, Ke J, Jia H, et al. Cancer cell membrane coated PLGA nanoparticles as biomimetic drug delivery system for improved cancer therapy. Colloids Surf B Biointerfaces. 2023;222:113131. doi:10.1016/j.colsurfb.2023.113131
133. Fan L, Wei A, Gao Z, Mu X. Current progress of mesenchymal stem cell membrane-camouflaged nanoparticles for targeted therapy. Biomed Pharmacother. 2023;161:114451. doi:10.1016/j.biopha.2023.114451
134. Khosravi N, Pishavar E, Baradaran B, Oroojalian F, Mokhtarzadeh A. Stem cell membrane, stem cell-derived exosomes and hybrid stem cell camouflaged nanoparticles: a promising biomimetic nanoplatforms for cancer theranostics. J Control Release. 2022;348:706–722. doi:10.1016/j.jconrel.2022.06.026
135. Gu X, Gao Y, Wang P, et al. Nano-delivery systems focused on tumor microenvironment regulation and biomimetic strategies for treatment of breast cancer metastasis. J Control Release. 2021;333:374–390. doi:10.1016/j.jconrel.2021.03.039
136. Hoang DM, Pham PT,Bach TQ et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7(1):272. doi:10.1038/s41392-022-01134-4
137. Suryaprakash S, Lao YH, Cho HY, et al. Engineered mesenchymal stem cell/nanomedicine spheroid as an active drug delivery platform for combinational glioblastoma therapy. Nano Lett. 2019;19(3):1701–1705. doi:10.1021/acs.nanolett.8b04697
138. Yang N, Ding Y, Zhang Y, et al. Surface functionalization of polymeric nanoparticles with umbilical cord-derived mesenchymal stem cell membrane for tumor-targeted therapy. ACS Appl Mater Interfaces. 2018;10(27):22963–22973. doi:10.1021/acsami.8b05363
139. Zhang M, Zhang F, Liu T, et al. Polydopamine nanoparticles camouflaged by stem cell membranes for synergistic chemo-photothermal therapy of malignant bone tumors. Int J Nanomed. 2020;15:10183–10197. doi:10.2147/IJN.S282931
140. Gao C, Lin Z, Wu Z, Lin X, He Q. Stem-cell-membrane camouflaging on near-infrared photoactivated upconversion nanoarchitectures for in vivo remote-controlled photodynamic therapy. Acs Appl Mater Interfaces. 2016;8(50):34252–34260. doi:10.1021/acsami.6b12865
141. Mu X, Li J, Yan S, et al. siRNA delivery with stem cell membrane-coated magnetic nanoparticles for imaging-guided photothermal therapy and gene therapy. ACS Biomater Sci Eng. 2018;4(11):3895–3905. doi:10.1021/acsbiomaterials.8b00858
142. Ho TC, Kim HS, Chen Y, et al. Scaffold-mediated CRISPR-Cas9 delivery system for acute myeloid leukemia therapy. Sci Adv. 2021;7(21):eabg3217. doi:10.1126/sciadv.abg3217
143. Tian W, Lu J, Jiao D. Stem cell membrane vesicle–coated nanoparticles for efficient tumor‐targeted therapy of orthotopic breast cancer. Polym Adv Technol. 2019;30(4):1051–1060. doi:10.1002/pat.4538
144. Gao C, Lin Z, Jurado-Sánchez B, Lin X, Wu Z, He Q. Stem cell membrane-coated nanogels for highly efficient in vivo tumor targeted drug delivery. Small. 2016;12(30):4056–4062. Doi:10.1002/smll.201600624
145. Zhou D, Chen Y, Bu W, et al. Modification of metal-organic framework nanoparticles using dental pulp mesenchymal stem cell membranes to target oral squamous cell carcinoma. J Colloid Interface Sci. 2021;601:650–660. doi:10.1016/j.jcis.2021.05.126
146. Xie L, Zhang C, Liu M, et al. Nucleus-targeting manganese dioxide nanoparticles coated with the human umbilical cord mesenchymal stem cell membrane for cancer cell therapy. ACS Appl Mater Interfaces. 2023;15(8):10541–10553. doi:10.1021/acsami.3c01176
147. Zhang J, Han M, Zhang J, et al. Syphilis mimetic nanoparticles for cuproptosis-based synergistic cancer therapy via reprogramming copper metabolism. Int J Pharm. 2023;640:123025. doi:10.1016/j.ijpharm.2023.123025
148. Taghavi S, Tabasi H, Zahiri M, et al. Surface engineering of hollow gold nanoparticle with mesenchymal stem cell membrane and muc-1 aptamer for targeted theranostic application against metastatic breast cancer. Eur J Pharm Biopharm. 2023;187:76–86. doi:10.1016/j.ejpb.2023.04.014
149. Li YS, Wu HH, Jiang XC, et al. Active stealth and self-positioning biomimetic vehicles achieved effective antitumor therapy. J Control Release. 2021;335:515–526. doi:10.1016/j.jconrel.2021.05.031
150. Chang Y, Cai X, Syahirah R, et al. CAR-neutrophil mediated delivery of tumor-microenvironment responsive nanodrugs for glioblastoma chemo-immunotherapy. Nature Commun. 2023;14(1):2266. doi:10.1038/s41467-023-37872-4
151. Lança T, Silva-Santos B. The split nature of tumor-infiltrating leukocytes: implications for cancer surveillance and immunotherapy. Oncoimmunology. 2012;1(5):717–725. doi:10.4161/onci.20068
152. Liu Y, Li H, Qiu Y-L, Li K-S, Lu Y, Wang W. Nanoscale biomimetic nano system for the co-delivery of SNS032 and tumor necrosis factor related apoptosis inducing ligand to enhance therapeutic efficacy in oral squamous cell carcinoma cell line SCC25. Materials Express. 2021;11(8):1321–1330. doi:10.1166/mex.2021.2055
153. Kloosterman DJ, Akkari L. Macrophages at the interface of the co-evolving cancer ecosystem. Cell. 2023;186(8):1627–1651. doi:10.1016/j.cell.2023.02.020
154. Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15(1):123–147. doi:10.1146/annurev-pathmechdis-012418-012718
155. Naitik J, Srinivasarao DA, Famta P, et al. The portrayal of macrophages as tools and targets: a paradigm shift in cancer management. Life Sci. 2023;316:121399. doi:10.1016/j.lfs.2023.121399
156. Choo YW, Kang M, Kim HY, et al. M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano. 2018;12(9):8977–8993. doi:10.1021/acsnano.8b02446
157. Zhang Y, Cai K, Li C, et al. Macrophage-membrane-coated nanoparticles for tumor-targeted chemotherapy. Nano Lett. 2018;18(3):1908–1915. doi:10.1021/acs.nanolett.7b05263
158. Xu L, Wang X, Wang R, Liu S, Xu M. Engineered macrophages: a safe-by-design approach for the tumor targeting delivery of sub-5 nm gold nanoparticles. Small. 2023;19(1):e2205474. doi:10.1002/smll.202205474
159. Liang B, Deng T, Li J, Ouyang X, Na W, Deng D. Biomimetic theranostic strategy for anti-metastasis therapy of breast cancer via the macrophage membrane camouflaged superparticles. Mater Sci Eng C Mater Biol Appl. 2020;115:111097. doi:10.1016/j.msec.2020.111097
160. Lai J, Deng G, Sun Z, et al. Scaffolds biomimicking macrophages for a glioblastoma NIR-Ib imaging guided photothermal therapeutic strategy by crossing blood-brain barrier. Biomaterials. 2019;211:48–56. doi:10.1016/j.biomaterials.2019.04.026
161. Wang D, Gao C, Zhou C, Lin Z, He Q. Leukocyte membrane-coated liquid metal nanoswimmers for actively targeted delivery and synergistic chemophotothermal therapy. Research (Wash D C). 2020;2020:3676954. doi:10.34133/2020/3676954
162. Ding J, Lu J, Zhang Q, et al. Camouflage nanoparticles enable in situ bioluminescence-driven optogenetic therapy of retinoblastoma. ACS Nano. 2023;17(8):7750–7764. doi:10.1021/acsnano.3c00470
163. Chen C, Song M, Du Y, et al. Tumor-associated-macrophage-membrane-coated nanoparticles for improved photodynamic immunotherapy. Nano Lett. 2021;21(13):5522–5531. doi:10.1021/acs.nanolett.1c00818
164. Wen X, Xiong X, Yang G, et al. A macrophage membrane-coated mesoporous silica nanoplatform inhibiting adenosine A2AR via in situ oxygen supply for immunotherapy. J Control Release. 2023;353:535–548. doi:10.1016/j.jconrel.2022.12.001
165. Li Y, Yan T, Chang W, Cao C, Deng D. Fabricating an intelligent cell-like nano-prodrug via hierarchical self-assembly based on the DNA skeleton for suppressing lung metastasis of breast cancer. BIOMATER SCI. 2019;7(9):3652–3661. doi:10.1039/c9bm00630c
166. Zhang F, Li F, Lu GH, et al. Engineering magnetosomes for ferroptosis/immunomodulation synergism in cancer. ACS Nano. 2019;13(5):5662–5673. doi:10.1021/acsnano.9b00892
167. Gungabeesoon J, Gort-Freitas NA, Kiss M, et al. A neutrophil response linked to tumor control in immunotherapy. Cell. 2023;186(7):1448–1464.e1420. doi:10.1016/j.cell.2023.02.032
168. Siwicki M, Pittet MJ. Versatile neutrophil functions in cancer. Semin Immunol. 2021;57:101538. doi:10.1016/j.smim.2021.101538
169. Coffelt SB, Kersten K, Doornebal CW, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345–348. doi:10.1038/nature14282
170. Kang T, Zhu Q, Wei D, et al. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano. 2017;11(2):1397–1411. doi:10.1021/acsnano.6b06477
171. Zhang C, Zhang L, Wu W, et al. Artificial super neutrophils for inflammation targeting and HClO generation against tumors and infections. Adv Mater. 2019;31(19):e1901179. doi:10.1002/adma.201901179
172. Cao X, Hu Y, Luo S, et al. Neutrophil-mimicking therapeutic nanoparticles for targeted chemotherapy of pancreatic carcinoma. Acta Pharm Sin B. 2019;9(3):575–589. doi:10.1016/j.apsb.2018.12.009
173. Xue J, Zhao Z, Zhang L, et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12(7):692–700. doi:10.1038/nnano.2017.54
174. Xia C, Bai W, Deng T, et al. Sponge-like nano-system suppresses tumor recurrence and metastasis by restraining myeloid-derived suppressor cells-mediated immunosuppression and formation of pre-metastatic niche. Acta Biomater. 2023;158:708–724. doi:10.1016/j.actbio.2023.01.009
175. Yu X, Xing G, Sheng S, et al. Neutrophil camouflaged stealth nanovehicle for photothermal-induced tumor immunotherapy by triggering pyroptosis. Adv Sci. 2023;10(15):e2207456. doi:10.1002/advs.202207456
176. Lancaster JN, Thyagarajan HM, Srinivasan J, Li Y, Hu Z, Ehrlich LIR. Live-cell imaging reveals the relative contributions of antigen-presenting cell subsets to thymic central tolerance. Nature Commun. 2019;10(1):2220. doi:10.1038/s41467-019-09727-4
177. Kurotaki D, Kawase W, Sasaki H, et al. Epigenetic control of early dendritic cell lineage specification by the transcription factor IRF8 in mice. Blood. 2019;133(17):1803–1813. doi:10.1182/blood-2018-06-857789
178. Carreno BM, Magrini V, Becker-Hapak M, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348(6236):803–808. doi:10.1126/science.aaa3828
179. Liang X, Cheng H, Liu C, Liu G. Antigen self-presenting nanovaccine for cancer immunotherapy. Sci Bull. 2022;67(16):1611–1613. doi:10.1016/j.scib.2022.07.018
180. Cheng S, Xu C, Jin Y, et al. Artificial mini dendritic cells boost T cell-based immunotherapy for ovarian cancer. Adv Sci (Weinh). 2020;7(7):1903301. doi:10.1002/advs.201903301
181. Liu C, Liu X, Xiang X, et al. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy. Nature Nanotechnol. 2022;17(5):531–540. doi:10.1038/s41565-022-01098-0
182. Ma X, Kuang L, Yin Y, et al. Tumor-antigen activated dendritic cell membrane-coated biomimetic nanoparticles with orchestrating immune responses promote therapeutic efficacy against glioma. ACS Nano. 2022;17(3):2341–2355. doi:10.1021/acsnano.2c09033
183. Yang X, Yang T, Liu Q, et al. Biomimetic aggregation‐induced emission nanodots with hitchhiking function for T cell‐mediated cancer targeting and NIR‐II fluorescence‐guided mild‐temperature photothermal therapy. Adv Funct Mater. 2022;32(45):2206346.1–2206346.13. doi:10.1002/adfm.202206346
184. Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717–734. doi:10.1038/nrclinonc.2017.101
185. Kang M, Hong J, Jung M, et al. T-cell-mimicking nanoparticles for cancer immunotherapy. Adv Mater. 2020;32(39):e2003368. doi:10.1002/adma.202003368
186. Zhang L, Li R, Chen H, et al. Human cytotoxic T-lymphocyte membrane-camouflaged nanoparticles combined with low-dose irradiation: a new approach to enhance drug targeting in gastric cancer. Int J Nanomed. 2017;12:2129–2142. doi:10.2147/IJN.S126016
187. Zhai Y, Wang J, Lang T, et al. T lymphocyte membrane-decorated epigenetic nanoinducer of interferons for cancer immunotherapy. Nat Nanotechnol. 2021;16(11):1271–1280. doi:10.1038/s41565-021-00972-7
188. Ma W, Zhu D, Li J, et al. Coating biomimetic nanoparticles with chimeric antigen receptor T cell-membrane provides high specificity for hepatocellular carcinoma photothermal therapy treatment. Theranostics. 2020;10(3):1281–1295. doi:10.7150/thno.40291
189. Hazeldine J, Lord JM. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res Rev. 2013;12(4):1069–1078. doi:10.1016/j.arr.2013.04.003
190. Baier C, Fino A, Sanchez C, et al. Natural killer cells modulation in hematological malignancies. Front Immunol. 2013;4:459. doi:10.3389/fimmu.2013.00459
191. Kim S, Poursine-Laurent J, Truscott SM, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–713. doi:10.1038/nature03847
192. Keener AB. Natural killers: cataloging immune cells for immunotherapy. Nat Med. 2015;21(3):207–208. doi:10.1038/nm0315-207
193. Zheng C, Zhong Q, Song W, et al. Membrane-fusion-mediated multiplex engineering of tumor cell surface glycans for enhanced NK cell therapy. Adv Mater. 2023;35(14):e2206989. doi:10.1002/adma.202206989
194. Pan S, Guan J, Xianyu B, Tan Y, Li T, Xu H. A nanotherapeutic strategy to reverse NK cell exhaustion. Adv Mater. 2023;35(23):e2211370. doi:10.1002/adma.202211370
195. Liu S, Galat V, Galat Y, Lee YKA, Wainwright D, Wu J. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol. 2021;14(1):7. doi:10.1186/s13045-020-01014-w
196. Deng G, Sun Z, Li S, et al. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12(12):12096–12108. doi:10.1021/acsnano.8b05292
197. Du W, Chen C, Sun P, et al. Eliciting an immune hot tumor niche with biomimetic drug-based multi-functional nanohybrids augments immune checkpoint blockade-based breast cancer therapy. Nanoscale. 2020;12(5):3317–3329. doi:10.1039/C9NR09835F
198. Pitchaimani A, Nguyen TDT, Aryal S. Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials. 2018;160:124–137. doi:10.1016/j.biomaterials.2018.01.018
199. Poetsch A, Wolters D. Bacterial membrane proteomics. Proteomics. 2008;8(19):4100–4122. doi:10.1002/pmic.200800273
200. Gao W, Fang RH, Thamphiwatana S, et al. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015;15(2):1403–1409. doi:10.1021/nl504798g
201. Patel RB, Ye M, Carlson PM, et al. Development of an in situ cancer vaccine via combinational radiation and bacterial-membrane-coated nanoparticles. Adv Mater. 2019;31(43):e1902626. doi:10.1002/adma.201902626
202. Naskar A, Cho H, Lee S, Kim KS. Biomimetic nanoparticles coated with bacterial outer membrane vesicles as a new-generation platform for biomedical applications. Pharmaceutics. 2021;13(11):1887. doi:10.3390/pharmaceutics13111887
203. Gao C, Wang Q, Li J, et al. In vivo hitchhiking of immune cells by intracellular self-assembly of bacteria-mimetic nanomedicine for targeted therapy of melanoma. Sci Adv. 2022;8(19):eabn1805. doi:10.1126/sciadv.abn1805
204. Chen W, Song Y, Bai S, et al. Cloaking mesoporous polydopamine with bacterial membrane vesicles to amplify local and systemic antitumor immunity. ACS Nano. 2023;17(8):7733–7749. doi:10.1021/acsnano.3c00363
205. Dong L, Huang CY, Johnson EJ, et al. High-throughput simultaneous mRNA profiling using nCounter technology demonstrates that extracellular vesicles contain different mRNA transcripts than their parental prostate cancer cells. Anal Chem. 2021;93(8):3717–3725. doi:10.1021/acs.analchem.0c03185
206. Sartorio MG, Pardue EJ, Feldman MF, Haurat MF. Bacterial outer membrane vesicles: from discovery to applications. Annu Rev Microbiol. 2021;75(1):609–630. doi:10.1146/annurev-micro-052821-031444
207. Deshmukh SK, Khan MA, Singh S, Singh AP. Extracellular nanovesicles: from intercellular messengers to efficient drug delivery systems. ACS Omega. 2021;6(3):1773–1779. doi:10.1021/acsomega.0c05539
208. Yellon DM, Davidson SM. Exosomes: nanoparticles involved in cardioprotection? Circ Res. 2014;114(2):325–332. doi:10.1161/CIRCRESAHA.113.300636
209. Weng Z, Zhang B, Wu C, et al. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J Hematol Oncol. 2021;14(1):136. doi:10.1186/s13045-021-01141-y
210. Xiao Q, Zhao W, Wu C, et al. Lemon-derived extracellular vesicles nanodrugs enable to efficiently overcome cancer multidrug resistance by endocytosis-triggered energy dissipation and energy production reduction. Adv Sci. 2022;9(20):e2105274. doi:10.1002/advs.202105274
211. Lim KM, Han JH, Lee Y, et al. Rapid production method with increased yield of high-purity extracellular vesicles obtained using extended mitochondrial targeting domain peptide. J Extracell Vesicles. 2022;11(10):e12274. doi:10.1002/jev2.12274
212. Zhao L, Gu C, Gan Y, Shao L, Chen H, Zhu H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Control Release. 2020;318:1–15. doi:10.1016/j.jconrel.2019.12.005
213. Pei W, Li X, Bi R, et al. Exosome membrane-modified M2 macrophages targeted nanomedicine: treatment for allergic asthma. J Control Release. 2021;338:253–267. doi:10.1016/j.jconrel.2021.08.024
214. Zuo L, Nie W, Yu S, et al. Biomimetic nanovesicle with mitochondria-synthesized sonosensitizer and mitophagy inhibition for cancer sono-immunotherapy. Nano Lett. 2023;23(7):3005–3013. doi:10.1021/acs.nanolett.3c00383
215. Zhu Z, Zhai Y, Hao Y, et al. Specific anti-glioma targeted-delivery strategy of engineered small extracellular vesicles dual-functionalised by Angiopep-2 and TAT peptides. J Extracell Vesicles. 2022;11(8):e12255. doi:10.1002/jev2.12255
216. Hosseini-Giv N, Basas A, Hicks C, El-Omar E, El-Assaad F, Hosseini-Beheshti E. Bacterial extracellular vesicles and their novel therapeutic applications in health and cancer. Front Cell Infect Microbiol. 2022;12:962216. doi:10.3389/fcimb.2022.962216
217. Liu H, Zhang H, Han Y, Hu Y, Geng Z, Su J. Bacterial extracellular vesicles-based therapeutic strategies for bone and soft tissue tumors therapy. Theranostics. 2022;12(15):6576–6594. doi:10.7150/thno.78034
218. Suri K, D’Souza A, Huang D, Bhavsar A, Amiji M. Bacterial extracellular vesicle applications in cancer immunotherapy. Bioact Mater. 2023;22:551–566. doi:10.1016/j.bioactmat.2022.10.024
219. Lusta KA, Poznyak AV, Litvinova L, Poggio P, Orekhov AN, Melnichenko AA. Involvement of bacterial extracellular membrane nanovesicles in infectious diseases and their application in medicine. Pharmaceutics. 2022;14(12):2597. doi:10.3390/pharmaceutics14122597
220. Lieberman LA. Outer membrane vesicles: a bacterial-derived vaccination system. Front Microbiol. 2022;13:1029146. doi:10.3389/fmicb.2022.1029146
221. Cheng K, Zhao R, Li Y, et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via plug-and-display technology. Nature Commun. 2021;12(1):2041. doi:10.1038/s41467-021-22308-8
222. Li Y, Zhang K, Wu Y, et al. Antigen capture and immune modulation by bacterial outer membrane vesicles as in situ vaccine for cancer immunotherapy post-photothermal therapy. Small. 2022;18(14):e2107461. doi:10.1002/smll.202107461
223. Wang S, Gao J, Wang Z. Outer membrane vesicles for vaccination and targeted drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(2):e1523. doi:10.1002/wnan.1523
224. Weyant KB, Oloyede A, Pal S, et al. A modular vaccine platform enabled by decoration of bacterial outer membrane vesicles with biotinylated antigens. Nat Commun. 2023;14(1):464. doi:10.1038/s41467-023-36101-2
225. Feng B, Wu J, Shen B, Jiang F, Feng J. Cancer-associated fibroblasts and resistance to anticancer therapies: status, mechanisms, and countermeasures. Cancer Cell Int. 2022;22(1):166. doi:10.1186/s12935-022-02599-7
226. Li H, Wan J. Lipid metabolism in tumor-associated fibroblasts. Adv Exp Med Biol. 2021;1316:117–131. doi:10.1007/978-981-33-6785-2_8
227. Li J, Zhen X, Lyu Y, Jiang Y, Huang J, Pu K. Cell membrane coated semiconducting polymer nanoparticles for enhanced multimodal cancer phototheranostics. ACS Nano. 2018;12(8):8520–8530. doi:10.1021/acsnano.8b04066
228. Zhang Y, Liao Y, Tang Q, Lin J, Huang P. Biomimetic nanoemulsion for synergistic photodynamic-immunotherapy against hypoxic breast tumor. Angew Chem Int Ed Engl. 2021;60(19):10647–10653. doi:10.1002/anie.202015590
229. Niu W, Xiao Q, Wang X, et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21(3):1484–1492. doi:10.1021/acs.nanolett.0c04753
230. Fang RH, Gao W, Zhang L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat Rev Clin Oncol. 2023;20(1):33–48. doi:10.1038/s41571-022-00699-x
231. Dehaini D, Wei X, Fang RH, et al. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv Mater. 2017;29(16). doi:10.1002/adma.201606209
232. Wang D, Dong H, Li M, et al. Erythrocyte–cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma. ACS Nano. 2018;12(6):5241–5252. doi:10.1021/acsnano.7b08355
233. Rezaei S, de Araújo Júnior RF, da Silva ILG, Schomann T, Eich C, Cruz LJ. Erythrocyte-cancer hybrid membrane-coated reduction-sensitive nanoparticles for enhancing chemotherapy efficacy in breast cancer. Biomater Adv. 2023;151:213456. doi:10.1016/j.bioadv.2023.213456
234. Xiong J, Wu M, Chen J, et al. Cancer-erythrocyte hybrid membrane-camouflaged magnetic nanoparticles with enhanced photothermal-immunotherapy for ovarian cancer. ACS nano. 2021;15(12):19756–19770. doi:10.1021/acsnano.1c07180
235. Sun M, Duan Y, Ma Y, Zhang Q. Cancer cell-erythrocyte hybrid membrane coated gold nanocages for near infrared light-activated photothermal/radio/chemotherapy of breast cancer. Int J Nanomed. 2020;15:6749–6760. doi:10.2147/IJN.S266405
236. Li H, Peng Q, Yang L, et al. High-performance dual combination therapy for cancer treatment with hybrid membrane-camouflaged mesoporous silica gold nanorods. ACS Appl Mater Interfaces. 2020;12(52):57732–57745. doi:10.1021/acsami.0c18287
237. Wu L, Li Q, Deng J, et al. Platelet-tumor cell hybrid membrane-camouflaged nanoparticles for enhancing therapy efficacy in Glioma. Int J Nanomed. 2021;16:8433–8446. doi:10.2147/IJN.S333279
238. Li Z, Yang G, Han L, Wang R, Gong C, Yuan Y. Sorafenib and triptolide loaded cancer cell-platelet hybrid membrane-camouflaged liquid crystalline lipid nanoparticles for the treatment of hepatocellular carcinoma. J Nanobiotechnology. 2021;19(1):360. doi:10.1186/s12951-021-01095-w
239. Ma J, Dai L, Yu J, et al. Tumor microenvironment targeting system for glioma treatment via fusion cell membrane coating nanotechnology. Biomaterials. 2023;295:122026. doi:10.1016/j.biomaterials.2023.122026
240. Rao L, Meng QF, Huang Q, et al. Platelet–leukocyte hybrid membrane‐coated immunomagnetic beads for highly efficient and highly specific isolation of circulating tumor cells. Adv. Funct. Mater. 2018;28(34):1803531.1–1803531.9. doi:10.1002/adfm.201803531
241. He H, Guo C, Wang J, et al. Leutusome: a biomimetic nanoplatform integrating plasma membrane components of leukocytes and tumor cells for remarkably enhanced solid tumor homing. Nano Lett. 2018;18(10):6164–6174. doi:10.1021/acs.nanolett.8b01892
242. Gong C, Yu X, You B, et al. Macrophage-cancer hybrid membrane-coated nanoparticles for targeting lung metastasis in breast cancer therapy. J Nanobiotechnology. 2020;18(1):92. doi:10.1186/s12951-020-00649-8
243. Liu WL, Zou MZ, Liu T, et al. Expandable immunotherapeutic nanoplatforms engineered from cytomembranes of hybrid cells derived from cancer and dendritic cells. Adv Mater. 2019;31(18):e1900499. doi:10.1002/adma.201900499
244. Hao W, Cui Y, Fan Y, et al. Hybrid membrane-coated nanosuspensions for multi-modal anti-glioma therapy via drug and antigen delivery. J Nanobiotechnology. 2021;19(1):378. doi:10.1186/s12951-021-01110-0
245. Bu LL, Rao L, Yu GT, et al. Cancer stem cell‐platelet hybrid membrane‐coated magnetic nanoparticles for enhanced photothermal therapy of head and neck squamous cell carcinoma. Adv. Funct. Mater. 2019;29(10):1807733–1807733. doi:10.1002/adfm.201807733
246. Yin Y, Tang W, Ma X et al. Biomimetic neutrophil and macrophage dual membrane-coated nanoplatform with orchestrated tumor-microenvironment responsive capability promotes therapeutic efficacy against glioma. Chem Eng J. 2022;433P3:133848,2–11.doi:10.1016/j.cej.2021.133848
247. Chen Q, Huang G, Wu W, et al. A hybrid eukaryotic-prokaryotic nanoplatform with photothermal modality for enhanced antitumor vaccination. Adv Mater. 2020;32(16):e1908185. doi:10.1002/adma.201908185
248. Wang D, Liu C, You S, et al. Bacterial vesicle-cancer cell hybrid membrane-coated nanoparticles for tumor specific immune activation and photothermal therapy. Acs Appl Mater Interfaces. 2020;12(37):41138–41147. doi:10.1021/acsami.0c13169
249. Zhang W, Gong C, Chen Z, Li M, Li Y, Gao J. Tumor microenvironment-activated cancer cell membrane-liposome hybrid nanoparticle-mediated synergistic metabolic therapy and chemotherapy for non-small cell lung cancer. J Nanobiotechnology. 2021;19(1):339. doi:10.1186/s12951-021-01085-y
250. Ye H, Wang K, Zhao J, et al. In situ sprayed nanovaccine suppressing exosomal PD-L1 by golgi apparatus disorganization for postsurgical melanoma immunotherapy. ACS Nano. 2023;17(11):10637–10650. doi:10.1021/acsnano.3c01733
251. Zhang J, Wei L, Ma X, et al. pH-sensitive tumor-tropism hybrid membrane-coated nanoparticles for reprogramming the tumor microenvironment and boosting the antitumor immunity. Acta Biomater. 2023;166:470–484. doi:10.1016/j.actbio.2023.05.040
252. Ma W, Yang Y, Zhu J, et al. Biomimetic nanoerythrosome-coated aptamer-DNA tetrahedron/maytansine conjugates: pH-responsive and targeted cytotoxicity for HER2-positive breast cancer. Adv Mater. 2022;34(46):e2109609. doi:10.1002/adma.202109609
253. Rao L, Wu L, Liu Z, et al. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. NAT COMMUN. 2020;11(1):4909. doi:10.1038/s41467-020-18626-y
254. Wang W, Liu S, Dai P, et al. Elucidating mechanisms of antitumor immunity mediated by live oncolytic vaccinia and heat-inactivated vaccinia. J Immunother Cancer. 2021;9(9):e002569. Doi:10.1136/jitc-2021-002569
255. Silva-Pilipich N, Blanco E, Lozano T, et al. Local delivery of optimized nanobodies targeting the PD-1/PD-L1 axis with a self-amplifying RNA viral vector induces potent antitumor responses. Cancer Lett. 2023;561:216139. doi:10.1016/j.canlet.2023.216139
256. Zhao X, Wang Y, Jiang W, et al. Herpesvirus-mimicking DNAzyme-loaded nanoparticles as a mitochondrial DNA stress inducer to activate innate immunity for tumor therapy. Adv Mater. 2022;34(37):e2204585. doi:10.1002/adma.202204585
257. Han S, Wang W, Wang S, et al. Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes. Theranostics. 2021;11(6):2892–2916. doi:10.7150/thno.50928
258. Xue C, Hu S, Gao ZH, et al. Programmably tiling rigidified DNA brick on gold nanoparticle as multi-functional shell for cancer-targeted delivery of siRNAs. Nat Commun. 2021;12(1):2928. doi:10.1038/s41467-021-23250-5
259. Yuan P, Chen X, Li X, et al. Effect of Cell Membrane-cloaked Nanoparticle Elasticity on Nano-Bio Interaction. Small Methods;2023.;7(6):e2201548. doi:10.1002/smtd.202201548
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
