Back to Journals » International Journal of Nanomedicine » Volume 18
Biomembrane-Derived Nanoparticles in Alzheimer’s Disease Therapy: A Comprehensive Review of Synthetic Lipid Nanoparticles and Natural Cell-Derived Vesicles
Authors Gao C, Liu Y, Zhang TL, Luo Y, Gao J, Chu JJ, Gong BF, Chen XH, Yin T, Zhang J, Yin Y
Received 20 September 2023
Accepted for publication 16 November 2023
Published 7 December 2023 Volume 2023:18 Pages 7441—7468
DOI https://doi.org/10.2147/IJN.S436774
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Chao Gao,1,* Yan Liu,2,* Ting-Lin Zhang,3,* Yi Luo,2,4,* Jie Gao,3,* Jian-Jian Chu,1 Bao-Feng Gong,1 Xiao-Han Chen,1 Tong Yin,1 Jian Zhang,2 You Yin1
1Department of Neurology, Second Affiliated Hospital (Shanghai Changzheng Hospital) of Naval Medical University, Shanghai, People’s Republic of China; 2Department of Clinical Pharmacy, Shanghai Jiao Tong University of Medicine, Shanghai, People’s Republic of China; 3Changhai Clinical Research Unit, Shanghai Changhai Hospital of Naval Medical University, Shanghai, People’s Republic of China; 4New Drug Discovery and Development, Biotheus Inc., Zhuhai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: You Yin; Jian Zhang, Tel +86-21-81885463 ; +86- 21-25077150, Email [email protected]; [email protected]
Abstract: Current therapies for Alzheimer’s disease used in the clinic predominantly focus on reducing symptoms with limited capability to control disease progression; thus, novel drugs are urgently needed. While nanoparticles (liposomes, high-density lipoprotein-based nanoparticles) constructed with synthetic biomembranes have shown great potential in AD therapy due to their excellent biocompatibility, multifunctionality and ability to penetrate the BBB, nanoparticles derived from natural biomembranes (extracellular vesicles, cell membrane-based nanoparticles) display inherent biocompatibility, stability, homing ability and ability to penetrate the BBB, which may present a safer and more effective treatment for AD. In this paper, we reviewed the synthetic and natural biomembrane-derived nanoparticles that are used in AD therapy. The challenges associated with the clinical translation of biomembrane-derived nanoparticles and future perspectives are also discussed.
Keywords: Alzheimer’s disease, nanoparticles, blood‒brain barrier, liposomes, bionanotechnology, neurodegenerative diseases
Graphical Abstract:
Introduction
Alzheimer’s disease (AD) is the most prevalent form of dementia, which accounts for approximately 50% to 80% of all cases of dementia and primarily manifests as memory loss, cognitive dysfunction, and impairment in daily activities. To be consistent with the world population aging, the number of people who suffer from dementia is rising exponentially.1 According to a global prospective study, 131.5 million new cases are estimated by 2050, which would significantly negatively impact on the economy and healthcare systems.2,3 Although the pathophysiology of AD is still unknown, theories about how AD develops have been proposed, including some well-known hypotheses such as amyloid accumulation, tau protein hyperphosphorylation, oxidative stress, and mitochondrial dysfunction.4,5 For instance, recent studies suggest that the “acidification” disorder of lysosomes in neuronal cells may cause increasing extracellular β-amyloid (Aβ) deposition and thus lead to disease progression,6 which has led to a new understanding of the pathogenesis of AD.
Our brain’s fragile structure is protected from outside injury, traumatic infections, and other irritants by the blood-brain barrier (BBB), which also helps to keep our brains stable and healthy. However, it also stops medications for AD treatment from accessing the brain, decreasing the efficacy.7 Only five oral medications—galantamine, donepezil, tacrine, rivastigmine, and memantine—have been given FDA approval to treat AD. However, these drugs only reduce the symptoms with minimal help in preventing disease progression, which may be primarily due to insufficient drug concentration at the lesions caused by BBB prevention. Moreover, long-term medication causes a high financial burden8 and significantly interferes with the efficacy of AD treatment.9 In the past few decades, scientists have developed various drug delivery systems, eg, nanoparticles, to improve the efficiency of drugs crossing the BBB and reaching lesions, which may start a new era of AD therapy. Although some reviews have discussed the development of one or a few nanoparticles (liposomes, high-density lipoprotein nanostructures, cell membrane nanostructures, or extracellular vesicles) carrying drugs for the treatment of AD, to our knowledge, there has not yet been a thorough and systematic review comparing most if not all the types of nanoparticles investigated in AD therapy. As a result, we thoroughly summarized nanoparticles used in AD therapy, including their biomembrane features, postmodification strategies, and mechanisms of action, to improve our understanding of current options for nanoparticles used in AD therapy, which in turn sheds light on directions for next-generation nanoparticle optimization that eventually cures AD.
Biomembrane-derived nanoparticles can be classified as synthetic membrane (lipids or proteins)-derived nanoparticles, such as liposomes and high-density lipoproteins, or natural biomembrane-derived nanoparticles, such as cell-derived nanovesicles. Compared to other drug carriers, biomembrane-derived nanoparticles show superior biocompatibility, lower immunogenicity, better drug-loading capability, and better stability. In this review, we discussed the three principal features of these nanoparticles in AD therapy: their biocompatibility, drug-loading capability, efficiency in delivering drugs across the BBB, and ability to target brain lesions. Additionally, we covered post-modification techniques for enhancing nanoparticle bioactivity and druggability and suggested using the most recent nanoparticle technology to treat AD.
Challenges of Conventional AD Therapies
Even though there have been several investigations on AD, the molecular mechanism of AD development remains unclear. The majority of society, however, favors the theory that AD development results from the accumulation of -amyloid at brain lesions, which causes a significant amount of neuronal death. Accordingly, drugs for treating AD have been developed. For instance, cholinesterase inhibitors (galantamine, donepezil, tacrine, and rivastigmine) were approved in all-stage AD patients, N-methyl-D-aspartic acid (NMDA) receptor antagonist (memantine) was approved for patients with moderate to severe stages of dementia, and Aβ monoclonal antibody (ducanumab) was recently approved by the FDA for use in mild dementia patients. However, the overall efficacy of these drugs on the market is poor. Therefore, significant investments are continuously spent on novel drug development for AD therapy. Currently, 143 drugs are under clinical investigation, spread across 172 clinical trials, mainly led by big pharma, including Boeing, Eli Lilly, Roche, and Novo Nordisk. Among them, 31 were at the Phase 3 stage, 82 were at the Phase 2 stage, and 30 were at the Phase 1 stage.10 Lecanemab, the second Aβ monoclonal antibody medication for AD in the world, which precisely binds with soluble Aβ protofibrils, significantly improved cognitive dysfunction during clinical tests. However, conventional drugs often face some inherent challenges, including instability in the body, poor BBB penetration, and lack of lesion-targeting capability, resulting in insufficient concentration at lesions and poor efficacy (Figure 1).
Instability of Drugs During Circulation
Conventional drugs, including proteins, nucleic acids, and large or small molecules, often face a stability problem during circulation in the body due to the complexity of the human body environment. This significantly decreases the therapeutic efficacy.11 For instance, conventional drugs enter the gastrointestinal tract by oral administration, and the intestinal mucosal epithelium will not wholly absorb these drugs into the blood. As a result of the liver’s first-pass metabolism and detoxification, as well as the reticuloendothelial system’s clearance action, drugs that are absorbed into the blood frequently experience low levels of drug concentration. Additionally, because most of the reported multifunctional cholinesterase inhibitors are carbamate-based compounds, they are easily hydrolyzed and thus unstable in vivo, significantly influencing the theoretical efficacy.12 Therefore, enhancing the stability of drugs during circulation in the body and developing carriers (eg, nanoparticles) that reduce the off-target systemic toxicity of medications may improve the efficacy of AD therapy.
Poor BBB Penetration
Drug penetration across the BBB is the predominant challenge in AD therapy since the BBB often prevents sufficient drug entry into brain lesions, which addresses the therapeutic effect. The anatomical structure of the BBB (Figure 2) and its biological function lead to the low permeability of the BBB via four main mechanisms. First, to maintain vascular stability and the integrity of the BBB, endothelial cells (ECs), which are the primary functional component of the BBB, form a thin layer via three kinds of junctions. These junctions increase EC tightness by 50–100-fold compared to that found in the peripheral microvascular wall, which prevents chemicals and cells from entering the brain.13,14 Second, the BBB offers an enzymatic barrier to metabolize substances, further preventing molecules from passing through the endothelial cell-based physical barrier and entering the brain parenchyma, which may interfere with neuronal activity.15 Third, the BBB prevents molecules from entering the brain by forming a barrier of efflux transport proteins on the luminal and extraluminal membranes.16 Last, the BBB behaves as an immune barrier via a close interaction system developed by Brain Microvascular Endothelial Cells (BMECs), mast cells, perivascular macrophages, T cells, and microglia, which in turn results in the unique immune environment in the brain. This immune barrier protects the brain from toxic substances and significantly diminishes the delivery of various nutrients, ions, and AD therapeutic drugs.17 More than 98% of small molecule medications, including recombinant proteins, peptides, nucleic acids, and monoclonal antibodies, cannot cross the BBB.18 Among those small molecules that can cross the BBB, most are used for treating emotional disorders, pain, insomnia, and epilepsy, while only a few drugs have been applied in AD therapy.
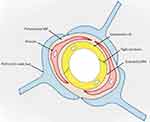 |
Figure 2 Schematic snapshot of the blood–brain barrier. Reprinted from Xu L, Nirwane A, Yao Y. Basement membrane and blood-brain barrier. Stroke Vasc Neurol. 2019;4(2):78–82. Creative Commons.14 |
Lack of Lesion-Targeting Capability
Another challenge in developing therapeutic medications for AD is the lack of lesion-targeting ability after entering the brain, which further results in insufficient drug concentrations at disease sites.11 To increase the on-site drug concentration, high doses of drugs and longer drug exposure times are needed; however, both may increase the risk of systemic toxicity.19,20 Polymeric and inorganic nano drug delivery systems may improve drug delivery efficiency at brain lesions to some extent; however, their poor specificity and biocompatibility still significantly limit therapeutic efficacy, and thus, new medicines with better targeting capability are needed.21 Here, we reviewed biomembrane-derived nanoparticles carrying drugs for AD therapy, which may overcome the challenges of conventional AD drugs.
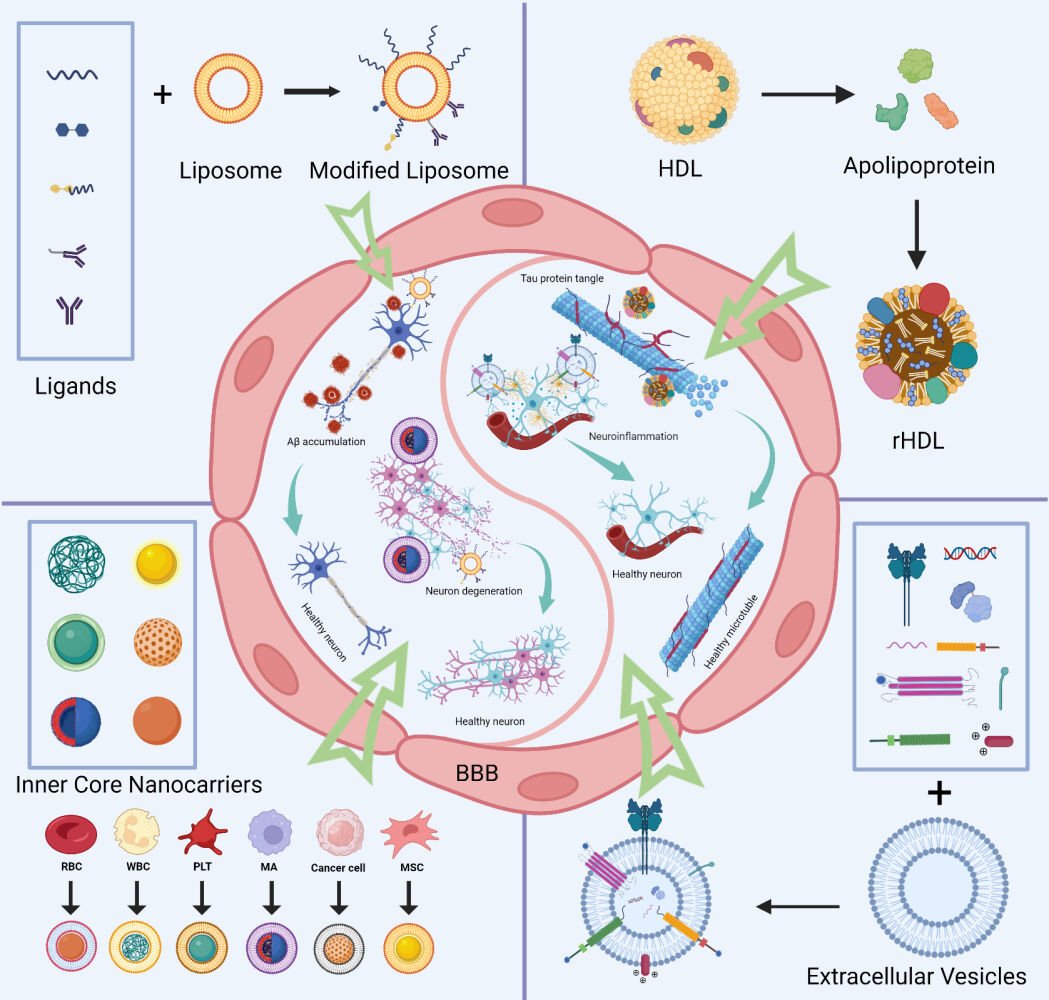
Biomembrane-Derived Nanoparticles for AD Therapy
Therapeutic or diagnostic medications could be delivered to AD lesions using nanoparticles, a well-proven drug delivery technology, for targeted therapy. Numerous biological restrictions imposed by conventional medicines, such as immune clearance, off-target buildup, poor blood circulation, and poor stability, can be addressed using nanoparticles. However, synthetic nanoparticles may raise biocompatibility problems since the body recognizes them as “foreign products”, which then induce an immune response. This restricts the application of artificial nanoparticles in AD therapy. Biomembrane-derived nanoparticles have the following advantages: (1) good biocompatibility: lipids and other natural substances are biodegradable with low or nontoxicity to humans; (2) multifunctional: suitable lesion-targeting capability, active penetration ability, various drug-loading capability, and high modification potential; and (3) high bioactivity: packing molecules that induce therapeutic effects on specific diseases. Therefore, biomembrane-derived nanoparticles may improve drug solubility, bioavailability, and drug accumulation in AD lesions. They also display diverse biofunctions and high bioactivity. These characteristics permit the development of nanoparticles for targeted AD therapy, paving the way for creating precise medications for the effective and safe treatment of AD.
To date, synthetic and natural biomembrane-derived nanoparticles have unique advantages and limitations, as summarized in Table 1. The nature of biomembranes enables them to be readily postmodified, multifunctionally specialized, and used to overcome the difficulties in AD-targeted therapy, although there are considerable variances in terms of origin, production process, biomolecular composition, etc. Biomembrane-derived nanoparticles can be modified to fulfill a range of needs related to medication delivery, disease therapy, and diagnosis. These methods include membrane hybridization, chemical approaches, postinsertion method, metabolic engineering, and gene engineering.22,23 Table 2 provides a summary of the benefits and drawbacks of various biomembrane engineering techniques.24
 |
Table 1 Characters of Biomembrane-Derived Nanoparticles for AD Treatment |
 |
Table 2 Summary of the Advantages and Disadvantages of Different Methods of Engineering Biomembranes |
Liposomes
An aqueous core is enclosed by one or more lipophilic bilayers inside the spherical, hollow structure known as a liposome.25,26 The diameter of liposomes can vary from 100 nm to 2.5 mm. Normally, liposomes form spontaneously by the interaction of water molecules with the hydrophilic head of lipids at one end and the attachment of the hydrophobic tails of lipids at the other end. As a result of their amphiphilicity, liposomes can combine hydrophilic and hydrophobic substances.27 Liposomes have emerged as an up-and-coming area for drug delivery in AD-targeted therapy due to their exceptional drug-loading capacity, excellent stability throughout the circulation, good BBB penetration capability, and lesion-targeting ability (Figure 3A).28,29
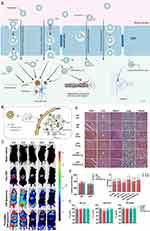 |
Figure 3 Schematic diagram and advantageous study of liposomes in AD-targeted therapy. (A) Schematic diagram of liposome-targeted therapy for AD through different pathways. Image created with Biorender.com. (B) Schematic illustration of the strategy for improving AD-related pathology by Tf-modified osthole liposomes. Reprinted with permission from Dove Medical Press. Kong L, Li XT, Ni YN, et al. Transferrin-modified osthole PEGylated liposomes travel the blood-brain barrier and mitigate Alzheimer’s disease-related pathology in APP/PS-1 mice. Int J Nanomedicine. 2020;15:2841–2858.28 (C) Real-time imaging observation after intravenous administration of varying liposomal formulations in APP/PS-1 mice (n=3). Reprinted with permission from Dove Medical Press. Kong L, Li XT, Ni YN, et al. Transferrin-modified osthole PEGylated liposomes travel the blood-brain barrier and mitigate Alzheimer’s disease-related pathology in APP/PS-1 mice. Int J Nanomedicine. 2020;15:2841–2858.28 (D–F) The biosafety of Tf-Pep63-Lip in vivo and in vitro: Tf-Pep63-Lip and Tf-Lip do not induce adverse effects in vivo and in vitro. Reprinted from Yang X, Li X, Liu L, et al. Transferrin-Pep63-liposomes accelerate the clearance of Abeta and rescue impaired synaptic plasticity in early Alzheimer’s disease models. Cell Death Discov. 2021;7(1):256. Creative Commons.29 |
Biosafety and Drug-Loading Capacity
Liposomes are regarded as a low/nontoxic and biocompatible drug delivery technology since they are made from phospholipids, which comprise the majority of biological membranes and may be produced naturally or synthetically.9 However, liposomes have poor serum stability and are readily absorbed by the reticuloendothelial system. These issues could be resolved by modifying the biomembrane and the surface of the liposomes.30 For instance, a biomembrane consisting of components similar to the erythrocyte cell membrane was generated and displayed resistance to monocyte macrophage system (MPS)-mediated clearance. Additionally, adding negatively charged molecules like monosialotetrahexosylganglioside (GM1), PI, or neutral or positive charges to the biomembrane of liposomes may increase their ability to cross the BBB. Still, they significantly inhibit MPS-mediated clearance, lengthen the drug’s half-life in circulation, all of which lead to adequate drug exposure at the targeted site.28,31–34 For another instance, the insertion of SM (sphingomyelin) into the biomembrane of liposomes stabilizes the membrane structure and increases the rigidity of liposomes, which can eventually diminish MPS-mediated clearance. It has been demonstrated that additional modifications, such as the addition of ligands or polyethyleneglycol (PEG)-conjugated antibodies to the biomembrane of liposomes, increase serum stability, lesion-targeting capability, and lower systemic toxicity.34–36 And there are no obvious morphological alterations after transferrin (Tf) modified liposomes was injected to the AD model mice (Figure 3D–F).29
Liposomes can load hydrophilic, lipophilic, or amphiphilic molecules. Under the protection of a hydrophobic lipid bilayer, soluble drugs encapsulated would not spread easily through the membrane, while lipophilic molecules bind to the lipid membrane via hydrophobic interactions. Liposomes can also load amphiphilic drugs at the junction between the aqueous phase and the interior lipid membrane. Although concerns regarding rapid drug release, plasma instability, and uptake to the reticuloendothelial system have been voiced, these issues can be resolved using postmodifications.35,37 For instance, adding cholesterol or 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine to liposomes’ biomembrane makes them more stable in plasma and eliminates the problem of quickly off-target drug release.38,39 Additionally, it has been demonstrated that conjugation of PEG to the liposome surface increases the circulation duration by up to 55 hours. Further, the mononuclear phagocyte system’s ability to clear substances (stealth liposomes) was significantly diminished by PEG decoration.31,40,41
BBB Penetrating Ability
Different engineering strategies have been investigated by focusing on different BBB transport pathways, including receptor-mediated transcytosis, carrier-mediated transcytosis, CPP (cell-penetrating peptide)-mediated endocytosis, and adsorptive transcytosis, to achieve effective BBB penetration of the nanoparticle and sufficient drug delivery at brain lesions.42 Modification strategies targeting these pathways could significantly improve the efficiency of AD drug delivery, increase the drug concentration at lesions, and maximize the therapeutic efficacy of AD.
First, the most effective and widely utilized method for modifying and optimizing liposomes is to target the receptor-mediated endocytosis pathway. Many receptors present on the surface of the BBB specifically recognize ligands such as proteins. When ligands bind to receptors, the receptors become activated and cause endocytosis, which makes it easier to transport cargo.43 For instance, to carry medications into the brain through the transferrin receptor (TfR), modified liposomes with Tf placed on their surface have been used.44,45 In a study by Liang Kong, they modified the targeted liposomes with Tf to increase the efficiency of BBB transmission (Figure 3B). Strong fluorescent signals were observed in the brain after the administration of DiR liposomes and Tf-modified DiR liposomes, and the signals in the Tf-modified DiR liposome group were maintained for up to 24 h, which can explain its brain-targeting ability to a certain extent (Figure 3C).28 In a separate study, Murtas et al demonstrated that anti-TfR-conjugated liposomes substantially enhanced the delivery of payload (a curcumin derivative) to the brain via TfR-mediated endocytosis compared to the unmodified control.43 Like these studies, engineered liposomes targeting other receptors, eg, lactoferrin receptor (LfR), also illustrated good BBB transport via receptor-mediated endocytosis.46,47
Second, carrier-mediated transcytosis is another vital pathway of BBB transport. Nutrients are transported to the brain via BBB-selective transport proteins continuously expressed on the BBB.43 Accordingly, the BBB transport capability of glucose-modified liposomes PEGylated with various lengths of PEG chains has been investigated in preclinical studies. Due to a lack of interaction between glucose and the BBB surface, the results demonstrated that liposomes containing short PEG chains possessed a low BBB penetration capacity. Long PEG-chained liposomes are also unsuitable for drug development because their highly flexible PEG chains promote self-aggregation, which hinders BBB crossing. The development of glucose-modified liposomes for drug delivery was supported by the high BBB-crossing efficiency of liposomes containing PEG chains of optimal length.48–51 In addition, Dan and others demonstrated that p-aminophenyl--d-mannopyranoside-modified liposomes (MAN-LIP) delivered drugs to the brain of a mouse model more efficiently than unmodified LIP.48 Furthermore, glutathione (GSH) is actively transported through the BBB by a highly expressed sodium-dependent GSH transporter protein on the BBB. GSH-modified liposomes also showed promising BBB transport capability in a double transgenic APPswe/PS1ΔE9 (APP/PS1) mouse model. Maarten et al encapsulated single domain llama antibody fragments (vHH) into GSH PEGlyated liposomes and demonstrated that GSH-PEG liposomes substantially improved vHH transport across the BBB, indicating that GSH-PEG liposomes may be an additional promising strategy for liposome-based drug delivery systems.52
Third, CPP-mediated transcytosis. Yang’s team generated CPP-modified liposomes (CPP-Lip) to improve the delivery of carbapenem across the BBB. They demonstrated that CPP-Lip effectively traversed the BBB via CPP-mediated endocytosis, significantly improving drug delivery to lesions.26 By using TAT-modified liposomes, the HIV-1 tat (TAT) protein has been shown to effectively deliver the payload to the brain, making it one of the promising CPP candidates.27 In addition, CPPs have demonstrated promising therapeutic efficacy in SAMP8 mice by delivering medications to the brain.53
Finally, adsorption-mediated endocytosis is also an essential pathway of BBB transport. Due to electrostatic interfaces, cationic liposomes can enhance the vesicle crossing of the BBB via adsorption-mediated endocytosis/transcytosis.54,55 Chen et al reported that lactoferrin cationic liposomes (Lf-PCL) bound to the Lf receptor on the BBB; furthermore, the positively charged biomembrane of liposomes facilitated membrane fusion, subsequently increasing BBB crossing and drug delivery.47 In addition, Wang et al utilized trimethylated chitosan-conjugated poly(d,l-lactide-co-glycolide) nanoparticles to efficiently deliver coenzyme Q to the brains of APP/PS1 double transgenic mice, thereby preventing cognitive impairment.56 However, this strategy may increase peripheral tissue uptake of cationic liposomes, leading to insufficient drug delivery at the disease site and high systemic toxicity.43
In conclusion, liposomes are lipid-based nanoscale spherical vesicles with intrinsically low BBB penetrability, necessitating postmodification to improve BBB transport in AD therapy. Engineered liposomes targeting these pathways have shown significant improvement in delivering drugs to the brain and promising potential in AD therapy. However, most studies on liposome-based drugs delivery are still in the preclinical phase and require additional proof in the clinic. Due to the high plasticity of liposomes, multiple postmodifications targeting various endocytosis pathways may be used to enhance BBB penetration synergistically.
Lesion-Targeting Ability
After delivering cargoes across the BBB, the next challenge in AD therapy is the targeted delivery of the drug to the disease site.57 The lesion-targeting capability of liposomes is minimal, and thus, Different strategies have been devised to enhance the ability of liposomes to target lesions. The biomembranes of liposomes are optimized and modified with lipid-associated components, peptides, polyethylene glycols, etc., which facilitate lesion-specific drug delivery of liposomes.
First, we discuss the application of using lipid-related components in liposome optimization. Curcumin (CRM) neutralizes free radicals and prevents lipid peroxidation in the brain.58 Due to the low bioavailability of curcumin, Mourtaset et al generated a liposome delivery system that incorporated curcumin into the biomembrane of liposomes. Their findings59,60 showed that curcumin-modified liposomes effectively inhibited the development of amyloid plaques in APPswe transgenic mice because of curcumin’s general affinity for the structure of amyloid proteins.61 The accumulation and aggregation of Aβ in the brain serve an important role, either directly or indirectly, in regulating synaptic damage and memory deficits, indicating a promising future for AD therapy. Furthermore, Dr. Balducci et al conducted a critical study using multifunctional liposomes in Aβ targeted therapy.62 A bifunctional liposome was engineered by decorations of liposomal biomembranes with apolipoprotein E (mApoE) and phosphatidic acid (PA). This engineered liposome (mApoE-PA-LIP) enhanced BBB penetration via mApoE-mediated endocytosis, which in turn allowed efficient accumulation of liposomes in the brain, where it actively induced Aβ aggregate clearance via PA-mediated blocking, eventually reversing memory impairment. Moreover, an Aβ-targeted study using engineered liposomes was also reported by Tanifum et al.42 They conjugated an Aβ-targeting lipid-like molecule, 1.2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy-XO4-(polyethylene glycol-3400)] sodium salt (DSPE-PEG3400-XO4), to the biomembrane of liposomes and then assessed amyloid plaque targeting and clearance in an AD mouse model. The data showed that modified liposomes interact with either Aβ aggregates or free forms, which in turn blocks aggregation formation. Furthermore, applying liposomes efficiently removed Aβ in APP/PSEN1 mice.63 The high flexibility of liposomes allows the simultaneous application of multiple postmodifications on one liposome that targets more than one therapeutic target, such as Aβ plaques and tau tangles. For instance, Kuo et al engineered a multifunctional liposome containing curcumin (CRM), cardiolipin (CL), nerve growth factor (NGF), and wheat germ agglutinin (WGA).42 In this multifunctional liposome, CRM inhibits Aβ aggregation and reduces inflammation; NGF reduces neuronal apoptosis by enhancing the activity of the type 1 tyrosine kinase receptor (TrkA); CL provides the ability to target lesions due to its high binding affinity for Aβ; WGA could facilitate targeted nanoparticle delivery across the BBB. Importantly, this research showed that in the hippocampus of AD rodents, WGA-CRM-CL/LIP reduced Aβ plaque deposition and lipid peroxidation, preserved neural survival, and reduced acetylcholinesterase (AChE) activity.33 One of the primary mechanisms of AD is the loss of cholinergic neurons in the brain, which results in decreased acetylcholine (ACh) levels, decreased ACh receptor (AChR) density, and overall reduced cholinergic neurotransmission. Several restrictions and side effects accompany existing AChEIs that aim to restore cholinergic neurotransmission in AD.42 To enhance acetylcholinesterase inhibition, Li’s team designed a liposome containing galanthamine hydrobromide (GH). Drug exposure was significantly increased after introducing GH into liposomes compared to the GH control group.64
Next, we discuss liposome modification with peptides. A short peptide termed OR2 (RGKLVFFGR-NH2) was created by Austen et al that potently inhibited the aggregation of amyloid beta and prevented the development of oligomers and protofibrils, which in turn decreased the toxicity of amyloid beta to neuronal cells.65 However, despite the significant effort put into optimizing this peptide, the leading candidate RI-OR2-TAT (AcrGffvlkGrrrrqrrrkkrGy-NH) alone still demonstrated only moderate efficacy after intravenous injection due to insufficient drug concentration in the brain.27 Therefore, a liposome delivery system was incorporated by decorating liposomes with the peptide RI-OR2-TAT, named peptide inhibitor nanoparticles (PINPs). PINPs showed a high binding affinity to Aβ, which prevented Aβ oligomer and fibril formation at a deficient concentration. It also protected neuronal cells from the toxic effect induced by pre-aggregated Aβ in vitro. PINPs also displayed good brain enrichment in vivo, preventing memory loss in transgenic rodents with APPSWE.66
Finally, we discussed the most commonly used modification strategy of liposomes, polyethylene glycosylation. A “nano scavenger” (M3) created by Luo et al consists of a cationic chitosan core with polyethylene PEGylated-GKLVFF that interacts with Aβ and Becln-1 directly and triggers Aβ degradation that is dependent on autophagy. Autophagy is a critical pathway for cells to degrade metabolites during AD development, for instance, Aβ clearance. A malfunction of the autophagy lysosome system results in the accumulation of Aβ and disease progression.67,68 The nano scavenger trapped extracellular Aβ, inhibited Aβ aggregation formation, and expressly directed Aβ degradation via autophagy. Using this method, they demonstrated that the insoluble form of Aβ in APPswe/PS1dE9 transgenic mice decreased from 1539 to 914 ng/mg, while the soluble form decreased from 585 to 190 ng/mg. This significantly increased the viability of neuronal cells.69
Various modification strategies have been approved to enhance the lesion-targeting capability of liposomes substantially, thereby enhancing the therapeutic efficacy in patients with AD. ACI-35 is a liposome-based vaccination that specifically targets phosphorylated tau in pathological conformations. A clinical trial (NCT04445831) was carried out to assess the immunogenicity, safety, and tolerability of different tau-targeting vaccination dosages, regimens, and combinations in people with early AD. Particularly in the mid- and low-dose groups, the data showed that participants had a robust and long-lasting immune response against pathogenic tau proteins and nonphosphorylated tau.70 However, AD is a complicated disease that may develop through the combination of multiple pathological mechanisms. It is important to explore the application of combination strategies that simultaneously target multiple therapeutic targets to synergistically and systemically treat AD. Moreover, liposomes still have some limitations, such as high cost, low solubility, poor stability, and unsuitable for complex biological modifications. To overcome these limitations, the development of liposomes combining multiple modifications and continuous optimization of the manufacturing process is required.
High-Density Lipoprotein Nanoparticles
High-density lipoproteins (HDLs) are particles consisting of phospholipids, cholesterol, and proteins of different sizes, shapes, and compositions. HDL’s primary protein component is apoA-I, which accounts for nearly 70% of the protein mass. Numerous studies have demonstrated that HDL71 reduces the generation of Reactive oxygen species (ROS) and intracellular oxidative stress and induces anti-inflammatory, anti-apoptosis, and anti-infection activities.72,73 In the body, it has been reported that HDL transports lipids, proteins, and microRNAs into cells by incorporating numerous cellular enzymes and receptors. Due to this unique nature, HDL has been explored and developed as a carrier for drug delivery. In the past few years, multiple HDL nanoparticles have been developed, including natural HDL or recombinant HDL (rHDL) composed of the recombinant ApoA/ApoE protein and phospholipid/cholesterol ester/cholesterol-rich emulsions. These rHDL nanoparticles inherit good drugability features from natural HDL, including good drug-loading capacity, excellent safety profile, and easy manufactory production.74 Moreover, rHDL nanoparticles could be further engineered to improve BBB penetration, lesion-targeting capabilities such as Aβ targeting, β-amyloid plaque clearance, and memory loss prevention (Figure 4A).75,76
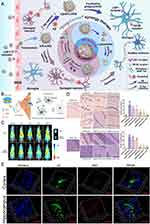 |
Figure 4 Schematic diagram and advantageous study of HDL-based nanoparticles delivering drugs in AD-targeted therapy. (A) Schematic diagram of HDL nanoparticles (exemplified by APLN/MB) delivering drugs through the BBB via receptor-mediated endocytosis with the help of LRP1 and other receptors to target AD. Reprinted from Han G, Bai K, Yang X, et al “Drug-Carrier” synergy therapy for amyloid-beta clearance and inhibition of tau phosphorylation via biomimetic lipid nanocomposite assembly. Adv Sci. 2022;9(14):e2106072. © 2022 The Authors. Advanced Science published by Wiley-VCH GmbH. Creative Commons.76 (B) After α-M loading, apolipoprotein E (ApoE)-reconstituted high-density lipoprotein nanocarriers (ANCs) can inhibit the formation of Aβ oligomers and fibrils and hasten Aβ cellular degradation. Reprinted from Song Q, Song H, Xu J, et al. Biomimetic ApoE-reconstituted high density lipoprotein nanocarrier for blood-brain barrier penetration and amyloid beta-targeting drug delivery. Mol Pharm. 2016;13(11):3976–3987, Copyright © 2016 American Chemical Society.75 (C) Brain distribution of ANC following intravenous administration. Reprinted from Song Q, Song H, Xu J, et al. Biomimetic ApoE-reconstituted high density lipoprotein nanocarrier for blood-brain barrier penetration and amyloid beta-targeting drug delivery. Mol Pharm. 2016;13(11):3976–3987, Copyright © 2016 American Chemical Society.75 (D) ANC-α-M decreased amyloid deposition and attenuated microgliosis in the brains of SAMP8 mice. (**p < 0.01, ***p < 0.001, significantly different from that in SAMP8 mice treated with NS; #p < 0.05, ###p < 0.001, significantly different from that in the SAMR1 mice treated with NS. Black arrows point to amyloid plaques and white arrows point to CD45-positive activated microglia.) Reprinted from Song Q, Song H, Xu J, et al. Biomimetic ApoE-reconstituted high density lipoprotein nanocarrier for blood-brain barrier penetration and amyloid beta-targeting drug delivery. Mol Pharm. 2016;13(11):3976–3987, Copyright © 2016 American Chemical Society.75 (E) In vivo accumulation of ANC (red) surrounding Aβ aggregates (green) in the cortex and hippocampus following intravenous administration. Reprinted from Song Q, Song H, Xu J, et al. Biomimetic ApoE-reconstituted high density lipoprotein nanocarrier for blood-brain barrier penetration and amyloid beta-targeting drug delivery. Mol Pharm. 2016;13(11):3976–3987, Copyright © 2016 American Chemical Society. |
Biosafety and Drug-Loading Capacity
Recombinant HDL comprises biocompatible and biodegradable lipids and proteins/peptides, and therefore typically demonstrates outstanding biosafety. Even without postmodification on its biomembrane, rHDL nanoparticles display immunogenicity comparable to natural HDL.77 In pioneering clinical studies, the tolerated dose of rHDL nanoparticles was found to be tens or even hundreds of times higher than that of conventional inorganic nanoparticles, although this may differ depending on the composition of each product.78 In general, rHDL nanoparticles are well tolerated at a Q1 W intravenous dosing schedule, with some common adverse events, including transient elevations of hepatic transaminases (Alanine aminotransferase and Aspartate aminotransferase) and another minor hepatotoxicity. Additionally, rHDL nanoparticles frequently exhibit a prolonged half-life of circulation in the body and a constrained immune response and/or clearance mediated by the MPS. Interestingly, the plasma half-life of apoA-I-derived rHDL could be dramatically increased by polyethylene glycosylation modification, as recently demonstrated by Murphy et al.79 Numerous clinical trials involving more than 800 patients and healthy volunteers have shown that humans tolerate rHDL nanoparticles well.78
Recombinant HDL can transport both hydrophobic and hydrophilic medications. Hydrophilic molecules can be adsorbed or conjugated to the hydrophilic surface of HDL, whereas hydrophobic molecules can be internalized or partially inserted into the interior of rHDL.78 For instance, some hydrophilic medicines, such as small interfering RNA (siRNA), can be employed to HDL for drug delivery after being chemically altered by cholesterol.80 However, rHDL nanoparticles are incapable of sustaining or controlling drug release. To improve it, Sanchez-Gaytan et al recently adapted Polylactic-co-glycolic acid (PLGA) in the hydrophobic core of rHDL, which demonstrated PLGA-HDL hybrid nanoparticles displaying traits of rHDL and controlled drug release capability.81
In conclusion, due to the natural features of HDL, rHDL nanoparticles display excellent biosafety in humans. Adjusting the composition of the biomembrane may interfere with the drug-loading capacity and biocompatibility of rHDL nanoparticles, which may require a high-quality control process during manufactory development. Nevertheless, rHDL nanoparticles could deliver various forms of drugs and could adjust the capability of drug release and loading through postmodifications; therefore, they illustrate great potential in AD therapy.
BBB Penetrating Ability
The structure of the BBB limits the entry of most molecules into the brain, resulting in significant interference with the therapeutic efficacy of AD treatment. To surmount BBB resistance, numerous post-modification strategies have been investigated to enhance the BBB penetrability of HDL nanoparticles, mainly via the receptor-mediated endocytosis pathway.82 Under physiological conditions, HDL interacts with LDLR or HDL receptors, such as SR-B1, ABCA1, and ABCG1, which in turn transport lipids, peptides/proteins, and nucleic acids to recipient cells.83,84 Importantly, different compositions of lipids and/or apolipoproteins on the biomembrane of HDL may determine its preferential interacting receptor that in turn alters HDL endocytosis and transport.85
Due to their natural characterization, HDL nanoparticles present good BBB penetration capability via receptor-mediated transcytosis. A recent study demonstrated that through interaction with LDLR, HDL nanoparticles efficiently transport cargo across the BBB via receptor-mediated endocytosis, which is subsequently released in the brain.86 The bioactive apoE peptides had a strong affinity for LDL receptor-related protein 1 (LRP1) in a pure system. In contrast, apoE could only bind to the LDLR family after being integrated into lipids.87 As a result, apoE-rHDL shows considerable potential for drug delivery and BBB penetration through LDLR-mediated transcytosis. As a biomimetic nanocarrier for AD treatment, Song et al made use of apoE3 to create apoE-rHDL, whose N-terminal domain interacted with LDLR to mediate transcytosis. This physiologically inspired nanocarrier easily traversed the BBB, encouraged Aβ breakdown in microglia and astrocytes, and reversed memory deficits in AD model mice (Figure 4C).75
By their inherent capability to interact with receptors (eg, LDLR), HDL nanoparticles effectively cross the BBB via the receptor-mediated transcytosis pathway, which in turn delivers cargo to the brain. Moreover, postmodifications on the biomembrane of HDL nanoparticles further enhance the BBB penetration efficiency, making HDL nanoparticles a high-potential drug delivery system in AD therapy.
Lesion-Targeting Ability
HDL is well known for its role in cholesterol transport and therefore may have some targeting capability by nature. To apply HDL nanoparticles in AD-targeted therapy as a drug delivery system, reconstitution or modifications to HDL nanoparticles are required to improve the disease lesion and intracellular organelle targeting capability of HDL nanoparticles, which in turn maximizes the therapeutic efficacy due to the complicated microenvironment of the brain.
One strategy is using apolipoproteins or lipids to reconstitute HDL, which can improve lesion-targeting capability. The group led by Gao Xiaoling designed a biomimetic nanocarrier called apolipoprotein E-reconstituted HDL nanocarrier (ANC) using recombinant ApoE and synthetic lipids to enhance the efficacy of drug delivery to Aβ-enriched lesions (Figure 4B). Immunofluorescence analysis was used to assess the in vivo Aβ-targeting effectiveness of ANC. Following the entry into the brain parenchyma, it suggested that ANC was highly accumulated around the Aβ aggregates, indicating ANC’s high Aβ-targeting ability (Figure 4E). And ANC-α-M decreased amyloid deposition and attenuated microgliosis in the brains of SAMP8 mice (Figure 4D).75 In addition, apolipoprotein J (ApoJ), a natural chaperone that interacts with Aβ, was used to assemble phospholipids with recombinant human ApoJ (rApoJ) to generate rApoJ-HDL nanoparticles. Sofía Fernández-de-Retana et al demonstrated that rApoJ-HDL displayed a better brain distribution pattern than liposome nanoparticles that delivered the same fluorescence-labeled cargo. Impotently, they further confirmed that rApoJ-HDL nanoparticles accumulated in the cranial region, particularly in aged transgenic mice with a heavy Aβ load.88
Another strategy to improve lesion-targeting capability is introducing postmodification on the biomembrane of HDL nanoparticles. For instance, brain endothelial cells can absorb vitamin E (-tocopherol), an essential molecule for tissue cells that the body cannot synthesize. To deliver siRNA targeting the lesion in the brain with AD, Yoshitaka Uno’s team inserted α-tocopherol into the biomembrane of HDL loaded with siRNA against β-site amyloid precursor protein cleaving enzyme 1 (BACE1), named Toc-siBACE/HDL. Seven days post administration of free Toc-siBACE or Toc-siBACE/HDL via intracerebroventricular infusion, Toc-siBACE/HDL distributed broadly within the brain at the posterior frontal, parietal, and temporal and hippocampal areas, whereas free Toc-siBACE was barely detectable in the brain. These results proved the lesion-targeting ability of HDL nanoparticles.89 Furthermore, to improve the lesion-targeting capability, Gao Xiaoling’s team designed an anionic amphiphilic lipid-monosialotetrahexosylganglioside (GM1)-modified HDL nanoparticle possessing Aβ-binding ability. They incorporated GM1 into the lipid membrane of rHDL to construct a novel nanostructure, GM1-modified rHDL (GM1-rHDL). Following intranasal administration, they found that the AUCall values of GM1-rHDL in the cortex and hippocampus were 0.85-fold higher than those of control rHDL, which suggested that GM1-rHDL had better targeting capability.90
The third strategy is targeting intracellular organelles that are critical for AD progression. When rHDL reaches the target region, it typically enters the target cell via receptor- or CPP-mediated internalization, leading to cargo degradation in the endosomes/lysosomes. Therefore, modification of rHDL that induces endosomal/lysosomal evasion could allow the delivery of cargo/drug targeting subcellular targets.82 Currently, such modification approaches are mainly used in cancer therapy and vaccine production. For example, Ding et al recently designed a pH-sensitive bifunctional nanoparticle cp-rHDL, of which ApoA-I provides tumor-targeting properties and lipophilic anchored R6H4 provides pH-dependent penetration ability. They demonstrated that cp-rHDL could efficiently deliver coumarin-6, a hydrophobic fluorescent probe, into the cytoplasm of tumor cells.91 Although the study of rHDL targeting intracellular organs used in treating AD has not been reported, mitochondrial dysfunction, one of the most essential pathological mechanisms of AD development, could be a valuable intracellular target for AD therapy.
rHDL, especially postmodified rHDL, has inherent lesion-targeting capability that provides many new directions in AD therapy. Although many of the current works focus on tumors and atherosclerosis, due to the unique features of rHDL, postmodified rHDL holds a great future as a multifunctional drug delivery system in AD therapy. Moreover, rHDL nanoparticles are relatively easy to manufacture on a large scale. In summary, as a highly promising targeted therapy for AD, a more efficient rHDL nanodrug delivery system using postmodification strategies that improve serum stability, BBB penetration, lesion-targeting capability and endosomal/lysosomal escape ability is continuously needed.
Cell Membrane-Based Nanoparticles
Due to its challenging nature to replicate artificially, the cell membrane has gained significant attention and allows for the preservation of the donor cell’s membrane proteins. This “top-down” approach92–94 facilitates the synthesis of nanoparticles that closely resemble cells, providing an ideal bilayer medium for anchoring transmembrane proteins.92,95 Researchers have utilized various cell membranes to create nanoparticles, enabling the selection of different membrane types and nanoparticle cores (Table 3).96–98 Several techniques, including coextrusion, sonication, microfluidic electroporation or sonication, and in situ packaging techniques, can be used to create cell membrane-based nanoparticles.22,99 Additionally, each of these approaches has benefits and drawbacks.99 In animal model studies, surface-modified cell membrane-based nanoparticles have demonstrated improved BBB penetration. They have also successfully delivered therapeutic medications to target the underlying pathways of AD, alleviating symptoms, and enhancing cognitive performance (Figure 5A).100
 |
Table 3 Summary of the Features Between Different Cell Membrane-Based Nanoparticles |
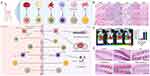 |
Figure 5 Schematic diagram and advantageous study of cell membrane-based nanoparticles delivering drugs in AD-targeted therapy. (A) Schematic diagram of cell membrane-based nanoparticles as drug carriers for targeted treatment of AD. Image created with Biorender.com. (B) Compared with the saline (control) group, no indicators of damage were observed for these organs after treatment. (C) The most intense distribution in the brain was displayed in the DIR-tagged RVG/TPP-MASLNs-treated mice and was further confirmed by the fluorescence intensity identified in the isolated brain homogenate. (*p < 0.05 compared with MASLNs.) (D) Administration of GS-loaded formulations slowed the alleviation of degenerative alterations of hippocampal neurons in AD mice to different extent. (Red arrows point to the damaged neurons.) Adapted with permission from KeAi Publishing. Han Y, Gao C, Wang H, et al. Macrophage membrane-coated nanocarriers Co-Modified by RVG29 and TPP improve brain neuronal mitochondria-targeting and therapeutic efficacy in Alzheimer’s disease mice. Bioact Mater. 2021;6(2):529–542.100 |
Biosafety and Drug-Loading Capacity
Cell membranes possess desirable characteristics such as biocompatibility, biodegradability, and nonimmunogenicity. These attributes make cell membrane carrier systems well-suited for use in humans, particularly when utilizing autologous cells. Regarding safety, cell membrane-based nanocarriers have shown a favorable profile without any myelosuppressive effects, unlike free drug formulations that can significantly reduce various immune cell subsets. For example, intravenous administration of MASLNs-GS for 14 days did not cause systemic toxicity to vital organs (Figure 5B).100 Notably, even after multiple administrations of RBC-NP, no anti-RBC antibodies were detected in mouse serum, suggesting the absence of acute or chronic immune responses elicited by these nanoparticles. Moreover, the safety and long-term viability of employing RBC membrane coatings were demonstrated using an iron oxide nanoparticle platform.101
The choice of nanoparticle core allows for customization, enabling the encapsulation of various drugs and providing protection to the cargo from inactivation. This feature offers a prolonged and controllable lifespan for the encapsulated drugs. Furthermore, the nanoparticle core can simultaneously encapsulate multiple drugs, directing them to the same site of action. This approach maximizes the potential synergistic effects resulting from the combination of these drugs.102–104 The data clearly demonstrate that drug-loading in WBC-NP was four times higher compared to bare nanoparticles.95
BBB Penetrating Ability
The abundant variety of cells in nature allows for the utilization of cell membrane-based nanoparticles derived from different cell membrane sources, each possessing unique natural characteristics. By harnessing these self-characteristics and employing various modification strategies, these nanoparticles can enhance the penetration ability across the blood-brain barrier (BBB), facilitate drug delivery to the brain, increase drug concentration in specific areas of the brain, and ultimately improve the effectiveness of AD treatment.
RBC membranes are widely used for nanoparticle encapsulation and are considered one of the most commonly employed cell membranes in this context. An example of such nanoparticles is the PLGA-encapsulated RBC membrane nanoparticles, which were the first reported and most comprehensive cell membrane-based nanoparticles.92 However, due to the inability of RBCs to cross the BBB, further modifications are required to enhance the targeting capabilities of RBC membrane-encapsulated nanoparticles. CDX, which is derived from candoxin, has a strong binding affinity for nicotinic acetylcholine receptors (nAChRs) present on the surface of brain endothelial cells. Chai et al successfully incorporated DCDX peptides onto the surface of RBCNPs using a simple yet effective approach that did not compromise the peptide’s ability to target the brain. In vitro and in vivo studies demonstrated promising brain-targeting efficiency of the resulting DCDX-RBCNPs.105 Additionally, Yang Han’s team developed macrophage (MA) membrane-coated solid lipid nanoparticles (SLNs) by attaching rabies virus glycoprotein (RVG29) and triphenylphosphine cation (TPP) molecules to the MA membrane surface. This modification aimed to deliver antioxidants specifically to the mitochondria of injured neurons in the brain. In an in vitro BBB model using bEnd.3/HT22 coculture, RVG/TPP-MASLN-Cou6 exhibited the highest BBB permeability among all groups, indicating efficient BBB crossing when employing these dual-modified formulations (Figure 5C).100 Similar conclusions were reached in experiments involving RBCs.106
Indeed, studies on other cell membrane-based nanoparticles are relatively limited. However, certain types of cell membranes themselves can cross the BBB. For instance, leukocytes and platelets can exploit their migration induced by inflammatory chemotaxis to traverse the BBB.94 Neutrophils naturally possess the ability to cross the BBB, and mesenchymal stem cells (MSCs) can transmigrate across brain microvascular endothelial cell monolayers by temporarily creating interendothelial gaps.107 Moreover, cancer cells exhibit distinct properties compared to blood cells, such as unlimited replicative potential, immune evasion, and homologous targeting abilities. Cancer cells can cross the BBB through their homologous targeting capability.108
Cell membrane-based nanoparticles have emerged as a promising strategy for drug delivery across the BBB. These nanoparticles, derived from various cell membranes, exhibit different structures and properties that influence their ability to penetrate the BBB. While some cell membranes possess inherent capabilities to cross the BBB, such as erythrocytes, most require specific modifications or conditions to achieve efficient BBB penetration. To advance this field, the focus is now on developing nanoparticles derived from diverse cell membranes that can effectively permeate the BBB under specified conditions. This entails understanding the factors that govern BBB permeability and optimizing the design and characteristics of the nanoparticles accordingly. By tailoring the properties of these nanoparticles, such as their surface modifications and targeting ligands, researchers aim to enhance their ability to traverse the BBB and deliver therapeutic agents to the brain more efficiently. Continued research and exploration in this area hold great potential for improving drug delivery across the BBB and advancing treatments for various neurological disorders.
Lesion-Targeting Ability
Cell membrane-based nanoparticles possess unique targeting capabilities, primarily achieved through the recognition of homotypic features and specific cell receptors. These mechanisms contribute to their intrinsic targeting properties and facilitate internalization by target cells.11 Nanoparticles of different cell membrane origins can acquire different targeting properties due to their different membrane characteristics. The targeting ability of cell membrane-based nanoparticles is achieved mainly through the following mechanisms.
First, various targeting ligand modifications play a crucial role in achieving targeted delivery using cell membrane-based nanoparticles, such as RBCs. RBCs themselves lack inherent targeting abilities; hence, modifications are necessary to enhance their targeting capabilities. One promising mitochondria-targeting ligand is TPP, which can exploit the negative mitochondrial membrane potential to drive nanoparticle entry into mitochondria.109 Gao et al prepared RBC-NPs and functionalized them with T807 (a ligand targeting tau protein) and TPP to deliver CRM specifically to neuronal mitochondria for anti-AD therapy. In vitro experiments they demonstrated that the ligand-modified formulations (T807-RBC-NPs, TPP-RBC-NPs, and T807/TPP-RBC-NPs) were significantly internalized by HT22 cells compared to nonmodified formulations (RBC-NPs). Among all the tested formulations, T807/TPP-RBC-NPs exhibited the highest intracellular fluorescence intensity due to dual-mediated endocytosis.110 Furthermore, when CRM was loaded onto RBC membrane-coated PLGA particles bearing T807 molecules on the RBC membrane surface (T807/RPCNP), it effectively inhibited tau aggregation by directly targeting p-tau. Notably, the T807/RPCNP-CRM group showed the most significant reduction in p-tau levels in the hippocampal CA1 region in vivo.111 Additionally, Yang Han’s team evaluated the targeting ability of an RVG29 and TPP-modified delivery system (RVG/TPP-MASLNs-Cou6) to specifically target neurons. They found that the fluorescence intensity in HT22 neurons was significantly higher than in astrocytes, indicating a relatively higher selectivity of RVG/TPP-MASLNs-Cou6 for neurons. In an in vivo setting, treatment with RVG/TPP-MASLN-GS significantly attenuated damage to hippocampal neurons and reduced the levels of Aβ1-42 in AD mice (Figure 5D).100
Second, lipids were inserted to enhance the cell membrane-based nanoparticle targeting capacity. To create erythro-NPs with functionalized erythrocyte membranes, Fang et al used a lipid insertion technique to insert the small molecules folate (MW441 Da) and nucleolin-targeting aptamer AS1411 (MW9000 Da) into erythrocyte membranes. They then validated their receptor-specific targeting ability in an in vitro cancer cell line model.112
Third, magnetic targeting. Magnetic erythrocytes, resulting from the coencapsulation of drugs with some ferrofluids such as cobalt–ferrite and magnetite, have been reported to direct the encapsulated drug predominantly to the desired sites of the body through an external magnetic field.113
The modification of targeting ligands is a primary strategy to enhance the targeting capability of cell membrane-based nanoparticles. By specifically binding to receptors at the lesion site, these modifications enable nanoparticles derived from cell membranes to target and treat various conditions effectively, including AD. This “top-down” approach holds significant potential in AD treatment. Currently, cell membrane-based nanoparticles find extensive use in antitumor and anti-inflammatory therapies, where they are designed to target cancer cells or inflammation sites. In these applications, strategies such as inflammation or homologous targeting are commonly employed to aggregate at lesion sites. While there is limited experimental research specifically focused on AD treatment using cell membrane-based nanoparticles, insights gained from studies on improving carrier efficacy in tumor settings can be applied to studying of cell membrane-based nanoparticles for treating AD. Though more research is needed, the principles and modifications explored in antitumor therapy can guide the development of targeted delivery systems for AD treatment. Applying similar approaches, such as utilizing specific ligands to target receptors associated with AD pathology, offers potential avenues for enhancing the effectiveness of cell membrane-based nanoparticles in delivering therapeutic agents to the brain for the treatment of AD.
Extracellular Vesicles
Extracellular vesicles (EVs) are nanosized membrane vesicles that are released by many, if not all, cell types.114 Based on their intracellular origin and size, they are Classified into three types of EVs: exosomes (30–150 nm in diameter), microvesicles (50–1000 nm), and apoptotic bodies (50–5000 nm).115 It has been shown that the ability of EVs to communicate among cells locally and systemically has prompted the evaluation of repurposing these vesicles as carriers of therapy for AD.116,117 Various experimental studies have discovered that using EVs as carriers for the targeted delivery of AD drugs can significantly improve the efficiency of crossing the BBB (Figure 6A), increase the bioavailability of medications in vivo, target drug aggregation to the site of the lesion, and increase the concentration of the medication in the targeted area, acting as a therapeutic agent to lower neuronal cell apoptosis and enhance cognitive function.118
Biosafety and Drug-Loading Capacity
EVs hold several advantageous characteristics that make them attractive as delivery vehicles. EVs, mainly produced by human cells, offer high biocompatibility, stability, low immunogenicity, and low toxicity compared to synthetic nanodrug delivery vehicles. This makes them potentially safe for therapeutic applications. Studies have shown that primary neurons can survive exposure to EVs generated by macrophages for 48 hours, indicating that these carriers do not exhibit neurotoxicity.119 However, it is important to acknowledge that potential safety concerns still exist. Primary considerations are the diversity in the composition of EVs derived from different cellular sources and our limited understanding of how EVs in the central nervous system (CNS) enable intercellular communication.11
Compared to synthetic vehicles, EVs can avoid phagocytosis and evade the immune system.120–122 Moreover, EVs are natural carrier systems with endogenous cellular tropism that allows efficient cell uptake, avoiding the endosomal pathway and lysosomal degradation, delivering their cargoes directly into the cytoplasm. This ability enables better efficacy in the delivery of therapeutic molecules, such as siRNAs.123 EVs in circulation are stable due to their inherent small size, endogenous origin and surface composition, which provide a natural targeting capacity.124 The hydrophobicity of the drug, the drug-loading technique, and the lipid makeup of the EVs may all affect how quickly they absorb drugs.125 EVs are rich in natural proteins and nucleic acids, which increase the difficulty of cargo loading and limit the loading efficiency of EVs.126 An increasing number of methods have been applied to improve the loading efficiency of EVs, including incubation, transfection, ultrasound, electroporation and surfactant treatment, but different loading methods have different advantages. For example, physical therapy significantly increased the loading capacity of exosomes compared to incubation;119,127–129 compared to extrusion, surfactant treatment, freeze-thaw treatment and incubation, ultrasound treatment produced higher loading efficiency on protein peroxidase. Therefore, in practical studies, a suitable loading method needs to be selected according to the physical or chemical properties of the drug. Despite several strategies to improve their loading capacity, the loading efficiency of extracellular vesicles remains much lower than that of unpackaged synthetic liposomes.130
BBB Penetrating Ability
Unmodified exosomes from various cell types show <1% delivery to the brain after systemic injection.131,132 Epitaxial alteration can significantly increase the BBB penetration of EVs, similar to liposomes. The following are some ways that BBB penetration of EVs can be accomplished.
First, receptor-mediated cytokinesis (RMT) is the most studied and applied pathway for drug transport through BBB endothelial cells. Recently, Choi et al used the LDLR-mediated transcytosis pathway to deliver ApoB-labeled EVs generated by ApoB binding to CD9. In contrast, CD9-ApoB EVs were observed to accumulate in the cortical vasculature and to be kept in the brain for an additional 24 hours, while CD9 EVs were not found in the vasculature.133 Based on reports that cur can treat AD by inhibiting tau phosphorylation, Hao Wang’s team tested the BBB penetration of fluorescent curcumin EVs (Exo-cur) and free EVs (free-cur) by using an in vitro BBB model and intercellular adhesion molecule-1 (ICAM-1). They discovered that only approximately 15% of free-cur and more than 60% of Exo-cur had penetrated the BBB layer, indicating a significant BBB-penetrating effect of Exo.134 Additionally, EVs have been altered to facilitate brain penetration using glucose transporter protein 1, insulin, lactoferrin, and transferrin receptors.135 In a mouse model, Yao Qi’s team developed plasma EVs loaded with Que (Exo-Que) (Figure 6B) to improve the bioavailability of the drug, enhance the brain targeting of Que (Figure 6C) and improve cognitive dysfunction in okadaic acid (OA)-induced AD mice.118
Second, CPPs induce the translocation of biologically active macromolecules across cell membranes. CPPs are usually classified as cationic, amphiphilic, and hydrophobic.136 The cationic class consists mainly of positively charged peptides, such as arginine and lysine, which can interact with negatively charged plasma membranes. The apical surface of brain capillaries is densely packed with negatively charged glycocalyx, which makes positively charged CPPs effective transporters of drugs through the BBB.137
Third, neuroviruses are used to cross the BBB. RVG-derived peptides showed efficient BBB penetration in many preclinical experiments.138 The CNS-specific RVG peptide interacts specifically with the acetylcholine receptor to enable viral entry into neuronal cells.139 Accordingly, Guo-hong Cui’s team proposed the use of CNS-specific RVG peptide to target intravenously infused exosomes derived from MSCs (MSC-Exo) to the brain of transgenic APP/PS1 mice. MSC-Exo was conjugated with RVG through a DOPE-NHS linker. According to the Morris water maze test, brain-targeted exosomes derived from MSCs were better than unmodified exosomes at improving cognitive function in APP/PS1 mice.140
The current focus of modifications in cell membrane-based nanoparticles is mainly on penetrating BBB for targeting brain tumors or inflammation, with limited studies specifically addressing AD therapy. However, the knowledge and insights gained from these studies provide a foundation for exploring the potential of EVs in penetrating the BBB for AD therapeutic applications. By combining expertise, innovation, and a deepening understanding of EV biology, we can work towards developing effective therapies for AD that harness the unique properties and capabilities of EVs for crossing the BBB and delivering therapeutic agents to the brain.
Lesion-Targeting Ability
EVs have a built-in ability to target, and studies have revealed that due to the differences in their characteristics, EVs from various cell types have variable organ and tissue tropism.131,141–143 We can maximize drug targeting by selecting EVs from different sources for drug delivery according to the treatment of different neurological diseases. Here, we present a description of the experiments related to AD treatment according to the different mechanisms of targeting pathology.
Many studies have focused on the neuroprotective function of EVs to reduce and clear Aβ, and extracellular vesicle-centered therapies for AD have been applied in mouse models. Glycosphingolipids (GSLs), a group of membrane glycolipids, are highly abundant in exosomes, and the enriched glycans of GSLs are essential for Aβ binding and assembly on exosomes both in vitro and in vivo. Yuyama et al plan to provide a novel therapeutic intervention for AD by improving Aβ clearance by exosome administration. They found that 50% less Aβ was observed in the ipsilateral injection side than in the contralateral injection side, which was similar when exosomes were administered into the lateral ventricles compared to direct administration of exosomes into the mouse hippocampus. The results suggested that exosomes in the brain mainly accumulate in the mouse hippocampus.144 In recent years, exosomes isolated from human umbilical cord mesenchymal stem cells (hucMSC-exosomes) have been demonstrated to mimic the therapeutic effects of hucMSCs in many inflammation-related diseases. In one study, Ding et al injected exosomes from the supernatant of hucMSCs into AD mouse models. By detecting the effects of hucMSC-exosome injection on Aβ deposition in AD mice, they found that the number of Aβ plaques in the cortex and hippocampal areas of the brain was significantly lower in the hucMSC-exosome-injected group than in the control group, which suggested that hucMSC-exosomes targeted these areas.145 Similarly, in experiments by Hao Wang’s team, the distribution of free-cur and Exo-cur in the brain was monitored after intraperitoneal injection administration into C57BL/6 mice. The intensity of green fluorescence emitted by cur in the hippocampus was more significant in the Exo-cur treatment group compared to the free-cur treatment group. These findings imply that Exo-cur increases the amount of cur that accumulates in the brain’s hippocampus in vivo, which aids in the neuroprotective effect.134 As mentioned in the previous section, MSC-RVG-Exo had excellent targeting capability to the AD lesion area. 5 h after injection, brain sections were observed under a fluorescence microscope to detect the presence of exosomes derived from MSCs in the brain and to determine whether RVG modification enhanced the engraftment of exosomes in the cortex and hippocampus. The cortex and hippocampus contained DiI-labeled exosomes in both the MSC-RVG-Exo and MSC-Exo groups. The number of injected exosomes was further compared by relative mean fluorescence intensity. Indeed, there were many more DiI-labeled exosomes in the cortex and hippocampus of the mice in the MSC-RVG-Exo group than in the MSC-Exo group.140
The hyperphosphorylation of the tau protein is one of the pathogenes of AD. A possible cognitive enhancer is quercetin (Que), a natural flavonoid molecule that has been shown to reduce tau pathology, limit amyloid development, and elicit neuroprotection linked to autophagy.146,147 Because of its weak bioavailability and brain targeting, it has limited clinical applicability.148 In Yao Qi’s research, their results showed that more exosomal Que was delivered into the hippocampus of the brain than free Que. Exo-Que dramatically enhanced the number of NeuN-positive neuron cells in the hippocampal CA1 area, CA3 region, and DG region as compared to the OA and OA + Que groups (Figure 6D). Additionally, the OA + Exo-Que group’s hippocampal neuronal layers displayed order and integrity, preserving the structure of the neuronal cells with a distinct line and lots of cytoplasm (Figure 6E).118
AD is not a disease caused by a single pathology. Many research, some of which were already mentioned, are trying to treat AD radical treatment by using extracellular vesicles as drug carriers in a multi-targeted, multi-pathway manner. In addition to phosphorylated tau proteins, neuronal fibrillary tangles, and Aβ amyloid plaques, these tests also target pathogenic variables such as inflammation,149 oxidative stress,150 mitochondrial dysfunction,151 and microglia activation.140,145,152 A multi-targeted therapeutic approach is more in line with the disease itself, and these studies have already yielded promising results at the laboratory stage.
EVs offer unparalleled advantages over other nanoparticles in drug delivery. Some modifications to the vesicle surface have made it possible for EVs to deliver pharmaceuticals across the BBB to CNS lesions, thereby enabling AD-targeted drug delivery. Despite this, the use of EVs for medication delivery is still in its infancy, and numerous obstacles, such as low yield and insufficient drug delivery efficacy, must be overcome. In addition, the specific secretion mechanism of EVs and the mechanism of crossing the BBB are still unclear. Still, at present, EVs are mainly applied in the targeted transport of anticancer drugs. Only one study (NCT04388982) related to EVs is in clinical trials, which is conducted by Xiaoling Gao’s team. The results of Phase I/II clinical trial indicates improved cognitive function in the medium-dose arm.153 Therefore, more preclinical studies on EV secretion, transportation and delivery safety are needed to promote the translation of extracellular vesicle technology from theory to the clinic.
Conclusion and Perspective
In the field of AD treatment, biomembrane nanoparticles have emerged as promising drug delivery systems, offering distinct advantages over other types of carriers. These nanoparticles are derived from natural biomembranes and possess the ability to mimic components found in vivo. As a result, they exhibit inherent biocompatibility and safety for human use. For instance, liposomes have been shown to enhance drug solubility, permeability, and bioavailability. Similarly, HDL nanoparticles demonstrate excellent biocompatibility. Cell-derived nanoparticles, on the other hand, offer stability, non-toxicity, and the potential for personalization based on the patient’s cell membrane source. These unique properties make them highly desirable as drug carriers. This review article aims to provide an overview of the advancements made in the utilization of both synthetic and natural biomembrane nanoparticles, such as liposomes, HDL, cell membrane-based nanoparticles, and extracellular vesicles (EVs), as drug carriers for the treatment of AD. By exploring the progress in this field, we aim to shed light on the potential of these nanoparticles for effective drug delivery in AD therapy.
By encapsulating drugs within their lipid bilayer or phospholipid structure, biomembrane nanoparticles facilitate transport across the BBB and enable targeted drug delivery to the CNS for AD treatment. Some nanoparticles may face challenges related to biosafety, drug-loading capacity, BBB penetration, and targeting ability. These limitations can be addressed through structural adjustments or surface modifications, such as composition ratio tuning, targeting ligand modification, lipid modification, charge modification, magnetic modification, and other strategies. These modified biomembrane nanoparticles can cross the BBB via receptor-mediated transcytosis, carrier-mediated transcytosis, adsorption-mediated transcytosis, etc., delivering drugs to target various AD pathological mechanisms like Aβ amyloid deposition, tau protein phosphorylation, and neurocholinergic disorders. The combination therapy of targeting multiple pathological mechanisms simultaneously shows potential for improving clinical symptoms in AD patients.
While the development of biomembrane nanoparticles holds great promise for AD treatment, it also presents certain challenges that need to be addressed in further research. These challenges are crucial to ensure optimal drug delivery and maximize the potential of biomembrane nanoparticles in AD therapy: (1) Although biomembrane nanoparticles have been extensively studied, most studies are still in the laboratory stage and it requires in-depth safety and toxicity assessments before translation to the clinic. (2) Cell membrane and EV technologies are just in their infancy and they have lower yields, and the preparation process and modification means need to be further skilled and improved to meet the realistic needs of respecting individual patient differences, individualized medical needs, and performing large-scale nanocarrier preparation. (3) The current research on biomembrane nanoparticles, especially cell membrane-based nanoparticles, is mainly for the treatment of tumors and inflammation, and there are fewer experiments related to AD drug delivery. In the future, we must promote the development of biomembrane nanoparticles in AD treatment with the knowledge gained from anti-tumor drug delivery experiments. (4) A single target can no longer meet the diversity of AD pathological mechanisms, so we need to increase the development of nanoparticles with strong drug-carrying capacity and adopt multidrug and multitarget combination therapy. There are already strategies to combine synthetic nanoparticles with natural biomaterials to achieve effective delivery of AD drugs using both advantages. Future advances in these issues will accelerate the translation of biomembrane nanoparticles to the clinic.
However, despite the challenges mentioned earlier, biomembrane nanoparticles for targeted drug delivery in AD still hold immense potential. With their unique advantages, it is possible to overcome these challenges in the future. A deeper understanding of the biology of nano-drug delivery systems, coupled with robust engineering of their functionality, will pave the way for exploring new strategies to achieve high BBB penetration, precise targeting, and enhanced drug delivery capabilities. In the coming years, research efforts will be directed towards selecting appropriate delivery vehicles that can accurately navigate the complex pathogenesis of AD. Collaborative approaches involving gene therapy and chemotherapy technologies will further enhance the potential of nano-drug delivery systems for multi-target and multi-mechanism therapy in AD. In conclusion, biomembrane nanoparticles offer a promising avenue for targeted drug delivery in AD treatment. Overcoming the current challenges through advancements in technology and a deeper understanding of their biological interactions will unlock their full potential. By combining various therapeutic approaches and leveraging the capabilities of nano-drug delivery systems, we can envision a future where effective and personalized treatments for AD become a reality.
Abbreviations
AD, Alzheimer’s disease; Aβ, β-amyloid; NMDA, N-methyl-D-aspartic acid; HDL, High-density lipoprotein; rHDL, reconstituted high density lipoprotein; RBC, red blood cell; WBC, white blood cell; PLT, platelet; MA, Macrophage; MSC, Mesenchymal stem cell; BBB, blood-brain barrier; FDA, Food and Drug Administration; CNS, central nervous system; GI, Gastrointestinal; BM, Basement membrane; ECs, endothelial cells; BMECs, Brain Microvascular Endothelial Cells; EVs, extracellular vehicles; MPS, monocyte macrophage system; GM1, monosialotetrahexosylganglioside; PI, propidium iodide; SM, sphingomyelin; PEG, polyethyleneglycol; CPP, cell-penetrating peptide; TfR, transferrin receptor; LfR, lactoferrin receptor; MAN-LIP, p-aminophenyl--d-mannopyranoside-modified liposomes; GSH, glutathione; PA, phosphatidic acid; CRM, curcumin; BACE1, β-site amyloid precursor protein cleaving enzyme 1; CL, cardiolipin; NGF, nerve growth factor; WGA, wheat germ agglutinin; TrkA, tyrosine kinase receptor; AChE, acetylcholinesterase; AChR, acetylcholine receptor; ROS, Reactive oxygen species; siRNA, small interfering RNA; PLGA, Polylactic-co-glycolic acid; ApoJ, apolipoprotein J; LDLR, Low-density lipoprotein receptor; LRP1, LDL receptor-related protein 1; ANC, apolipoprotein E-reconstituted HDL nanocarrier; RBC-NP, red blood cell-nanoparticle; SLNs, solid lipid nanoparticles; RVG, rabies virus glycoprotein; TPP, triphenylphosphine cation; EVs, Extracellular vesicles; RMT, receptor-mediated cytokinesis; GSLs, Glycosphingolipids; Que, quercetin; OA, okadaic acid.
Ethics Approval and Consent to Participate
This article does not involve ethics or consent.
Acknowledgments
Thanks to Biorender for giving the drawing support. Thanks to Home for Researchers for the language polish.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the Natural Science Foundation of Shanghai Municipal Science and Technology Commission, grant number #22ZR147750; Science and Technology Support Projects in Biomedicine Field of Shanghai Science and Technology Commission, grant number #19441907500; National Natural Science Foundation of China, grant number 82072051 and National Natural Science Foundation of China, grant number 81771964; Innovative Clinical Research Project of Changzheng Hospital, grant number 2020 YLCYJ-Y 02; Characteristic Medical Service Project for the Army of Changzheng Hospital, grant number 2020 CZWJFW 12; Doctor Innovation Fund of Shanghai Changzheng Hospital, grant numbers BSCX2022-34, BSCX2023-19, and BSCX2022-35; Science and Technology Innovation Action Plan of Shanghai Science and Technology Commission (23Y11906600).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nichols E, Szoeke CEI, Vollset SE.; Collaborators GBDD. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88–106. doi:10.1016/S1474-4422(18)30403-4
2. Cano A, Turowski P, Ettcheto M, et al. Nanomedicine-based technologies and novel biomarkers for the diagnosis and treatment of Alzheimer’s disease: from current to future challenges. J Nanobiotechnology. 2021;19(1):122. doi:10.1186/s12951-021-00864-x
3. Liu Z, Sun W, Chen H, et al. Caregiver burden and its associated factors among family caregivers of persons with dementia in Shanghai, China: a cross-sectional study. BMJ Open. 2022;12(5):e057817.
4. Ettcheto M, Cano A, Busquets O, et al. A metabolic perspective of late onset Alzheimer’s disease. Pharmacol Res. 2019;145:104255. doi:10.1016/j.phrs.2019.104255
5. Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270–276. doi:10.1038/s41591-018-0297-y
6. Lee JH, Yang DS, Goulbourne CN, et al. Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Abeta in neurons, yielding senile plaques. Nat Neurosci. 2022;25(6):688–701. doi:10.1038/s41593-022-01084-8
7. Tumani H, Huss A, Bachhuber F. The cerebrospinal fluid and barriers - anatomic and physiologic considerations. Handb Clin Neurol. 2017;146:21–32.
8. Ji W, Gong B, Jin H, et al. Recent progress towards vaccines and antibody-based therapies against Alzheimer’s disease. Mini Rev Med Chem. 2021;21(19):3062–3072. doi:10.2174/1389557521666210805110920
9. Agrawal M, Prathyusha E, Ahmed H, et al. Biomaterials in treatment of Alzheimer’s disease. Neurochem Int. 2021;145:105008. doi:10.1016/j.neuint.2021.105008
10. Cummings J, Lee G, Nahed P, et al. Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement. 2022;8(1):e12295. doi:10.1002/trc2.12295
11. Chen YX, Wei CX, Lyu YQ, Chen HZ, Jiang G, Gao XL. Biomimetic drug-delivery systems for the management of brain diseases. Biomater Sci. 2020;8(4):1073–1088.
12. Zhang H, Peng Y, Zhuo L, et al. Recent advance on pleiotropic cholinesterase inhibitors bearing amyloid modulation efficacy. Eur J Med Chem. 2022;242:114695. doi:10.1016/j.ejmech.2022.114695
13. Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17(1):69. doi:10.1186/s12987-020-00230-3
14. Xu L, Nirwane A, Yao Y. Basement membrane and blood-brain barrier. Stroke Vasc Neurol. 2019;4(2):78–82. doi:10.1136/svn-2018-000198
15. Pardridge WM. Molecular biology of the blood-brain barrier. Mol Biotechnol. 2005;30(1):57–70. doi:10.1385/MB:30:1:057
16. Furtado D, Bjornmalm M, Ayton S, Bush AI, Kempe K, Caruso F. Overcoming the blood-brain barrier: the role of nanomaterials in treating neurological diseases. Adv Mater. 2018;30(46):e1801362. doi:10.1002/adma.201801362
17. Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. doi:10.1602/neurorx.2.1.3
18. Pardridge WM. Blood–brain barrier delivery. Drug Discovery Today. 2007;12(1):54–61. doi:10.1016/j.drudis.2006.10.013
19. Ajay BGW, Murcko MA, Murcko MA. Designing libraries with CNS activity. J Med Chem. 1999;42(24):4942–4951. doi:10.1021/jm990017w
20. Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb Chem. 1999;1(1):55–68. doi:10.1021/cc9800071
21. Ouyang Q, Meng Y, Zhou W, Tong J, Cheng Z, Zhu Q. New advances in brain-targeting nano-drug delivery systems for Alzheimer’s disease. J Drug Target. 2022;30(1):61–81. doi:10.1080/1061186X.2021.1927055
22. Wu Z, Zhang H, Yan J, Wei Y, Su J. Engineered biomembrane-derived nanoparticles for nanoscale theranostics. Theranostics. 2023;13(1):20–39. doi:10.7150/thno.76894
23. Yan H, Shao D, Lao YH, Li M, Hu H, Leong KW. Engineering cell membrane-based nanotherapeutics to target inflammation. Adv Sci. 2019;6(15):1900605. doi:10.1002/advs.201900605
24. Sato YT, Umezaki K, Sawada S, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6(1):21933. doi:10.1038/srep21933
25. Zhang CX, Zhao WY, Liu L, et al. A nanostructure of functional targeting epirubicin liposomes dually modified with aminophenyl glucose and cyclic pentapeptide used for brain glioblastoma treatment. Oncotarget. 2015;6(32):32681–32700. doi:10.18632/oncotarget.5354
26. Yang ZZ, Zhang YQ, Wang ZZ, Wu K, Lou JN, Qi XR. Enhanced brain distribution and pharmacodynamics of rivastigmine by liposomes following intranasal administration. Int J Pharm. 2013;452(1–2):344–354. doi:10.1016/j.ijpharm.2013.05.009
27. Parthsarathy V, McClean PL, Holscher C, et al. A novel retro-inverso peptide inhibitor reduces amyloid deposition, oxidation and inflammation and stimulates neurogenesis in the APPswe/PS1DeltaE9 mouse model of Alzheimer’s disease. PLoS One. 2013;8(1):e54769. doi:10.1371/annotation/57e0a947-8600-4658-b04c-cf7a45c8bd8d
28. Kong L, Li XT, Ni YN, et al. Transferrin-modified osthole PEGylated liposomes travel the blood-brain barrier and mitigate Alzheimer’s disease-related pathology in APP/PS-1 mice. Int J Nanomedicine. 2020;15:2841–2858. doi:10.2147/IJN.S239608
29. Yang X, Li X, Liu L, et al. Transferrin-Pep63-liposomes accelerate the clearance of Abeta and rescue impaired synaptic plasticity in early Alzheimer’s disease models. Cell Death Discov. 2021;7(1):256. doi:10.1038/s41420-021-00639-1
30. Hernandez C, Shukla S. Liposome based drug delivery as a potential treatment option for Alzheimer’s disease. Neural Regen Res. 2022;17(6):1190–1198. doi:10.4103/1673-5374.327328
31. Harilal S, Jose J, Parambi DGT, et al. Advancements in nanotherapeutics for Alzheimer’s disease: current perspectives. J Pharm Pharmacol. 2019;71(9):1370–1383. doi:10.1111/jphp.13132
32. Mourtas S, Christodoulou P, Klepetsanis P, Gatos D, Barlos K, Antimisiaris SG. Preparation of benzothiazolyl-decorated nanoliposomes. Molecules. 2019;24(8):1540. doi:10.3390/molecules24081540
33. Kuo YC, Lin CY, Li JS, Lou YI. Wheat germ agglutinin-conjugated liposomes incorporated with cardiolipin to improve neuronal survival in Alzheimer’s disease treatment. Int J Nanomedicine. 2017;12:1757–1774. doi:10.2147/IJN.S128396
34. Kuo YC, Tsao CW. Neuroprotection against apoptosis of SK-N-MC cells using RMP-7- and lactoferrin-grafted liposomes carrying quercetin. Int J Nanomedicine. 2017;12:2857–2869. doi:10.2147/IJN.S132472
35. Kuo YC, Lin CY. Targeting delivery of liposomes with conjugated p-aminophenyl-alpha-d-manno-pyranoside and apolipoprotein E for inhibiting neuronal degeneration insulted with beta-amyloid peptide. J Drug Target. 2015;23(2):147–158. doi:10.3109/1061186X.2014.965716
36. Balklava Z, Niehage C, Currinn H, et al. The amyloid precursor protein controls PIKfyve function. PLoS One. 2015;10(6):e0130485. doi:10.1371/journal.pone.0130485
37. Hubin E, Verghese PB, van Nuland N, Broersen K. Apolipoprotein E associated with reconstituted high-density lipoprotein-like particles is protected from aggregation. FEBS Lett. 2019;593(11):1144–1153. doi:10.1002/1873-3468.13428
38. Corace G, Angeloni C, Malaguti M, et al. Multifunctional liposomes for nasal delivery of the anti-Alzheimer drug tacrine hydrochloride. J Liposome Res. 2014;24(4):323–335. doi:10.3109/08982104.2014.899369
39. Dreier J, Sorensen JA, Brewer JR, Santos HA. Superresolution and fluorescence dynamics evidence reveal that intact liposomes do not cross the human skin barrier. PLoS One. 2016;11(1):e0146514. doi:10.1371/journal.pone.0146514
40. Ostro MJ, Cullis PR. Use of liposomes as injectable-drug delivery systems. Am J Hosp Pharm. 1989;46(8):1576–1587.
41. Morgan D. Immunotherapy for Alzheimer’s disease. J Intern Med. 2011;269(1):54–63. doi:10.1111/j.1365-2796.2010.02315.x
42. Ross C, Taylor M, Fullwood N, Allsop D. Liposome delivery systems for the treatment of Alzheimer’s disease. Int J Nanomedicine. 2018;13:8507–8522. doi:10.2147/IJN.S183117
43. Agrawal M, Tripathi DK, Saraf S, et al. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J Control Release. 2017;260:61–77. doi:10.1016/j.jconrel.2017.05.019
44. Nicastro MC, Spigolon D, Librizzi F, et al. Amyloid beta-peptide insertion in liposomes containing GM1-cholesterol domains. Biophys Chem. 2016;208:9–16. doi:10.1016/j.bpc.2015.07.010
45. Selkoe DJ. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3(1):75–80. doi:10.3233/JAD-2001-3111
46. Chen H, Qin Y, Zhang Q, et al. Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas. Eur J Pharm Sci. 2011;44(1–2):164–173. doi:10.1016/j.ejps.2011.07.007
47. Chen H, Tang L, Qin Y, et al. Lactoferrin-modified procationic liposomes as a novel drug carrier for brain delivery. Eur J Pharm Sci. 2010;40(2):94–102. doi:10.1016/j.ejps.2010.03.007
48. Qu B, Li X, Guan M, Li X, Hai L, Wu Y. Design, synthesis and biological evaluation of multivalent glucosides with high affinity as ligands for brain targeting liposomes. Eur J Med Chem. 2014;72:110–118. doi:10.1016/j.ejmech.2013.10.007
49. Xie F, Yao N, Qin Y, et al. Investigation of glucose-modified liposomes using polyethylene glycols with different chain lengths as the linkers for brain targeting. Int J Nanomedicine. 2012;7:163–175. doi:10.2147/IJN.S23771
50. Patching SG. Glucose transporters at the blood-brain barrier: function, regulation and gateways for drug delivery. Mol Neurobiol. 2017;54(2):1046–1077. doi:10.1007/s12035-015-9672-6
51. Rotman M, Welling MM, Bunschoten A, et al. Enhanced glutathione PEGylated liposomal brain delivery of an anti-amyloid single domain antibody fragment in a mouse model for Alzheimer’s disease. J Control Release. 2015;203:40–50. doi:10.1016/j.jconrel.2015.02.012
52. Kamei N, Tanaka M, Choi H, et al. Effect of an enhanced nose-to-brain delivery of insulin on mild and progressive memory loss in the senescence-accelerated mouse. Mol Pharm. 2017;14(3):916–927. doi:10.1021/acs.molpharmaceut.6b01134
53. Joshi S, Singh-Moon RP, Ellis JA, et al. Cerebral hypoperfusion-assisted intra-arterial deposition of liposomes in normal and glioma-bearing rats. Neurosurgery. 2015;76(1):92–100. doi:10.1227/NEU.0000000000000552
54. Joshi S, Singh-Moon RP, Wang M, et al. Transient cerebral hypoperfusion assisted intraarterial cationic liposome delivery to brain tissue. J Neurooncol. 2014;118(1):73–82. doi:10.1007/s11060-014-1421-6
55. Du D, Chang N, Sun S, et al. The role of glucose transporters in the distribution of p-aminophenyl-alpha-d-mannopyranoside modified liposomes within mice brain. J Control Release. 2014;182:99–110. doi:10.1016/j.jconrel.2014.03.006
56. Wang ZH, Wang ZY, Sun CS, Wang CY, Jiang TY, Wang SL. Trimethylated chitosan-conjugated PLGA nanoparticles for the delivery of drugs to the brain. Biomaterials. 2010;31(5):908–915. doi:10.1016/j.biomaterials.2009.09.104
57. Khan J, Alexander A, Saraf S, Saraf S, Saraf S. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J Control Release. 2013;168(1):50–60. doi:10.1016/j.jconrel.2013.02.025
58. Martin-Aragon S, Benedi JM, Villar AM. Modifications on antioxidant capacity and lipid peroxidation in mice under fraxetin treatment. J Pharm Pharmacol. 1997;49(1):49–52. doi:10.1111/j.2042-7158.1997.tb06751.x
59. Taylor M, Moore S, Mourtas S, et al. Effect of curcumin-associated and lipid ligand-functionalized nanoliposomes on aggregation of the Alzheimer’s Abeta peptide. Nanomedicine. 2011;7(5):541–550. doi:10.1016/j.nano.2011.06.015
60. Mourtas S, Lazar AN, Markoutsa E, Duyckaerts C, Antimisiaris SG. Multifunctional nanoliposomes with curcumin-lipid derivative and brain targeting functionality with potential applications for Alzheimer disease. Eur J Med Chem. 2014;80:175–183. doi:10.1016/j.ejmech.2014.04.050
61. Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21(21):8370–8377. doi:10.1523/JNEUROSCI.21-21-08370.2001
62. Balducci C, Mancini S, Minniti S, et al. Multifunctional liposomes reduce brain beta-amyloid burden and ameliorate memory impairment in Alzheimer’s disease mouse models. J Neurosci. 2014;34(42):14022–14031. doi:10.1523/JNEUROSCI.0284-14.2014
63. Tanifum EA, Dasgupta I, Srivastava M, et al. Intravenous delivery of targeted liposomes to amyloid-beta pathology in APP/PSEN1 transgenic mice. PLoS One. 2012;7(10):e48515. doi:10.1371/journal.pone.0048515
64. Li W, Zhou Y, Zhao N, Hao B, Wang X, Kong P. Pharmacokinetic behavior and efficiency of acetylcholinesterase inhibition in rat brain after intranasal administration of galanthamine hydrobromide loaded flexible liposomes. Environ. Toxicol. Pharmacol. 2012;34(2):272–279. doi:10.1016/j.etap.2012.04.012
65. Austen BM, Paleologou KE, Ali SA, Qureshi MM, Allsop D, El-Agnaf OM. Designing peptide inhibitors for oligomerization and toxicity of Alzheimer’s beta-amyloid peptide. Biochemistry. 2008;47(7):1984–1992. doi:10.1021/bi701415b
66. Gregori M, Taylor M, Salvati E, et al. Retro-inverso peptide inhibitor nanoparticles as potent inhibitors of aggregation of the Alzheimer’s Abeta peptide. Nanomedicine. 2017;13(2):723–732. doi:10.1016/j.nano.2016.10.006
67. Nixon RA. Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci. 2007;120(Pt 23):4081–4091. doi:10.1242/jcs.019265
68. Cai Z, Zhao B, Li K, et al. Mammalian target of rapamycin: a valid therapeutic target through the autophagy pathway for Alzheimer’s disease? J Neurosci Res. 2012;90(6):1105–1118. doi:10.1002/jnr.23011
69. Luo Q, Lin YX, Yang PP, et al. A self-destructive nanosweeper that captures and clears amyloid beta-peptides. Nat Commun. 2018;9(1):1802. doi:10.1038/s41467-018-04255-z
70. Huang LK, Kuan YC, Lin HW, Hu CJ. Clinical trials of new drugs for Alzheimer disease: a 2020–2023 update. J Biomed Sci. 2023;30(1):83. doi:10.1186/s12929-023-00976-6
71. Sviridov D, Remaley AT. High-density lipoprotein mimetics: promises and challenges. Biochem J. 2015;472(3):249–259. doi:10.1042/BJ20150832
72. Tabet F, Lambert G, Cuesta Torres LF, et al. Lipid-free apolipoprotein A-I and discoidal reconstituted high-density lipoproteins differentially inhibit glucose-induced oxidative stress in human macrophages. Arterioscler Thromb Vasc Biol. 2011;31(5):1192–1200. doi:10.1161/ATVBAHA.110.222000
73. Yvan-Charvet L, Pagler TA, Seimon TA, et al. ABCA1 and ABCG1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circ Res. 2010;106(12):1861–1869. doi:10.1161/CIRCRESAHA.110.217281
74. Navab M, Anantharamaiah GM, Reddy ST, et al. Human apolipoprotein A-I and A-I mimetic peptides: potential for atherosclerosis reversal. Curr Opin Lipidol. 2004;15(6):645–649. doi:10.1097/00041433-200412000-00004
75. Song Q, Song H, Xu J, et al. Biomimetic ApoE-reconstituted high density lipoprotein nanocarrier for blood-brain barrier penetration and amyloid beta-targeting drug delivery. Mol Pharm. 2016;13(11):3976–3987. doi:10.1021/acs.molpharmaceut.6b00781
76. Han G, Bai K, Yang X, et al. “Drug-Carrier” synergy therapy for amyloid-beta clearance and inhibition of tau phosphorylation via biomimetic lipid nanocomposite assembly. Adv Sci. 2022;9(14):e2106072. doi:10.1002/advs.202106072
77. Zhang X, Huang G. Synthetic lipoprotein as nano-material vehicle in the targeted drug delivery. Drug Deliv. 2017;24(sup1):16–21. doi:10.1080/10717544.2017.1384518
78. Kuai R, Li D, Chen YE, Moon JJ, Schwendeman A. High-density lipoproteins: nature’s multifunctional nanoparticles. ACS Nano. 2016;10(3):3015–3041. doi:10.1021/acsnano.5b07522
79. Murphy AJ, Funt S, Gorman D, Tall AR, Wang N. Pegylation of high-density lipoprotein decreases plasma clearance and enhances antiatherogenic activity. Circ Res. 2013;113(1):e1–e9. doi:10.1161/CIRCRESAHA.113.301112
80. Wolfrum C, Shi S, Jayaprakash KN, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25(10):1149–1157. doi:10.1038/nbt1339
81. Sanchez-Gaytan BL, Fay F, Lobatto ME, et al. HDL-mimetic PLGA nanoparticle to target atherosclerosis plaque macrophages. Bioconjug Chem. 2015;26(3):443–451. doi:10.1021/bc500517k
82. Ma X, Song Q, Gao X. Reconstituted high-density lipoproteins: novel biomimetic nanocarriers for drug delivery. Acta Pharm Sin B. 2018;8(1):51–63. doi:10.1016/j.apsb.2017.11.006
83. Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest. 2006;116(12):3090–3100. doi:10.1172/JCI30163
84. Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7(5):365–375. doi:10.1016/j.cmet.2008.03.001
85. Schwendeman A, Sviridov DO, Yuan W, et al. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J Lipid Res. 2015;56(9):1727–1737. doi:10.1194/jlr.M060285
86. Molino Y, David M, Varini K, et al. Use of LDL receptor-targeting peptide vectors for in vitro and in vivo cargo transport across the blood-brain barrier. FASEB J. 2017;31(5):1807–1827. doi:10.1096/fj.201600827R
87. Croy JE, Brandon T, Komives EA. Two apolipoprotein E mimetic peptides, ApoE(130-149) and ApoE(141-155)2, bind to LRP1. Biochemistry. 2004;43(23):7328–7335. doi:10.1021/bi036208p
88. Fernandez-de-Retana S, Cano-Sarabia M, Marazuela P, et al. Characterization of ApoJ-reconstituted high-density lipoprotein (rHDL) nanodisc for the potential treatment of cerebral beta-amyloidosis. Sci Rep. 2017;7(1):14637. doi:10.1038/s41598-017-15215-w
89. Uno Y, Piao W, Miyata K, Nishina K, Mizusawa H, Yokota T. High-density lipoprotein facilitates in vivo delivery of alpha-tocopherol-conjugated short-interfering RNA to the brain. Hum Gene Ther. 2011;22(6):711–719. doi:10.1089/hum.2010.083
90. Huang M, Hu M, Song Q, et al. GM1-modified lipoprotein-like nanoparticle: multifunctional nanoplatform for the combination therapy of Alzheimer’s disease. ACS Nano. 2015;9(11):10801–10816. doi:10.1021/acsnano.5b03124
91. Ding Y, Wang Y, Opoku-Damoah Y, et al. Dual-functional bio-derived nanoparticulates for apoptotic antitumor therapy. Biomaterials. 2015;72:90–103. doi:10.1016/j.biomaterials.2015.08.051
92. Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108(27):10980–10985. doi:10.1073/pnas.1106634108
93. Zhen X, Cheng P, Pu K. Recent advances in cell membrane-camouflaged nanoparticles for cancer phototherapy. Small. 2019;15(1):e1804105. doi:10.1002/smll.201804105
94. Hu CM, Fang RH, Wang KC, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–121. doi:10.1038/nature15373
95. Luk BT, Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release. 2015;220(Pt B):600–607. doi:10.1016/j.jconrel.2015.07.019
96. Li R, He Y, Zhang S, Qin J, Wang J. Cell membrane-based nanoparticles: a new biomimetic platform for tumor diagnosis and treatment. Acta Pharm Sin B. 2018;8(1):14–22. doi:10.1016/j.apsb.2017.11.009
97. Vijayan V, Uthaman S, Park IK. Cell membrane-camouflaged nanoparticles: a promising biomimetic strategy for cancer theragnostics. Polymers. 2018;10(9):983. doi:10.3390/polym10090983
98. Liu Y, Luo J, Chen X, Liu W, Chen T. Cell membrane coating technology: a promising strategy for biomedical applications. Nanomicro Lett. 2019;11(1):100. doi:10.1007/s40820-019-0330-9
99. Zhong X, Na Y, Yin S, et al. Cell membrane biomimetic nanoparticles with potential in treatment of Alzheimer’s disease. Molecules. 2023;28(5):2336. doi:10.3390/molecules28052336
100. Han Y, Gao C, Wang H, et al. Macrophage membrane-coated nanocarriers Co-Modified by RVG29 and TPP improve brain neuronal mitochondria-targeting and therapeutic efficacy in Alzheimer’s disease mice. Bioact Mater. 2021;6(2):529–542. doi:10.1016/j.bioactmat.2020.08.017
101. Rao L, Bu LL, Xu JH, et al. Red blood cell membrane as a biomimetic nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small. 2015;11(46):6225–6236. doi:10.1002/smll.201502388
102. Calabro F, Lorusso V, Rosati G, et al. Gemcitabine and paclitaxel every 2 weeks in patients with previously untreated urothelial carcinoma. Cancer. 2009;115(12):2652–2659. doi:10.1002/cncr.24313
103. Hu CM, Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacol. 2012;83(8):1104–1111. doi:10.1016/j.bcp.2012.01.008
104. McDaid HM, Johnston PG. Synergistic interaction between paclitaxel and 8-chloro-adenosine 3’,5’-monophosphate in human ovarian carcinoma cell lines. Clin Cancer Res. 1999;5(1):215–220.
105. Chai Z, Hu X, Wei X, et al. A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery. J Control Release. 2017;264:102–111. doi:10.1016/j.jconrel.2017.08.027
106. Han Y, Chu X, Cui L, et al. Neuronal mitochondria-targeted therapy for Alzheimer’s disease by systemic delivery of resveratrol using dual-modified novel biomimetic nanosystems. Drug Deliv. 2020;27(1):502–518. doi:10.1080/10717544.2020.1745328
107. Matsushita T, Kibayashi T, Katayama T, et al. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Neurosci Lett. 2011;502(1):41–45. doi:10.1016/j.neulet.2011.07.021
108. Wang J, Zhu M, Nie G. Biomembrane-based nanostructures for cancer targeting and therapy: from synthetic liposomes to natural biomembranes and membrane-vesicles. Adv Drug Deliv Rev. 2021;178:113974. doi:10.1016/j.addr.2021.113974
109. Marrache S, Dhar S. Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc Natl Acad Sci U S A. 2012;109(40):16288–16293. doi:10.1073/pnas.1210096109
110. Gao C, Wang Y, Sun J, et al. Neuronal mitochondria-targeted delivery of curcumin by biomimetic engineered nanosystems in Alzheimer’s disease mice. Acta Biomater. 2020;108:285–299. doi:10.1016/j.actbio.2020.03.029
111. Gao C, Chu X, Gong W, et al. Neuron tau-targeting biomimetic nanoparticles for curcumin delivery to delay progression of Alzheimer’s disease. J Nanobiotechnology. 2020;18(1):71. doi:10.1186/s12951-020-00626-1
112. Fang RH, Hu CM, Chen KN, et al. Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles. Nanoscale. 2013;5(19):8884–8888. doi:10.1039/c3nr03064d
113. Hamidi M, Zarrin A, Foroozesh M, Mohammadi-Samani S. Applications of carrier erythrocytes in delivery of biopharmaceuticals. J Control Release. 2007;118(2):145–160.
114. Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106(Pt A):148–156.
115. Rufino-Ramos D, Albuquerque PR, Carmona V, Perfeito R, Nobre RJ, Pereira de Almeida L. Extracellular vesicles: novel promising delivery systems for therapy of brain diseases. J Control Release. 2017;262:247–258. doi:10.1016/j.jconrel.2017.07.001
116. Sarko DK, McKinney CE. Exosomes: origins and therapeutic potential for neurodegenerative disease. Front Neurosci. 2017;11:82. doi:10.3389/fnins.2017.00082
117. Vashist A, Manickam P, Raymond AD, et al. Recent advances in nanotherapeutics for neurological disorders. ACS Appl Bio Mater. 2023;6(7):2614–2621. doi:10.1021/acsabm.3c00254
118. Qi Y, Guo L, Jiang Y, Shi Y, Sui H, Zhao L. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Deliv. 2020;27(1):745–755. doi:10.1080/10717544.2020.1762262
119. Haney MJ, Klyachko NL, Zhao Y, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. doi:10.1016/j.jconrel.2015.03.033
120. Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10(7):521–535. doi:10.1038/nrd3499
121. Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287–296. doi:10.1016/j.apsb.2016.02.001
122. van den Boorn JG, Schlee M, Coch C, Hartmann G. SiRNA delivery with exosome nanoparticles. Nat Biotechnol. 2011;29(4):325–326. doi:10.1038/nbt.1830
123. Mehrotra N, Tripathi RM. Short interfering RNA therapeutics: nanocarriers, prospects and limitations. IET Nanobiotechnol. 2015;9(6):386–395. doi:10.1049/iet-nbt.2015.0018
124. Lakhal S, Wood MJ. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. Bioessays. 2011;33(10):737–741. doi:10.1002/bies.201100076
125. Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015;205:35–44. doi:10.1016/j.jconrel.2014.11.029
126. Das CK, Jena BC, Banerjee I, et al. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol Pharm. 2019;16(1):24–40. doi:10.1021/acs.molpharmaceut.8b00901
127. Kim MS, Haney MJ, Zhao Y, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12(3):655–664. doi:10.1016/j.nano.2015.10.012
128. Park O, Choi ES, Yu G, et al. Efficient delivery of Tyrosinase Related Protein-2 (TRP2) peptides to lymph nodes using serum-derived exosomes. Macromol Biosci. 2018;18(12):e1800301. doi:10.1002/mabi.201800301
129. Sancho-Albero M, Encabo-Berzosa MDM, Beltran-Visiedo M, et al. Efficient encapsulation of theranostic nanoparticles in cell-derived exosomes: leveraging the exosomal biogenesis pathway to obtain hollow gold nanoparticle-hybrids. Nanoscale. 2019;11(40):18825–18836. doi:10.1039/C9NR06183E
130. Fu S, Wang Y, Xia X, Zheng JC. Exosome engineering: current progress in cargo loading and targeted delivery. NanoImpact. 2020;20:100261. doi:10.1016/j.impact.2020.100261
131. Wiklander OP, Nordin JZ, O’Loughlin A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4(1):26316. doi:10.3402/jev.v4.26316
132. Mirzaaghasi A, Han Y, Ahn SH, Choi C, Park JH. Biodistribution and pharmacokinectics of liposomes and exosomes in a Mouse Model of sepsis. Pharmaceutics. 2021;13(3):427. doi:10.3390/pharmaceutics13030427
133. Choi H, Choi K, Kim DH, et al. Strategies for targeted delivery of exosomes to the brain: advantages and challenges. Pharmaceutics. 2022;14(3):672. doi:10.3390/pharmaceutics14030672
134. Wang H, Sui H, Zheng Y, et al. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3beta pathway. Nanoscale. 2019;11(15):7481–7496. doi:10.1039/C9NR01255A
135. Terstappen GC, Meyer AH, Bell RD, Zhang W. Strategies for delivering therapeutics across the blood-brain barrier. Nat Rev Drug Discov. 2021;20(5):362–383.
136. Xie J, Bi Y, Zhang H, et al. Cell-penetrating peptides in diagnosis and treatment of human diseases: from preclinical research to clinical application. Front Pharmacol. 2020;11:697.
137. Ando Y, Okada H, Takemura G, et al. Brain-Specific Ultrastructure of Capillary Endothelial Glycocalyx and Its Possible Contribution for Blood Brain Barrier. Sci Rep. 2018;8(1):17523. doi:10.1038/s41598-018-35976-2
138. Wang Q, Cheng S, Qin F, Fu A, Fu C. Application progress of RVG peptides to facilitate the delivery of therapeutic agents into the central nervous system. RSC Adv. 2021;11(15):8505–8515. doi:10.1039/D1RA00550B
139. Lentz TL, Burrage TG, Smith AL, Crick J, Tignor GH. Is the acetylcholine receptor a rabies virus receptor? Science. 1982;215(4529):182–184. doi:10.1126/science.7053569
140. Cui GH, Guo HD, Li H, et al. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun Ageing. 2019;16(1):10. doi:10.1186/s12979-019-0150-2
141. Sancho-Albero M, Navascues N, Mendoza G, et al. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J Nanobiotechnology. 2019;17(1):16. doi:10.1186/s12951-018-0437-z
142. Qiao L, Hu S, Huang K, et al. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics. 2020;10(8):3474–3487. doi:10.7150/thno.39434
143. Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015;199:145–155. doi:10.1016/j.jconrel.2014.12.013
144. Yuyama K, Sun H, Sakai S, et al. Decreased amyloid-beta pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J Biol Chem. 2014;289(35):24488–24498. doi:10.1074/jbc.M114.577213
145. Ding M, Shen Y, Wang P, et al. Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer’s disease. Neurochem Res. 2018;43(11):2165–2177. doi:10.1007/s11064-018-2641-5
146. Kuo YC, Chen CL, Rajesh R. Optimized liposomes with transactivator of transcription peptide and anti-apoptotic drugs to target hippocampal neurons and prevent tau-hyperphosphorylated neurodegeneration. Acta Biomater. 2019;87:207–222. doi:10.1016/j.actbio.2019.01.065
147. Khan A, Ali T, Rehman SU, et al. Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain. Front Pharmacol. 2018;9:1383. doi:10.3389/fphar.2018.01383
148. Vinayak M, Maurya AK. Quercetin loaded nanoparticles in targeting cancer: recent development. Anticancer Agents Med Chem. 2019;19(13):1560–1576. doi:10.2174/1871520619666190705150214
149. Cui GH, Wu J, Mou FF, et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018;32(2):654–668. doi:10.1096/fj.201700600R
150. de Godoy MA, Saraiva LM, de Carvalho LRP, et al. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. J Biol Chem. 2018;293(6):1957–1975. doi:10.1074/jbc.M117.807180
151. Xu F, Wu Y, Yang Q, et al. Engineered extracellular vesicles with SHP2 high expression promote mitophagy for Alzheimer’s disease treatment. Adv Mater. 2022;34(49):e2207107. doi:10.1002/adma.202207107
152. Bodart-Santos V, de Carvalho LRP, de Godoy MA, et al. Extracellular vesicles derived from human Wharton’s jelly mesenchymal stem cells protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. Stem Cell Res Ther. 2019;10(1):332. doi:10.1186/s13287-019-1432-5
153. Xie X, Song Q, Dai C, et al. Clinical safety and efficacy of allogenic human adipose mesenchymal stromal cells-derived exosomes in patients with mild to moderate Alzheimer’s disease: a phase I/II clinical trial. Gen Psychiatr. 2023;36(5):e101143. doi:10.1136/gpsych-2023-101143
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.