Back to Journals » Journal of Multidisciplinary Healthcare » Volume 13
Biomedical Data Sharing Among Researchers: A Study from Jordan
Authors Al-Ebbini L , Khabour OF , Alzoubi KH , Alkaraki AK
Received 28 September 2020
Accepted for publication 22 October 2020
Published 23 November 2020 Volume 2020:13 Pages 1669—1676
DOI https://doi.org/10.2147/JMDH.S284294
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Lina Al-Ebbini,1 Omar F Khabour,2 Karem H Alzoubi,3 Almuthanna K Alkaraki4
1Department of Biomedical Systems and Informatics Engineering, Hijjawi for Engineering Technology, Yarmouk University, Irbid 21163, Jordan; 2Department of Medical Laboratory Sciences, Faculty of Applied Medical Sciences, Jordan University of Science and Technology, Irbid 22110, Jordan; 3Department of Clinical Pharmacy, Jordan University of Science and Technology, Irbid 22110, Jordan; 4Department of Biological Sciences, Faculty of Science, Yarmouk University, Irbid 21163, Jordan
Correspondence: Almuthanna K Alkaraki
Department of Biological Sciences, Faculty of Science, Yarmouk University, Irbid 21163, Jordan
Tel +962 2 72 11111 ext. 2833
Fax +962 27211117
Email [email protected]
Lina Al-Ebbini
Department of Biomedical Systems and Informatics Engineering, Hijjawi for Engineering Technology, Yarmouk University, Irbid 21163, Jordan
Tel +962 2 7211111 ext. 4188
Email [email protected]
Background: Data sharing is an encouraged practice to support research in all fields. For that purpose, it is important to examine perceptions and concerns of researchers about biomedical data sharing, which was investigated in the current study.
Methods: This is a cross-sectional survey study that was distributed among biomedical researchers in Jordan, as an example of developing countries. The study survey consisted of questions about demographics and about respondent’s attitudes toward sharing of biomedical data.
Results: Among study participants, 46.9% (n=82) were positive regarding making their research data available to the public, whereas 53.1% refused the idea. The reasons for refusing to publicly share their data included “lack of regulations” (33.5%), “access to research data should be limited to the research team” (29.5%), “no place to deposit the data” (6.5%), and “lack of funding for data deposition” (6.0%). Agreement with the idea of making data available was associated with academic rank (P=0.003). Moreover, gender (P-value=0.043) and number of publications (P-value=0.005) were associated with a time frame for data sharing (ie, agreeing to share data before vs after publication).
Conclusion: About half of the respondents reported a positive attitude toward biomedical data sharing. Proper regulations and facilitation data deposition can enhance data sharing in Jordan.
Keywords: data sharing, responsible conduct of research, ethical issues, Jordan
Introduction
Data is important as it represents the framework of science and the basis for valuable scientific decisions and utilization of resources.1 Data collection, analysis, storage, and sharing have increased considerably due to the developments in information technology, automated data acquisition, and computational modeling.2,3 In fact, as science – especially healthcare sciences – becomes more data informative, research data sharing becomes more influential.4,5 Research data sharing reflects the practices whereby data collected in a specific research project are made available to others to be used for new research purposes. Data sharing has many advantages, such as time saving,6,7 efficient utilization,8 and data availability, which eases the contribution in different studies.9 In addition, analysis of the same data using different approaches can participate in scientific progress and enrich literature for new researchers.10,11 Moreover, data sharing can reduce redundancy and cost.12 Finally, data sharing can enhance transparency and the subsequent minimization of false or invalid conclusions, as well as the potential of hiding “undesirable” findings.13
Awareness of data sharing has been the subject of well-established debate among scientists including clinical and ethical experts.14–16 For instance, a study has presented qualitative interviews with different bio-banking stakeholders in Switzerland regarding data sharing.17 Many interviewees pointed out that data sharing between different biobanks can significantly advance biomedical research in the country and save the time needed to obtain ethical approval and informed consent.17 Another study pointed out to ethically acceptable data sharing options such as sending data for reanalysis by inviting researchers to undertake a joint re-analysis, and being co-author of any resulting publications of the shared data.18 An increased trend in the adoption of open data sharing policies among journals in the biomedical fields, and making unprocessed data available to others in publications was reported. On the other hand, there are many barriers that might impede the possibility of data sharing.19 These include lack of guidelines, restrictive policies, lack of resources, protection of privacy, ownership issues, lack of motivations, and restrictive data formatting.19
Among the issues that are still controversial in data sharing of biomedical research is who owns collected data, and should research participants consent to data sharing.20,21 It is broadly agreed that the utmost responsibility for any data set lies with the organization or persons who collected the data, and they have the authority to decide with whom the data can be shared.22,23 Therefore, researchers may have certain reasons to share data, under suitable cases, or not to do so if the proposed usage of the data is not clear or not suitable.24,25 However, on some occasions researchers may unsuitably decide not to share their data with others because they do not believe in the principle of data sharing.9,26 The attitude of biomedical researchers in Jordan, as an example of developing countries, toward sharing their data with others has not yet been investigated. Herein, the experiences and attitudes of researchers toward data sharing were investigated.
Methodology
A questionnaire was developed to examine the attitude of biomedical researchers toward data sharing. This study was carried out during the second half of summer 2019. The developed questionnaire was distributed among biomedical researchers in different Jordanian universities and it was ethically approved by the Institutional Review Board (IRB) of Jordan University of Science and Technology. The kind of data was defined in the questionnaire as medical, experimental, genomic sequences, surveys etc.The participants of the study consented before the self-administered questionnaire was administered. The aims and objectives of the study were illustrated to the participants and explanations were delivered upon request.
Research Instrument
The survey instrument consisted of two sections: questions about demographics and general data, and questions about attitude and concerns toward biomedical data sharing. The demographic/general data included gender, marital status, and sector of work (governmental or private), researcher’s subject discipline (medical vs biology), academic rank, and funding source. Biomedical researchers’ in Jordan reported their beliefs and perceptions toward data sharing via several questions. Participants were asked about the way of storing data (using regular names or coded) and they were asked if they would be willing to make their datasets available to others, then they were directed to the reasons for withholding datasets. Respondents could select one of six responses (concerns about future publishing opportunities, concerns about patient privacy, data should not be available to the public, lack of funding, lack of regulations, or no place to deposit data). The six options were based on common reasons for not sharing, as identified in the available literature.10,27 Moreover, researchers’ attitudes toward data sharing and reuse were assessed through the questionnaire. The participants were asked about the possibility of data sharing before or after publication, and if they would share data with academic and/or industrial institutions.
Statistical Analysis
Participants’ responses were described using frequency distribution for variables. Pearson Chi-square test was used to analyze attitudes toward data sharing according to demographic variables. A P-value<0.05 indicated statistical significance.
Results
Demographic information of participants (n=195) is shown in Table 1, where 56.5% (n=113) of respondents were males, and 41.0% (n=82) were females. The mean age of the participants was 39.9±10.3. The majority of participants were married (69.0%, n=138), whereas 22.0% (n=44) were single. About 63% (n=126) of the participants worked in governmental institutions, and the remaining 35% (n=35) worked in private institutions. Similarly, 63% (n=126) of the respondents reported their role in medical departments (ie, either medicine, pharmacy, dentistry, or nursing) while 34.5% (n=69) specified themselves as biologists. Around 15% (n=30) of the participants were lecturers, 39% (n=78) were assistant professors, 26% (n=52) were associate professors, and 17.5% (n=35) were full professors. Approximately half of the participants (50.5%, n=101) got institutional funds for research purposes while 24.5% (n=49) got external (eg, industrial) funds and only 11.5% (n=23) reported that they self-funded their research.
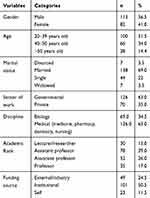 |
Table 1 Demographic Information |
Table 2 shows the reported strategy by which researchers deal with medical data in Jordan. Most participants (53.1%, n=103) rejected the idea of their data beingavailable to the public, whereas 42.3% (n=82) indicated that they would agree to share their data upon request, and only 4.6% (n=9) indicated an agreement to let their data be available to others. Participants specified the reason for refusing to publicly share their data as “lack of regulations” (33.5%), which was the leading reason. The second reason was “data should not be available to public” (29.5%), followed by “no place to deposit the data” (6.5%), then “lack of funding for data deposit” (6.0%). Few participants (2.5%) indicated their concerns about patient privacy, and only 1.0% pointed out their concerns about future publishing opportunities. Regarding the time frame of data sharing, 77.0% (n=154) indicated an agreement to share their data after publications, whereas 5.0% (n=10) showed the possibility of data sharing before publication. Meanwhile, 15.5% (n=31) denied the idea of data sharing either before or after publication. Participants also specified the opportunity of sharing data with academic and/or industrial institutions. Notably, most of participants (69.5%, n=139) preferred data sharing with both academia and industry. However, 13% preferred to share their data with industry and only 9.5% preferred to share their data with academic members only. Yet, 5.5% of participants absolutely refused the idea of sharing with either industry or academy sectors.
 |
Table 2 Attitude Toward Researchers About Data Sharing |
Both Tables 3 and 4 show the relationship between data sharing and demographic/general characteristics of the study participants. Agreement with the idea of making data available was associated with academic rank (P=0.003), but not with gender, number of publications, or work sector. Additionally, the number of publications (P-value=0.033) and the academic rank (P-value=0.044) were associated with reasons for withholding data. Moreover, gender (P-value=0.043) and number of publications (P-value=0.005) were associated with a time frame for data sharing, ie, sharing data before or after publication.
 |
Table 3 Relationship of Gender, Number of Publications, and Work Sector with Researchers’ Attitude Toward Data Sharing (Data are Presented as n (%)) |
 |
Table 4 Relationship of Academic Rank, Primary Source of Funding, and Discipline of Researcher with Attitude Toward Data Sharing, (Data are Presented as n (%)) |
Discussion
In the current investigation, the attitude of Jordanian biomedical researchers regarding data sharing was examined. About half of the researchers had negative attitudes regarding data sharing. Reported barriers of data sharing include lack of regulations, belief that data should not be available to the public, no place to deposit the data, or lack of funding to cover costs of data deposition.
Despite the fact that the importance of data sharing and its potential positive impacts,28,29 no research has been carried out to gain perception into the concerns that restrict the sharing of data, especially among researchers from a developing country such as Jordan. For instance, it was reported that researchers related to biobanks in the US were very motivated to make their data easily accessible and available to others.30
The majority of responders pointed out that they use coding aspects instead of the subject’s regular name for data storing. Thus, anonymization is highly practiced among the study participants, which protects subjects’ privacy and encourages participation in medical research. The importance of anonymization in maintaining the privacy of research participants was highlighted in a recent review.31 In clinical studies, a model that balances anonymity with efficiency of data sharing/utilization was suggested.32 In the US, iDASH (integrating data for analysis, anonymization, and sharing) is part of the National Center for Biomedical Computing that focuses on algorithms and tools for sharing data in a privacy-preserving manner.33 Therefore, anonymization in biomedical data sharing should be considered when establishing research databases in the country.
In this study, respondents were asked about the main reasons for not allowing their data to be available to others. The most common reason was “lack of regulations”. This is a common ethical issue that faces research in most developing countries34–36 which may be resolved by institutional initiatives. The second most common reason was the opinion that data should not be available to the public, which could be solved by providing more orientation to researchers about the importance as well as advantages of data sharing. In addition, incentives can be provided to encourage researchers to deposit their data in the national public databases,37 as data sharing can significantly limit redundancy in research and reduce the cost.12 The third ranked reasons were “no place to deposit the data” and “lack of funding for data deposition,” which might be solved by creating governmental databases that can be used to deposit data without costs.38 Few participants indicated their concerns about patient privacy and future publishing opportunities. Patients’ privacy can be maintained by having proper regulations and approaches.39,40 Thus, willingness to data sharing among researchers can be significantly enhanced by overcoming the obstacles including having proper regulations and approaches to maintain the integrity of research.41
Most of the participants pointed out that they can agree to share data only after publication. Most likely, the reason for sharing after publication is to receive appropriate citation credit when others make benefit of their data.13 In contrast, few participants denied the idea of data sharing either before or after publication. This study proposes that scientists’ motivation to achieve credit and academic recognition can enhance their data-sharing behaviors, and we can encourage scientists’ data-sharing behaviors by better developing the reward system in scientific societies.42 However, we did not find any significant relationship between academic rank and scientists’ data-sharing strategies. One possible reason for this is that data sharing is most probably hypothesized as sharing the data of published articles, rather than the data of unpublished articles.
Current results indicated that not all participants share data equally or have the same views and concerns of data sharing. The number of publications and the academic rank showed significant differences among participants who had answered reasons for withholding their data. The most common reason for not sharing data was the lack of regulations by the respondents with less than 15 publications, while most of the participants with a higher number of publications (more than 30 publications) indicated that data should not be available to the public. This argued that scientists with a higher number of publications view data sharing as a possible loss of publication opportunities, so they are reluctant to share; claiming that data should not be available to the public. The present findings are in agreement with previous literature that the exertion (eg, time, cost, and additional work) elaborated in data sharing discourage scientists to share their data.10,43
In conclusion, this study illustrates, biomedical researchers in Jordan are not identical in their reservations towards data sharing. Helpful strategies for encouraging data sharing must consider the requirements and demands of various scientific communities.
Acknowledgments
The study was conducted as part of “The Research Ethics Education Program in Jordan” and has been supported by NIH grant number (1R25TW010026-01). The authors also thank Jordan University of Science and Technology and Yarmouk University for their support.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Bergren MD, Johnson K. Data sharing. NASN Sch Nurse. 2019;34(4):211–213. doi:10.1177/1942602X19852934
2. Tenopir C, Rice NM, Allard S, et al. Data sharing, management, use, and reuse: practices and perceptions of scientists worldwide. PLoS One. 2020;15(3):e0229003. doi:10.1371/journal.pone.0229003
3. Hughes G. Sharing research data. Emerg Med Australas. 2017;29(1):4–5. doi:10.1111/1742-6723.12722
4. Joly Y, Dyke SOM, Knoppers BM, Pastinen T. Are data sharing and privacy protection mutually exclusive? Cell. 2016;167(5):1150–1154. doi:10.1016/j.cell.2016.11.004
5. Byrd JB, Greene CS. Data-sharing models. N Engl J Med. 2017;376(23):2305.
6. Green AK, Reeder-Hayes KE, Corty RW, et al. The project data sphere initiative: accelerating cancer research by sharing data. Oncologist. 2015;20(5):464–e420. doi:10.1634/theoncologist.2014-0431
7. Sampson MG, Kang HM. Using and producing publicly available genomic data to accelerate discovery in nephrology. Nat Rev Nephrol. 2019;15(9):523–524. doi:10.1038/s41581-019-0166-z
8. Hughes DR. can you do health disparities research with publicly available datasets? Acad Radiol. 2018;25(5):552–555. doi:10.1016/j.acra.2017.06.019
9. Sardanelli F, Alì M, Hunink MG, Houssami N, Sconfienza LM, Di Leo G. To share or not to share? Expected pros and cons of data sharing in radiological research. Eur Radiol. 2018;28(6):2328–2335. doi:10.1007/s00330-017-5165-5
10. Tenopir C, Allard S, Douglass K, et al. Data sharing by scientists: practices and perceptions. PloS One. 2011;6(6):e21101.
11. Alter G, Gonzalez R. Responsible practices for data sharing. Am Psychol. 2018;73(2):146–156. doi:10.1037/amp0000258
12. Saldanha IJ, Smith BT, Ntzani E, Jap J, Balk EM, Lau J. The Systematic Review Data Repository (SRDR): descriptive characteristics of publicly available data and opportunities for research. Syst Rev. 2019;8(1):334. doi:10.1186/s13643-019-1250-y
13. Barnhart KT, Legro RS, Scott RT
14. Broes S, Lacombe D, Verlinden M, Huys I. Sharing human samples and patient data: opening pandora’s box. J Cancer Policy. 2017;13:65–69.
15. Bezuidenhout L. Data sharing and dual-use issues. Sci Eng Ethics. 2013;19(1):83–92. doi:10.1007/s11948-011-9298-7
16. She M, Li Z, Ma L. User-defined information sharing for team situation awareness and teamwork. Ergonomics. 2019;62(8):1098–1112. doi:10.1080/00140139.2019.1607910
17. Colledge F, Persson K, Elger B, Shaw D. Sample and data sharing barriers in biobanking: consent, committees, and compromises. Ann Diagn Pathol. 2014;18(2):78–81.
18. Pearce N, Smith AH. Data sharing: not as simple as it seems. Environ Health. 2011;10(1):107.
19. van Panhuis WG, Paul P, Emerson C, et al. A systematic review of barriers to data sharing in public health. BMC Public Health. 2014;14(1):1144.
20. McGuire AL, Hamilton JA, Lunstroth R, McCullough LB, Goldman A. DNA data sharing: research participants’ perspectives. Genet Med. 2008;10(1):46–53. doi:10.1097/GIM.0b013e31815f1e00
21. Ross MW, Iguchi MY. Ethical aspects of data sharing and research participant protections. Am Psychol. 2018;73(2):138–145.
22. Kalkman S, Mostert M, Gerlinger C, van Delden JJM, van Thiel G. Responsible data sharing in international health research: a systematic review of principles and norms. BMC Med Ethics. 2019;20(1):21. doi:10.1186/s12910-019-0359-9
23. Sotos JG, Huyen Y, Borrelli A. Data-sharing models. N Engl J Med. 2017;376(23):2305.
24. Abbasi K. Data sharing and opening up research. J R Soc Med. 2019;112(6):211. doi:10.1177/0141076819855176
25. Raza S, Hall A. Genomic medicine and data sharing. Br Med Bull. 2017;123(1):35–45. doi:10.1093/bmb/ldx024
26. Acharya G. Data sharing revolution: adventurism, utopia or reality? Acta Obstet Gynecol Scand. 2018;97(1):5–6. doi:10.1111/aogs.13267
27. Federer LM, Lu Y-L, Joubert DJ, Welsh J, Brandys B, Kanungo J. Biomedical data sharing and reuse: attitudes and practices of clinical and scientific research staff. PLoS One. 2015;10(6):e0129506. doi:10.1371/journal.pone.0129506
28. Mello MM, Francer JK, Wilenzick M, Teden P, Bierer BE, Barnes M. Preparing for responsible sharing of clinical trial data. Mass Med Soc. 2013.
29. Knoppers BM. The importance and challenges of data sharing. Nat Nanotechnol. 2020;15(2):83. doi:10.1038/s41565-020-0646-0
30. Pereira SJ. Motivations and barriers to sharing biological samples: a case study. J Pers Med. 2013;3(2):102–110.
31. Kayaalp M. Patient privacy in the era of big data. Balkan Med J. 2018;35(1):8–17. doi:10.4274/balkanmedj.2017.0966
32. Keerie C, Tuck C, Milne G, Eldridge S, Wright N, Lewis SC. Data sharing in clinical trials - practical guidance on anonymising trial datasets. Trials. 2018;19(1):25. doi:10.1186/s13063-017-2382-9
33. Ohno-Machado L, Bafna V, Boxwala AA, et al. iDASH: integrating data for analysis, anonymization, and sharing. J Am Med Inform Assoc. 2012;19(2):196–201. doi:10.1136/amiajnl-2011-000538
34. Karampela M, Ouhbi S. Connected health user willingness to share personal health data: questionnaire study. J Med Internet Res. 2019;21(11):e14537.
35. Khabour OF, Alomari MA, Al-Sheyab NA. Parental perceptions about informed consent/assent in pediatric research in Jordan. J Empir Res Hum Res Ethics. 2017;12(4):261–268. doi:10.1177/1556264617718937
36. Rababa’h AM, Alzoubi KH, Ababneh M, Khabour OF. Awareness of jordanian investigators about the importance of ethics review committees: a Pilot Study. Sci Eng Ethics. 2020;26(2):821–831. doi:10.1007/s11948-019-00139-7
37. Orsini LS, Berger M, Crown W, et al. Improving transparency to build trust in real-world secondary data studies for hypothesis testing-why, what, and how: recommendations and a road map from the real-world evidence transparency initiative. Value Health. 2020;23(9):1128–1136. doi:10.1016/j.jval.2020.04.002
38. Min S, Martin LT, Rutter CM, Concannon TW. Are publicly funded health databases geographically detailed and timely enough to support patient-centered outcomes research? J Gen Intern Med. 2019;34(3):467–472. doi:10.1007/s11606-018-4673-6
39. Scheel H, Dathe H, Franke T, Scharfe T, Rottmann T. A privacy preserving approach to feasibility analyses on distributed data sources in biomedical research. Stud Health Technol Inform. 2019;267:254–261.
40. Denny SG, Silaigwana B, Wassenaar D, Bull S, Parker M. Developing ethical practices for public health research data sharing in South Africa: the views and experiences from a diverse sample of research stakeholders. J Empir Res Hum Res Ethics. 2015;10(3):290–301. doi:10.1177/1556264615592386
41. Parker M, Bull S. Sharing public health research data: toward the development of ethical data-sharing practice in Low- and Middle-Income Settings. J Empir Res Hum Res Ethics. 2015;10(3):217–224. doi:10.1177/1556264615593494
42. Levesque RJR. Data sharing mandates, developmental science, and responsibly supporting authors. J Youth Adolesc. 2017;46(12):2401–2406. doi:10.1007/s10964-017-0741-1
43. Foster N, Nancy F, Gibbons S. Understanding faculty to improve content recruitment for institutional repositories. Dlib Mag. 2005;11. doi:10.1045/january2005-foster
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
