Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Bioinformatics and Systems Biology Approach to Identify the Pathogenetic Link Between Psoriasis and Cardiovascular Disease
Authors Shi L , Du X, Li J, Zhang G
Received 2 June 2023
Accepted for publication 11 August 2023
Published 22 August 2023 Volume 2023:16 Pages 2283—2295
DOI https://doi.org/10.2147/CCID.S421193
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Jeffrey Weinberg
Liping Shi,1,2 Xiaoqing Du,3 Jing Li,1,2 Guoqiang Zhang1,2
1Department of Dermatology, The First Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 2Candidate Branch of National Clinical Research Center for Skin Diseases, Shijiazhuang, People’s Republic of China; 3Department of Dermatology, Bethune International Peace Hospital, Shijiazhuang, People’s Republic of China
Correspondence: Guoqiang Zhang, The First Hospital of Hebei Medical University, 89 Donggang Road, Yuhua District, Shijiazhuang City, Hebei Province, People’s Republic of China, Tel +8618633888122, Email [email protected]
Objective: This study aimed to identify hub genes and common pathways shared between psoriasis and cardiovascular disease (CVD) using bioinformatics analysis and predict the transcription factors (TFs) of hub genes.
Methods: GSE133555 data from the Gene Expression Omnibus (GEO) database were used to identify differentially expressed genes (DEGs) between involved and uninvolved skin lesions in psoriasis, employing the limma package in R. Additionally, CVD-related genes were obtained from the GeneCards database. The intersection of DEGs and CVD-related genes yielded CVD-DEGs. Gene Ontology and signaling pathway analyses were performed using the clusterProfiler package in R. Hub genes were identified by intersecting six algorithms in the CytoHubba plugin of Cytoscape. To identify potential biomarkers, the GSE14905 dataset was subjected to receiver operating characteristic analysis, resulting in the identification of eight central hub genes. Finally, the NetworkAnalyst web tool was used to identify the TFs of the eight hub genes.
Results: We identified 92 significant DEGs out of 1825 CVD-related genes in psoriasis obtained from the GSE13355 and GeneCard data. Functional enrichment analysis revealed the involvement of these genes in various signaling pathways, including the interleukin-17 signaling, tumor necrosis factor signaling, lipid and atherosclerosis, chemokine signaling, and cytokine signaling pathways in the immune system. The eight hub genes identified included interleukin-1 beta, C-X-C motif chemokine ligand 8, signal transducer and activator of transcription 3, C-C motif chemokine ligand 2, arginase 1, C-X-C motif chemokine receptor 4, cyclin D1, and matrix metallopeptidase 9, with forkhead box C1 also identified as an associated TF of these genes. These hub genes and TF may act as key regulators in the context of CVD.
Conclusion: This study identified several hub genes and signaling pathways associated with both CVD and psoriasis. These findings lay the groundwork for potential therapeutic interventions for patients with psoriasis affected by CVD.
Keywords: psoriasis, cardiovascular disease, signaling pathway, gene expression omnibus
Introduction
Psoriasis is a chronic inflammatory immune-mediated disease with a 3% worldwide prevalence. It is associated with various comorbidities, including cardiometabolic diseases, gastrointestinal tract issues, kidney disease, malignant tumors, infections, and mood disorders.1 Owing to its high prevalence, recurrence, tendency to cause disfigurement, and numerous comorbidities, psoriasis poses substantial challenges to clinicians, with the World Health Organization recognizing it as a major global health issue.2 The visible skin manifestations and recurrent episodes of psoriasis can also lead to depression and suicidal tendencies, imposing marked physiological, psychological, and economic burdens on patients.
Cardiovascular disease (CVD), also known as circulatory system disease, primarily affects the heart and blood vessels, encompassing conditions such as coronary heart disease, stroke, heart failure, myocardial infarction, and arrhythmia. CVD shares common risk factors with psoriasis, including smoking, excessive alcohol consumption, metabolic syndrome, hypertension, dyslipidemia, abdominal obesity, and insulin resistance.3–6 For instance, psoriasis combined with hyperlipidemia may result in increased tumor necrosis factor (TNF)-α levels, leading to elevated proatherogenic oxidized low-density lipoprotein and decreased high-density lipoprotein concentrations.7 Psoriatic individuals face approximately a 50% higher risk of cardiovascular events compared with the general population,8 with greater risks observed in younger patients, severe cases, and those with longer disease duration.9–11 A meta-analysis including 66,509 patients with psoriasis and 1,790,757 patients without psoriasis confirmed that psoriasis, especially severe cases, can exacerbate adverse cardiovascular outcomes, indicating that psoriasis itself is an independent risk factor for such outcomes.12
Despite numerous studies confirming the association between psoriasis and CVD, their shared etiology and pathogenesis remain unclear. Therefore, there is an urgent need to elucidate the common molecular networks involved in both psoriasis and CVD to enable physicians to develop targeted interventions.
Methods
Microarray Data
The gene expression profiles of GSE13355 and GSE14905 were obtained from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/). This study included fifty-eight psoriasis lesional skin (LS) samples and fifty-eight nonlesional skin (NL) samples from GSE13355. For receiver operating characteristic (ROC) analysis, thirty-three LS and twenty-eight NL samples from GSE14905 were used.
Screening of Differentially Expressed Genes and CVD-Related Differentially Expressed Genes
Differentially expressed genes (DEGs) between psoriasis lesional and normal skin were identified using the limma package in R, with criteria set as follows: adjusted P < 0.05 and |Log2fold change (Log FC)| > 1. Volcano and principal component analysis maps of DEGs were generated using the ggplot package in R. Subsequently, CVD-related genes were obtained from the GeneCards database (http://www.genecards.org) using the retrieval strategy “cardiovascular diseases” OR “Cardiovascular Disease” OR “Disease, Cardiovascular” OR “Diseases, Cardiovascular”. The categories of genes encoding proteins and genes with a relevance score >1 were selected for further analysis. The intersection of these genes with the genes from GSE13355 was considered to represent CVD-related genes in psoriasis, where the DEGs were considered CVD-DEGs.
Gene Set Enrichment Analysis
To gain insights into the biological mechanisms associated with psoriasis and CVD, gene set enrichment analysis (GSEA) was performed using the clusterProfiler package in R. A false discovery rate of <0.25 and an adjusted P value of <0.05 were used as the cutoff criteria to identify candidate molecular pathways of CVD-related genes in psoriasis.
Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Analyses
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional enrichment analysis of CVD-DEGs was conducted using the clusterProfiler package in R, and the results were visualized using the ggplot2 package in R. The significance cutoff value for enrichment analysis was set as adjusted P < 0.05.
Construction of a Protein–Protein Interaction Network
The online STRING database (http://string-db.org) was used to construct a protein–protein interaction (PPI) network, and interactions with a combined score > 0.4 were considered statistically significant. Cytoscape (version 3.9.1) was used to visualize the PPI network, and the most closely connected modules were selected using the Molecular Complex Detection (MCODE) plugin with the following parameters: degree cutoff = 2; node score = 0.2; k-core = 2; and maximum depth = 100. Subsequently, KEGG enrichment analysis was conducted on modules with a score > 3. The CytoHubba plugin in Cytoscape was used to identify the top 20 node genes in 6 different ways, and the intersection of these genes was selected as the hub genes.
ROC Curve Analysis
To assess the specificity and sensitivity of hub genes for diagnosing psoriasis, ROC curve analysis was performed using the pROC package in R. The results were visualized using the ggplot2 package in R.
Transcription Factor–Gene Interactions
To gain further insights into the regulation of hub genes, we identified the transcription factors (TFs) of these genes and constructed a network map using NetworkAnalyst (https://www.networkanalyst.ca) with the JASPAR database.
Results
Screening of Candidate Genes
From the analysis of GSE13355, 21,655 genes were identified, including 542 DEGs. Furthermore, 1944 genes related to CVD were screened from the GeneCards database, with 1825 genes matching the GSE13355 expression profile and 92 genes overlapping with the DEGs. The principal component analysis map and volcano map of GSE13355 are presented in Figure 1A and B. Additionally, the Venn diagram and heat map of the top 20 upregulated and downregulated CVD-DEGs are shown in Figure 1C and D.
GSEA
Among the 1825 CVD genes in psoriasis, the results indicated that a majority were significantly enriched in pathways such as signaling by interleukins (ILs), cytokine signaling in the immune system, and the chemokine signaling pathway (Figure 2A).
 |
Figure 2 (A) GSEA of 1825 CVD-genes in psoriasis. (B) GO enrichment map of CVD-DEGs. (C) TOP15 KEGG pathway enrichment map of CVD-DEGs. |
GO and KEGG Analyses
GO functional enrichment analysis of CVD-DEGs revealed the top five biological processes in patients with both psoriasis and CVD: cytokine-mediated signaling pathway, leukocyte migration, response to lipopolysaccharide, response to molecule of bacterial origin, and cell chemotaxis. The top five GO molecular function terms were cytokine activity, receptor ligand activity, cytokine receptor binding, signaling receptor activator activity, and IL-1 receptor binding. The top five GO cell component terms were external side of the plasma membrane, secretory granule lumen, cytoplasmic vesicle lumen, vesicle lumen, and collagen-containing extracellular matrix (Figure 2B).
KEGG pathway analysis revealed the top 5 enriched pathways to be the IL-17 signaling pathway, lipid and atherosclerosis, viral protein interaction with cytokine and cytokine receptor, AGE-RAGE signaling pathway in diabetic complications, and cytokine–cytokine receptor interaction (Figure 2C).
PPI Network Analysis
The constructed PPI network consisted of 92 CVD-DEGs, with 84 nodes and 462 edges (Figure 3). Using the MCODE plugin, two critical modules with scores > 5 were identified from the PPI network. According to KEGG analysis, the first module was primarily enriched in pathways such as coronavirus disease-COVID-19, lipid and atherosclerosis, IL-17 signaling pathway, TNF signaling pathway, and prolactin signaling pathway. The second module was enriched in pathways such as chemokine signaling pathway, cytokine–cytokine receptor interaction, viral protein interaction with cytokine and cytokine receptor, and Toll-like receptor signaling pathway (Figure 4A–D).
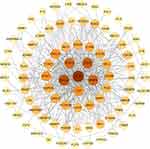 |
Figure 3 PPI network diagram of CVD-DEGs. The darker the red color, the higher the degree of nodes. |
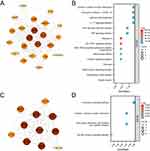 |
Figure 4 (A) PPI Network diagram of module 1. (B) The bubble plot of module 1 KEGG analysis. (C) PPI Network diagram of module 2. (D) The bubble plot of module 2 KEGG analysis. |
The top 20 hub genes were determined using six algorithms via CytoHubba. By taking the intersection, nine common hub genes were identified: IL-1 beta (IL1B), C-X-C motif chemokine ligand 8 (CXCL8), signal transducer and activator of transcription 3 (STAT3), C-C motif chemokine ligand 2 (CCL2), matrix metallopeptidase 9 (MMP9), arginase 1 (ARG1), C-X-C motif chemokine receptor 4 (CXCR4), cyclin D1 (CCND1), and leptin (LEP) (Table 1).
 |
Table 1 The Top 20 Hub Genes Rank in CytoHubba |
Assessment of Hub Gene Diagnostic Accuracy
ROC curve analyses were conducted to assess the diagnostic accuracy of the nine hub genes. The area under the curve (AUC) values for the ROC curves of IL1B, CXCL8, STAT3, CCL2, MMP9, ARG1, CXCR4, CCND1, and LEP were 0.989, 0.969, 0.925, 0.947, 0.782, 0.957, 0.949, 0.989, and 0.921 for GSE13355, respectively, and 0.973, 0.962, 0.891, 0.960, 0.496, 0.862, 0.871, 0.846, and 0.816 for GSE14905, respectively (Figure 5A and B). Except for LEP, the AUC values of these genes were >0.7, indicating that eight hub genes have diagnostic significance.
 |
Figure 5 (A) ROC curve analysis of 9 common hub genes in GSE13355. (B) ROC curve analysis of 9 common hub genes in GSE14905. |
TF–Gene Pairs
One TF, forkhead box C1 (FOXC1), exhibited a degree ≥5, and a TF–gene network diagram was constructed (Figure 6). FOXC1 had a degree of 5 and a betweenness of 232.85.
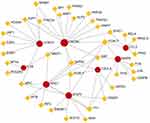 |
Figure 6 TFs-genes network diagram. The red circle represents the hub genes, and the yellow quadrilateral represents TFs. |
Discussion
Psoriasis is a systemic inflammatory disease involving both the adaptive and innate immune systems in its pathogenesis. Patients with psoriasis are at an increased risk of developing CVD. The presence of cardiovascular risk factors and metabolic abnormalities can further exacerbate the cardiovascular burden in patients with psoriasis, potentially contributing to cardiovascular events. Moreover, psoriasis and CVD share not only common risk factors but also key pathways and systemic inflammation.13–15 For instance, atherosclerosis, a CVD recognized as a chronic immune-mediated inflammatory disease characterized by endothelial dysfunction, lipid deposition in arterial walls, and infiltration of monocyte-derived macrophages,16 shares similar immune-inflammatory mechanisms with psoriasis, both involving type 17 activation and decreased T-regulatory cell function.17,18 In recent years, the therapeutic efficacy of various biological agents for psoriasis and CVD have shown promising results. Biological agents have demonstrated superior efficacy in resolving psoriatic lesions and improving Psoriasis Area and Severity Index (PASI) scores compared with traditional therapies. Furthermore, some studies have indicated that biological therapies can improve cardiovascular function by affecting, for example, flow-mediated dilation (an endothelial function marker)19 and the carotid plaque burden.20,21 However, despite these advancements, psoriasis remains incurable, and biological agents may be cost-prohibitive for some patients as well as carrying a risk of infection. Therefore, there is a pressing need for development of highly effective and specific treatments for psoriasis and CVD.
In the present study, we identified 1825 overlapping genes associated with both psoriasis and CVD, including 92 CVD-DEGs. We conducted bioinformatics analyses on these genes, with GO analysis results revealing that they mostly function on the external side of the plasma membrane and within secretory granule lumens. The associated proteins were mainly involved in cytokine-mediated signaling pathways and cell chemotaxis. Importantly, aberrant cytokine expression plays a crucial role in the development of psoriasis.22 KEGG analysis and GSEA revealed that the genes were primarily enriched in pathways such as the IL-17 signaling, TNF signaling, lipid and atherosclerosis, chemokine signaling, and cytokine signaling pathways in the immune system. In psoriatic lesions, activated plasmacytoid dendritic cells contribute to the production of IL-12, TNF-α, and IL-23, along with the maturation of myeloid dendritic cells. This leads to the activation of Th1 and Th17 cells, which subsequently secrete various cytokines, including IL-17, IL-21, IL-22, and TNF-α.23 These cytokines then activate keratinocytes to produce peptides, cytokines, and chemokines, further promoting inflammation. The chemokine signaling pathway and cytokine–cytokine receptor interaction pathway, including the IL-23–IL-17 immune axis, as well as the TNF pathway, play pivotal roles in the pathogenesis of psoriasis.24 Immune mediators involved in IL-23 and IL-17 signaling trigger hyperproliferation and abnormal differentiation of epidermal keratinocytes, leading to psoriatic skin lesions.25 Targeted interventions against these cytokines have shown excellent efficacy during psoriasis treatment. IL-17 may induce vascular inflammation and stiffness by triggering oxidative stress, as well cardiac and renal fibrosis, potentially leading to hypertension.26,27 TNF-α can degrade endothelial nitric oxide synthase mRNA, alter vascular function, and induce oxidative stress, resulting in an imbalance between microvascular dilation and contraction,28–30 thereby increasing the risk of hypertension and coronary heart disease.31 Additionally psoriasis is associated with type 2 diabetes,32 and TNF-α, IL-23/IL-17, and adipokines can influence the signaling pathway between insulin receptors, cytokines, and adipokines, affecting insulin sensitivity regulation.33,34
Based on our PPI network analysis, we identified nine common genes by taking the intersection of six algorithms. ROC verification in both GSE13355 and GSE14905 datasets demonstrated that the AUC for all genes, except LEP, was >0.7. As a result, we identified eight hub genes: CXCL8, MMP9, IL-1B, ARG1, CCND1, STAT3, CXCR4, and CCL2.
Activated keratinocytes in psoriatic patients can recruit neutrophils to the inflamed epidermis by producing CXCL8.35 Additionally, activated keratinocytes produce CCL2, which is crucial for keratinocyte proliferation, a hallmark of psoriasis.36 CXCL8 and CCL2 concentrations are increased in cardiovascular patients,37 suggesting their involvement in CVD. CXCL8 also plays an important role in hypertension and atherosclerosis by increasing endothelial cell proliferation, reducing apoptosis,38 and stimulating leukocyte migration to subendothelial vessels.39 Elevated CCL2 expression and synthesis are observed in various cardiovascular conditions,40,41 exacerbating disease progression through leukocyte migration to sites of inflammation.42 Matrix metalloproteinases (MMPs) are associated with loss of cell-to-cell interactions and increased capillary permeability, both essential features in psoriasis development.43,44 MMP9, in particular, serves as a marker of psoriasis, even in patients without active skin lesions.45 Although MMPs generally play a vital role in CVD,46 MMP9 is closely related to vascular lesions,47,48 including acute myocardial infarction, atherosclerosis, heart failure, and aortic aneurysm.49,50 Elevated serum MMP9 levels are an independent risk factor for increased CVD risk.51 IL-1B, abundant in the tissue fluid of patients with psoriasis, plays a dual role in the disease’s development,52 impacting insulin resistance and contributing to keratinocyte proliferation. IL-1B is also associated with CVD,53 with its levels being elevated in coronary atherosclerotic disease, influencing disease severity and outcome.54,55 IL-1B inhibitors can significantly reduce the risk of recurrent cardiovascular events, confirming its crucial role in atherosclerotic disease.56 ARG1 is involved in the regulation of Th17 cell proliferation and the pathogenesis of psoriasis,57,58 and its overexpression contributes to the hyperproliferation of psoriatic keratinocytes.59 ARG1 also plays a key role in vascular pathology and ischemic heart disease,60 and ARG1-expressing macrophages are associated with the of repair of cardiovascular tissue damage.61 Dysregulation of apoptosis contributes to the pathogenesis of psoriasis, and CCND1, an apoptosis inhibitor, regulates mitochondrial apoptosis by arresting the cell cycle in response to intracellular DNA damage. CCND1 is an independent risk factor for the development of metabolic syndrome in patients with psoriasis.62 Stroke is a leading cause of death associated with CVD, and ischemic stress can increase CCND1 levels in mature neurons, with the gene CAMTA1 known to regulate CCND1 expression levels and affect ischemic reperfusion injury during stroke.63 STAT3 is involved in cell survival, differentiation, proliferation, angiogenesis, and immune activation, playing a vital role in the pathogenesis and development of psoriasis.64,65 STAT3 activation is high in keratinocytes of human psoriasis lesions and aggravates psoriasis by promoting the proliferation, differentiation, and cytokine production of T cells. In patients with cardiovascular events, STAT3 activation contributes to vasculopathy.66 CXCR4, a receptor of the angiogenic chemokine stromal cell–derived factor-1, is involved in skin inflammation and inflammatory angiogenesis in psoriasis,67 playing a role in thrombopoiesis in CVD.68
Our TF–gene network revealed that FOXC1 plays a vital role in psoriasis and CVD. FOXC1 levels are known to be negatively correlated with KRT6B and KRT16, markers of hyperproliferation of abnormal keratinocytes.69,70 Moreover, inhibition of FOXC1 expression and activity contributes to the development of psoriasis.71 FOXC1 is also important in promoting angiogenesis, as shown during mouse embryonic development, and its deficiency leads to cardiovascular defects in mouse embryos.72,73
This study has some limitations. For example, information on smoking, drinking, and other patient factors was not extracted from the GEO database, preventing analysis of their potential influence on gene and protein expression. Additionally, rigorous experimental validation is required to confirm our preliminary findings.
Conclusion
In this study, we successfully identified common genes shared between psoriasis and CVD, namely IL-1B, CXCL8, STAT3, CCL2, ARG1, CXCR4, CCND1, and MMP9. These genes hold potential as therapeutic targets for treating psoriasis with CVD. The pathogenesis of psoriasis with CVD was found to be closely associated with several pathways, including the IL-17 signaling, TNF signaling, lipid and atherosclerosis, chemokine signaling, and cytokine signaling pathways in the immune system. By providing insights into the mechanisms underlying the emergence of psoriasis with CVD, our study identifies probable target genes for the treatment of this complex condition.
Abbreviations
GEO, Gene expression omnibus; DEGs, differentially expressed genes; CVD, cardiovascular disease; GSEA, Gene Set Enrichment Analysis; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; PPI, protein–protein interaction; ROC, Receiver Operating Characteristic; TF, transcriptional factor; TNF, tumor necrosis factor; LS, lesional skin; NL, non-lesional; Log FC, Log2fold change; FDR, false discovery rate; ILs, interleukins; MCODE, Molecular Complex Detection; IL1B, IL-1 beta; CXCL8, C-X-C motif chemokine ligand 8; STAT3, signal transducer and activator of transcription 3; CCL2, C-C motif chemokine ligand 2; MMP9, matrix metallopeptidase 9; ARG1, arginase 1; CXCR4, C-X-C motif chemokine receptor 4; CCND1, cyclin D1; LEP, leptin; FOXC1, forkhead box C1.
Ethics Approval
All procedures performed in this study involving human participants was approved by the Ethics Committee of the First Hospital of Hebei Medical University (20230601).
Consent for Publication
All of the authors have agreed to the publication of the article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work is supported by Medical science research project of Hebei Province Health Commission (20190470 and 20231294).
Disclosure
All authors report no conflicts of interest in this work.
References
1. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76(3):377–390. doi:10.1016/j.jaad.2016.07.064
2. Lai YC, Yew YW. Psoriasis as an independent risk factor for cardiovascular disease: an epidemiologic analysis using a national database. J Cutan Med Surg. 2016;20(4):327–333. doi:10.1177/1203475415602842
3. Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125(1):61–67. doi:10.1111/j.0022-202X.2005.23681.x
4. Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298(7):321–328. doi:10.1007/s00403-006-0703-z
5. Gisondi P, Tessari G, Conti A, et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case-control study. Br J Dermatol. 2007;157(1):68–73. doi:10.1111/j.1365-2133.2007.07986.x
6. Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18(10):2211. doi:10.3390/ijms18102211
7. Shih CM, Chen CC, Chu CK, Wang KH, Huang CY, Lee AW. The roles of lipoprotein in psoriasis. Int J Mol Sci. 2020;21(3):859. doi:10.3390/ijms21030859
8. Egeberg A, Thyssen JP, Jensen P, Gislason GH, Skov L. Risk of myocardial infarction in patients with psoriasis and psoriatic arthritis: a nationwide cohort study. Acta Derm Venereol. 2017;97(7):819–824. doi:10.2340/00015555-2657
9. Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. doi:10.1001/jama.296.14.1735
10. Armstrong AW, Harskamp CT, Ledo L, Rogers JH, Armstrong EJ. Coronary artery disease in patients with psoriasis referred for coronary angiography. Am J Cardiol. 2012;109(7):976–980. doi:10.1016/j.amjcard.2011.11.025
11. Li WQ, Han JL, Manson JE, et al. Psoriasis and risk of nonfatal cardiovascular disease in U.S. women: a cohort study. Br J Dermatol. 2012;166(4):811–818. doi:10.1111/j.1365-2133.2011.10774.x
12. Liu L, Cui S, Liu M, Huo X, Zhang G, Wang N. Psoriasis increased the risk of adverse cardiovascular outcomes: a new systematic review and meta-analysis of cohort study. Front Cardiovasc Med. 2022;9:829709. doi:10.3389/fcvm.2022.829709
13. Sajja AP, Joshi AA, Teague HL, Dey AK, Mehta NN. Potential immunological links between psoriasis and cardiovascular disease. Front Immunol. 2018;9:1234. doi:10.3389/fimmu.2018.01234
14. Lockshin B, Balagula Y, Merola JF. Interleukin 17, inflammation, and cardiovascular risk in patients with psoriasis. J Am Acad Dermatol. 2018;79(2):345–352. doi:10.1016/j.jaad.2018.02.040
15. von Stebut E, Boehncke WH, Ghoreschi K, et al. IL-17A in psoriasis and beyond: cardiovascular and metabolic implications. Front Immunol. 2019;10:3096. doi:10.3389/fimmu.2019.03096
16. Puig L. Cardiometabolic comorbidities in psoriasis and psoriatic arthritis. Int J Mol Sci. 2017;19(1):58. doi:10.3390/ijms19010058
17. Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270(2):147–157. doi:10.1111/j.1365-2796.2010.02310.x
18. Alexandroff AB, Pauriah M, Camp RD, Lang CC, Struthers AD, Armstrong DJ. More than skin deep: atherosclerosis as a systemic manifestation of psoriasis. Br J Dermatol. 2009;161(1):1–7. doi:10.1111/j.1365-2133.2009.09281.x
19. von Stebut E, Reich K, Thaçi D, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Invest Dermatol. 2019;139(5):1054–1062. doi:10.1016/j.jid.2018.10.042
20. Elnabawi YA, Dey AK, Goyal A, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. 2019;115(4):721–728. doi:10.1093/cvr/cvz009
21. Eder L, Joshi AA, Dey AK, et al. Association of tumor necrosis factor inhibitor treatment with reduced indices of subclinical atherosclerosis in patients with psoriatic disease. Arthritis Rheumatol. 2018;70(3):408–416. doi:10.1002/art.40366
22. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. doi:10.1056/NEJMra0804595
23. Yamanaka K, Yamamoto O, Honda T. Pathophysiology of psoriasis: a review. J Dermatol. 2021;48(6):722–731. doi:10.1111/1346-8138.15913
24. Kim J, Krueger JG. The immunopathogenesis of psoriasis. Dermatol Clin. 2015;33(1):13–23. doi:10.1016/j.det.2014.09.002
25. Ghoreschi K, Balato A, Enerbäck C, Sabat R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. 2021;397(10275):754–766. doi:10.1016/S0140-6736(21)00184-7
26. Wu J, Saleh MA, Kirabo A, et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2016;126(1):50–67. doi:10.1172/JCI80761
27. Huang LH, Zinselmeyer BH, Chang CH, et al. Interleukin-17 drives interstitial entrapment of tissue lipoproteins in experimental psoriasis. Cell Metab. 2019;29(2):475–487.e477. doi:10.1016/j.cmet.2018.10.006
28. Chen M, Ma L, Hall JE, Liu X, Ying Z. Dual regulation of tumor necrosis factor-α on myosin light chain phosphorylation in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2015;308(5):H398–H406. doi:10.1152/ajpheart.00691.2014
29. Yoshizumi M, Perrella MA, Burnett JC, Lee ME. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res. 1993;73(1):205–209. doi:10.1161/01.RES.73.1.205
30. Zhang H, Park Y, Wu J, et al. Role of TNF-alpha in vascular dysfunction. Clin Sci. 2009;116(3):219–230. doi:10.1042/CS20080196
31. Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25–30. doi:10.1016/j.atherosclerosis.2016.05.036
32. Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149(1):84–91. doi:10.1001/2013.jamadermatol.406
33. Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130(7):1785–1796. doi:10.1038/jid.2010.103
34. Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13(6):465–476. doi:10.1038/nrd4275
35. Albanesi C, Scarponi C, Giustizieri ML, Girolomoni G. Keratinocytes in inflammatory skin diseases. Curr Drug Targets Inflamm Allergy. 2005;4(3):329–334. doi:10.2174/1568010054022033
36. Gillitzer R, Wolff K, Tong D, et al. MCP-1 mRNA expression in basal keratinocytes of psoriatic lesions. J Invest Dermatol. 1993;101(2):127–131. doi:10.1111/1523-1747.ep12363613
37. Hedayati-Moghadam M, Hosseinian S, Paseban M, et al. The role of chemokines in cardiovascular diseases and the therapeutic effect of curcumin on CXCL8 and CCL2 as pathological chemokines in atherosclerosis. Adv Exp Med Biol. 2021;1328:155–170.
38. Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170(6):3369–3376. doi:10.4049/jimmunol.170.6.3369
39. Boekholdt SM, Peters RJ, Hack CE, et al. IL-8 plasma concentrations and the risk of future coronary artery disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2004;24(8):1503–1508. doi:10.1161/01.ATV.0000134294.54422.2e
40. Martynowicz H, Janus A, Nowacki D, Mazur G. The role of chemokines in hypertension. Adv Clin Exp Med. 2014;23(3):319–325. doi:10.17219/acem/37123
41. Ganjali S, Sahebkar A, Mahdipour E, et al. Investigation of the effects of curcumin on serum cytokines in obese individuals: a randomized controlled trial. ScientificWorldJournal. 2014;2014:898361. doi:10.1155/2014/898361
42. Aukrust P, Berge RK, Ueland T, et al. Interaction between chemokines and oxidative stress: possible pathogenic role in acute coronary syndromes. J Am Coll Cardiol. 2001;37(2):485–491. doi:10.1016/S0735-1097(00)01110-4
43. Jaiswal AK, Sadasivam M, Archer NK, et al. Syndecan-1 regulates psoriasiform dermatitis by controlling homeostasis of IL-17-producing γδ T cells. J Immunol. 2018;201(6):1651–1661. doi:10.4049/jimmunol.1800104
44. Starodubtseva NL, Sobolev VV, Soboleva AG, Nikolaev AA, Bruskin SA. [Expression of genes for metalloproteinases (MMP-1, MMP-2, MMP-9, and MMP-12) associated with psoriasis]. Genetika. 2011;47(9):1254–1261. Russian.
45. Wagner M, Theodoro TR, Filho C, Oyafuso LKM, Pinhal MAS. Extracellular matrix alterations in the skin of patients affected by psoriasis. BMC Mol Cell Biol. 2021;22(1):55. doi:10.1186/s12860-021-00395-1
46. Hopps E, Caimi G. Matrix metalloproteases as a pharmacological target in cardiovascular diseases. Eur Rev Med Pharmacol Sci. 2015;19(14):2583–2589.
47. Mirhafez SR, Avan A, Tajfard M, et al. Relationship between serum cytokines receptors and matrix metalloproteinase 9 levels and coronary artery disease. J Clin Lab Anal. 2017;31(5):e22100. doi:10.1002/jcla.22100
48. El-Aziz TAA, Mohamed RH. Matrix metalloproteinase −9 polymorphism and outcome after acute myocardial infarction. Int J Cardiol. 2017;227:524–528. doi:10.1016/j.ijcard.2016.10.109
49. Briasoulis A, Tousoulis D, Papageorgiou N, et al. Novel therapeutic approaches targeting matrix metalloproteinases in cardiovascular disease. Curr Top Med Chem. 2012;12(10):1214–1221. doi:10.2174/1568026611208011214
50. Cai H, Ma Y, Jiang L, et al. Hypoxia response element-regulated MMP-9 promotes neurological recovery via glial scar degradation and angiogenesis in delayed stroke. Mol Ther. 2017;25(6):1448–1459. doi:10.1016/j.ymthe.2017.03.020
51. Rathnayake N, Gustafsson A, Norhammar A, et al. Salivary matrix metalloproteinase-8 and -9 and myeloperoxidase in relation to coronary heart and periodontal diseases: a subgroup report from the PAROKRANK Study (Periodontitis and its relation to coronary artery disease). PLoS One. 2015;10(7):e0126370. doi:10.1371/journal.pone.0126370
52. Buerger C, Richter B, Woth K, et al. Interleukin-1β interferes with epidermal homeostasis through induction of insulin resistance: implications for psoriasis pathogenesis. J Invest Dermatol. 2012;132(9):2206–2214. doi:10.1038/jid.2012.123
53. Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40(1):91–104. doi:10.1016/j.immuni.2013.11.019
54. Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci. 2018;132(12):1243–1252. doi:10.1042/CS20180306
55. Zhu Y, Xian X, Wang Z, et al. Research progress on the relationship between atherosclerosis and inflammation. Biomolecules. 2018;8(3):80. doi:10.3390/biom8030080
56. Ain QU, Sarfraz M, Prasesti GK, Dewi TI, Kurniati NF. Confounders in identification and analysis of inflammatory biomarkers in cardiovascular diseases. Biomolecules. 2021;11(10):1464. doi:10.3390/biom11101464
57. Deng J, Tan S, Liu R, et al. Chinese medicine formula PSORI-CM02 alleviates psoriatic dermatitis via M-MDSCs and Th17 crosstalk. Front Pharmacol. 2020;11:563433. doi:10.3389/fphar.2020.563433
58. Ayala-Fontánez N, Soler DC, McCormick TS. Current knowledge on psoriasis and autoimmune diseases. Psoriasis. 2016;6:7–32. doi:10.2147/PTT.S64950
59. Bruch-Gerharz D, Schnorr O, Suschek C, et al. Arginase 1 overexpression in psoriasis: limitation of inducible nitric oxide synthase activity as a molecular mechanism for keratinocyte hyperproliferation. Am J Pathol. 2003;162(1):203–211. doi:10.1016/S0002-9440(10)63811-4
60. Zhu M, Goetsch SC, Wang Z, et al. FoxO4 promotes early inflammatory response upon myocardial infarction via endothelial Arg1. Circ Res. 2015;117(11):967–977. doi:10.1161/CIRCRESAHA.115.306919
61. Shiraishi M, Shintani Y, Shintani Y, et al. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest. 2016;126(6):2151–2166. doi:10.1172/JCI85782
62. Korkmaz S, Korkmaz H. Effect of alterations in apoptotic pathway on development of metabolic syndrome in patients with psoriasis vulgaris. Br J Dermatol. 2017;176(6):1549–1557. doi:10.1111/bjd.15185
63. Liu Y, Shang G, Zhang X, et al. CAMTA1 gene affects the ischemia-reperfusion injury by regulating CCND1. Front Cell Neurosci. 2022;16:868291. doi:10.3389/fncel.2022.868291
64. Calautti E, Avalle L, Poli V. Psoriasis: a STAT3-centric view. Int J Mol Sci. 2018;19(1):171. doi:10.3390/ijms19010171
65. Wang A, Wei J, Lu C, et al. Genistein suppresses psoriasis-related inflammation through a STAT3-NF-κB-dependent mechanism in keratinocytes. Int Immunopharmacol. 2019;69:270–278. doi:10.1016/j.intimp.2019.01.054
66. Dutzmann J, Daniel JM, Bauersachs J, Hilfiker-Kleiner D, Sedding DG. Emerging translational approaches to target STAT3 signalling and its impact on vascular disease. Cardiovasc Res. 2015;106(3):365–374. doi:10.1093/cvr/cvv103
67. Zgraggen S, Huggenberger R, Kerl K, Detmar M, Ribatti D. An important role of the SDF-1/CXCR4 axis in chronic skin inflammation. PLoS One. 2014;9(4):e93665. doi:10.1371/journal.pone.0093665
68. Petzold T, Zhang Z, Ballesteros I, et al. Neutrophil “plucking” on megakaryocytes drives platelet production and boosts cardiovascular disease. Immunity. 2022;55(12):2285–2299.e2287. doi:10.1016/j.immuni.2022.10.001
69. Rubin AJ, Barajas BC, Furlan-Magaril M, et al. Lineage-specific dynamic and pre-established enhancer-promoter contacts cooperate in terminal differentiation. Nat Genet. 2017;49(10):1522–1528. doi:10.1038/ng.3935
70. Bin L, Deng L, Yang H, et al. Forkhead box C1 regulates human primary keratinocyte terminal differentiation. PLoS One. 2016;11(12):e0167392. doi:10.1371/journal.pone.0167392
71. Zeng F, Liu H, Lu D, Liu Q, Chen H, Zheng F. Integrated analysis of gene expression profiles identifies transcription factors potentially involved in psoriasis pathogenesis. J Cell Biochem. 2019;120(8):12582–12594. doi:10.1002/jcb.28525
72. Kume T, Jiang H, Topczewska JM, Hogan BL. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 2001;15(18):2470–2482. doi:10.1101/gad.907301
73. Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol. 2006;294(2):458–470. doi:10.1016/j.ydbio.2006.03.035
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

