Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Biodegradable mesoporous calcium–magnesium silicate-polybutylene succinate scaffolds for osseous tissue engineering
Authors Zhang X, Zhang C, Xu W, Zhong B, Lin F, Zhang J, Wang Q, Ji J, Wei J, Zhang Y
Received 17 July 2015
Accepted for publication 20 September 2015
Published 28 October 2015 Volume 2015:10(1) Pages 6699—6708
DOI https://doi.org/10.2147/IJN.S92598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lei Yang
Xinxin Zhang,1,2,* Chi Zhang,3,* Wei Xu,1,* Biao Zhong,3 Feng Lin,3 Jian Zhang,3 Quanxiang Wang,4 Jiajin Ji,4 Jie Wei,4 Yang Zhang1
1TongRen Hospital, School of Medicine, Shanghai Jiao Tong University, 2Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 3Shanghai Sixth People’s Hospital, Shanghai Jiao Tong University, 4Key Laboratory for Ultrafine Materials of Ministry of Education, East China University of Science and Technology, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Abstract: The structural features of bone engineering scaffolds are expected to exhibit osteoinductive behavior and promote cell adhesion, proliferation, and differentiation. In the present study, we employed synthesized ordered mesoporous calcium–magnesium silicate (om-CMS) and polybutylene succinate (PBSu) to develop a novel scaffold with potential applications in osseous tissue engineering. The characteristics, in vitro bioactivity of om-CMS/PBSu scaffold, as well as the cellular responses of MC3T3-E1 cells to the composite were investigated. Our results showed that the om-CMS/PBSu scaffold possesses a large surface area and highly ordered channel pores, resulting in improved degradation and biocompatibility compared to the PBSu scaffold. Moreover, the om-CMS/PBSu scaffold exhibited significantly higher bioactivity and induced apatite formation on its surface after immersion in the simulated body fluid. In addition, the om-CMS/PBSu scaffold provided a high surface area for cell attachment and released Ca, Mg, and Si ions to stimulate osteoblast proliferation. The unique surface characteristics and higher biological efficacy of the om-CMS/PBSu scaffold suggest that it has great potential for being developed into a system that can be employed in osseous tissue engineering.
Keywords: bone repair, polybutylene succinate, calcium–magnesium silicate, ordered mesoporous, proliferation
Introduction
The treatment of bone defects resulting from trauma, infections, tumors, or genetic malformations represents a major challenge for clinicians. A three-dimensional structure known as a “scaffold”, fabricated from a suitable artificial or natural material and exhibiting high porosity and pore interconnectivity, has been widely studied to regenerate the lost bone and provide treatment modalities for bone defects.1,2 Scaffolds should ideally not only provide a passive structural support for bone cells but they should also favorably modulate bone formation by simulating osteoblastic cell proliferation and differentiation.3 Scaffolds have certain advantages over autologous grafts, such as easy shaping, limitless supply, and avoidance of donor-site morbidity. Biodegradable scaffolds even allow new tissues to take over the biological functions; therefore, potential chronic problems associated with biostable implants can be avoided.4,5
Multiple attempts have been made to construct scaffolds exhibiting the desired porosity and mechanical performance by using inorganic bioactive ceramics and glass or from biodegradable polymers.6,7 Polybutylene succinate (PBSu) is a biodegradable biopolymer with good processability, excellent mechanical properties, and harmless degradation products (CO2 and H2O).8 PBSu has been studied extensively for its potential to be used as a conventional plastic and is also proposed to be a good candidate for use as a supporting material that has various applications in the field of tissue engineering. Notably, Wang et al have reported the use of PBSu based scaffolds in artificial bone implants.9 Although PBSu is an excellent material for use as a bone substitute and possesses certain controllable manufacturing characteristics on the large scale, its osteocompatibility is still insufficient. Some clinical studies have reported that the degradation rate of PBSu polymer material varies greatly between patients, and the void left after polymer degradation was not filled by regenerating bone.10–12
Currently, the research on the use of bioglass for biomedical purposes has largely focused on new compositional modifications, processing, and potential biomedical applications. Silicate glasses that are based primarily on the SiO2-CaO-Na2O-P2O5 system exhibit unique biological properties, such as osteoinductive behavior and the ability to bind to soft tissue as well as to hard tissue when exposed to biological fluids.13 The ionic dissolution products from bioglass (eg, Si, Ca, P) stimulate the expression of osteolastic cells and angiogenesis, leading to the formation of strong bonds between the bioactive glass and human bone.14 Further, many trace elements present in the human body such as Mg, Cu, Zn, and Sr are known for their anabolic effects on bone metabolism.15 Therefore, novel techniques that enhance bioactivity of scaffold materials by introducing therapeutic ions are now being investigated. The release of these ions upon exposure to a physiological environment is believed to favorably affect the behavior of human cells and also enhance the bioactivity of these scaffolds in stimulating both osteogenesis and angiogenesis.
Herein, we report the development of a novel scaffold comprising a combination of active ordered mesoporous calcium–magnesium silicate (om-CMS) material with PBSu. This om-CMS/PBSu scaffold was expected to exhibit sustained release of the mesoporous cargo from the polymeric scaffold and stimulate cell adhesion, proliferation, and differentiation into osteoblasts. We have also evaluated the characteristics of the scaffold material, its mechanical properties, and cellular responses.
Materials and methods
Preparation of om-CMS/PBSu scaffold
om-CMS was synthesized using the typical sol-gel method.16 Briefly, 4 g of Pluronic P123 (PEO20-PPO70-PEO20, Sigma-Aldrich Co., St Louis, MO, USA) was dissolved in 30 g of H2O and 120 g of HCl solution (2 M), and mixed well. This solution was then stirred until clear and then 4.8 g of MgNO3·6H2O and 5.2 g of CaNO3 were added to the solution. This was followed by addition of 8.5 g of tetraethyl orthosilicate dropwise using an injection pump. The reaction mixture was vigorously stirred at 50°C for 5 hours and then incubated for another 24 hours at 80°C to obtain precipitation. Finally, the precipitate was filtered out, washed thoroughly with deionized water, and dried in vacuum at 60°C to obtain the om-CMS powder.
The om-CMS/PBSu scaffold was prepared by mixing the om-CMS and PBSu powders in a ratio of 40:60 by weight at 130°C for 10 minutes. Disc-shaped scaffold samples (Φ15×2 mm) were then prepared at 130°C using a mold. The samples were then washed with ultrasounds for 10 minutes and dried in an oven at 37°C to obtain the composites.
Characterization
The morphology and microstructure of om-CMS and om-CMS/PBSu were observed by transmission electron microscopy. The transmission electron microscopy micrographs were obtained on a JEM1400F electron microscope (JEOL, Tokyo, Japan) with an accelerating voltage of 200 kV. The porosities and specific surface area of the om-CMS were measured through nitrogen adsorption-desorption isotherms by using a Macromeritics ASAP2010N analyzer. The surface areas of the powders were calculated by the Brunauer–Emmett–Teller equation.17 The relative pressure P/Po of the isotherms was studied between 0.01 and 1.0. The pore parameters were calculated by employing the Barrett–Joyner–Halenda model.18 The chemical structure and functional groups of the om-CMS, PBSu, and om-CMS/PBSu composites were characterized by Fourier transform infrared spectroscopy (FTIR, Thermo Nicolet 6700, Waltham, MA, USA). The phase and crystallographic structures of om-CMS, PBSu, and om-CMS/PBSu were characterized by X-ray diffraction using the D/max 2550VB/PC diffractometer (Rigaku, Tokyo, Japan) with a scan range from 10° to 80°.
In vitro biodegradation
An immersion test was performed to evaluate degradation, bioactivity, and mechanical stability of the om-CMS/PBSu scaffold by immersing the samples in simulated body fluid (SBF). The standard SBF solution was prepared by dissolving NaCl, NaHCO3, KCl, K2HPO4·3H2O, MgCl2·6H2O, CaCl2·6H2O, and Na2SO4 in distilled water and buffering it to a pH of 7.4 with Tris buffer and 1 N HCl as described previously by Kokubo and Takadama.19 The ionic concentration of SBF simulates that of human plasma. Circular tablets of om-CMS/PBSu were immersed in flasks containing SBF at 37°C. The morphologies of the scaffolds before and after the SBF immersion test were observed under a scanning electron microscope (VEGA TS 5163MM, TESCAN, Brno, Czech Republic) at an accelerating voltage of 20 kV. A pH meter was used to monitor changes of pH in the solution. The amount of weight lost by the samples during the experiment was calculated as the difference in weight of samples before and after immersion in the SBF.
Structural and element stability
The phase structure of the om-CMS/PBSu scaffold after the SBF immersion test was evaluated by using X-ray diffraction. To evaluate the exchange of ions between the samples and the solutions, the chemical composition of the soaking solutions was analyzed by using an inductively coupled plasma spectrometer (JY70 Plus, Horiba Jobin Yvon, Palaiseau, France) to measure the amount of Ca, Mg, and Si in the soaking solutions.
Cell culture
The mouse osteoblastic cell line MC3T3-E1 was used to investigate the interaction between osteoblasts and the om-CMS/PBSu composite. The cells were cultured in modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 μg/mL penicillin, and streptomycin at 37°C in an environment of 5% CO2 and saturated humidity. The culture medium was replaced with fresh medium every 3 days.
Cell adhesion and imaging
To study the influence of serum proteins fibronectin (FN) and scaffold on the osteoblast cell attachment and proliferation, we have processed the om-CMS/PBSu composite using a simple treatment. The om-CMS/PBSu samples were placed in a 24-well plate and sterilized in 75% ethanol for 1 hour and then washed twice with phosphate buffered saline liquid to remove residual ethanol. The substrates were then incubated with either 10% FBS or serum levels of FN (20 μg/mL) for 4 hours at 37°C. After this time period, each substrate was gently washed with phosphate buffered saline just before seeding with cells.
The MC3T3-E1 cells were seeded on these substrates at a density of 104 cells/cm2 and incubated for 6 hours. The substrates were then washed twice with serum-free medium. Cells were fixed with 4% paraformaldehyde for 10 minutes, then washed three times with phosphate buffered saline, and permeabilized with 0.1% Triton X-100 for 10 minutes. Cell-seeded samples were stained with 20 μg/mL of Acti-stain 488 phalloidin (Sigma-Aldrich) and then counterstained with 1 μg/mL of DAPI for 5 minutes at room temperature. The cells were imaged by using a confocal laser-scanning microscope (Leica Microsystems, Wetzlar, Germany).
Cell proliferation
Cells were cultured on the PBSu and om-CMS/PBSu substrates at a density of 104 cells/cm2 and cell proliferation was measured at 1, 4, and 7 days using the Cell Counting Kit-8.20 After culturing on substrates, the Cell Counting Kit-8 solution (5 mg/mL) was added to each well (100 μL/well), and the cells were incubated for another 4 hours. The optical density (OD) of each well at 450 nm was determined using a spectrophotometer (BioTek, Winooski, VT, USA).
Statistical analysis
The data were analyzed by a one-way analysis of variance using SPSS 10.0 (SPSS Inc., Chicago, IL, USA). The results of the statistical analysis are expressed as mean ± standard deviation. Differences were considered statistically significant at a P-value <0.05.
Results and discussion
Characterization of om-CMS/PBSu
Figure 1A shows the images of PBSu and om-CMS/PBSu scaffolds. The density of the PBSu scaffold increases from 1.26 to 1.42 g/cm3 after blending with the om-CMS powder. Figure 1B and C represents transmission electron microscopy images revealing the morphology and microstructure of the om-CMS/PBSu scaffold. An ordered mesoporous structure with a uniform pore size can be clearly observed. N2 adsorption-desorption isotherms of the om-CMS/PBSu scaffold (Figure 2A) revealed that the om-CMS/PBSu scaffold exhibits a type IV adsorption-desorption isotherm with H1-type hysteresis loops at high relative pressures according to International Union of Pure and Applied Chemistry classification. This would indicate the presence of independent cylindrical slim pore channels with a highly uniform pore size distribution.21 Both branches of the adsorption-desorption isotherms showed a sharp adsorption-desorption step in the P/P0 region between 0.8 and 1.0. These results suggest that the om-CMS/PBSu scaffold possesses a well-defined array of regular mesopores. The pore size distribution calculated from the adsorption-desorption isotherm by the Barrett–Joyner–Halenda method is shown in Figure 2B. It was found that the om-CMS/PBSu scaffold exhibits a narrow pore size distribution with an average pore size of approximately 13 nm and a relatively large Brunauer–Emmett–Teller surface area of 891 m2/g. This ordered mesoporous structure and large specific surface area of the scaffold are conducive for the transmission of body fluids to the site of bone defect, and thus promote cell adhesion and proliferation.22 Furthermore, the large specific surface area of the scaffold enhances the probability of contact with body fluids ions, which accelerate the degradation of the scaffold material. This results in excellent biological activity and osteocompatibility.
FTIR spectra of PBSu, om-CMS, and om-CMS/PBSu are shown in Figure 3. The IR bands at 1,717 and 1,148 cm−1 represent the vibrational mode of the ester base of PBSu (Figure 3A). The stretching vibration peak of the Si-O structure in om-CMS was seen at 1,047 cm−1 (Figure 3B). All these peaks were observed in the FTIR spectrum of om-CMS/PBSu (Figure 3C), suggesting that the effects of PBSu and om-CMS get compounded when mixed together. Figure 4 shows the X-ray diffraction pattern of PBSu, om-CMS, and om-CMS/PBSu. It was observed that om-CMS had a broad peak at approximately 22°, which is typical for amorphous silicate materials.23 Strong diffraction peaks at 19° and 22° were observed for PBSu. Notably, the diffraction peaks obtained from om-CMS/PBSu were broader and less intense than the sample PBSu, indicating that om-CMS/PBSu is less crystalline than PBSu.
  | Figure 4 Wide-angle XRD patterns of PBSu, om-CMS and om-CMS/PBSu. |
Degradability of om-CMS/PBSu in the SBF
Degradability of a biomaterial in a physiological environment is crucial for its application in tissue engineering. Immersion tests in SBF are the standard procedure to estimate the in vitro biodegradability and bioactivity of scaffolds. This provides information on the long-term degradation characteristics of the implants, including degradation rate, pH variation, and changes in surface morphology.
Figure 5 shows the Scanning Electron Microscopy micrographs and photographs of PBSu and om-CMS/PBSu scaffolds before and after immersion in SBF for 5 days. The obvious difference in surface morphology was found before and after soaking the scaffold in SBF. The PBSu with a rough surface contained some columnar crystals (Figure 5A) before it was soaked in SBF. After soaking in SBF, it can be observed that the surface morphology of the PBSu scaffold has been degraded and the crystals were dissolved by the SBF (Figure 5C). The surface morphology of the om-CMS/PBS scaffold was similar to PBSu but with less columnar crystals prior to soaking it in SBF (Figure 5B). This could be due to the blending of the amorphous om-CMS powder. After soaking in SBF, however, the surfaces of om-CMS/PBSu were covered with a faveolate structure (Figure 5D); this was different than that observed in PBSu upon soaking in SBF. Notably, the degradation of om-CMS/PBSu was more severe than that of PBSu indicating that the degradation rate of the PBSu scaffold is increased by addition of om-CMS. These results suggested that the mesoporous structure of the om-CMS/PBSu scaffold enhances the biocompatibility as well as the chances of osseointegrated interface formation after implantation.
The changes in pH of the SBF solution after immersion of the PBSu and om-CMS/PBSu scaffolds for various durations are shown in Figure 6A. It was found that the pH of the PBSu solution decreased slowly from 7.4 to 7.1 during the first 8 weeks. A sharp decrease was noticed from week 9 onward and finally a pH of 5.6 was detected at the end of week 12. However, the pH value of the om-CMS/PBSu containing SBF increased slowly in the first 2 weeks and then showed a slow decrease in the following 10 weeks. The pH of the SBF solution containing om-CMS/PBSu was 7.0 at week 12. Polyester is chemically degraded by hydrolysis and pH is one of the factors that influences the velocity of this hydrolysis through autocatalysis.24 The PBSu scaffold might be degrading by a random chain scission by ester hydrolysis in a process autocatalyzed by the generation of carboxylic acid end groups that accelerate degradation and pH decrease by acid catalysis after 8 weeks of immersion. The degradation of om-CMS in the om-CMS/PBSu scaffold is known to occur within the first few days of exposure to the physiological media. This is mainly due to the mesoporous structure providing a high contact interface for the SBF. The degradation of Mg and Ca in the om-CMS is described by the following reactions.25
Mg/Ca + 2H2O → Mg(OH)2/Ca(OH)2 + H2; | (1) |
Mg(OH)2/Ca(OH)2 + 2Cl− → MgCl2/CaCl2 + 2OH− | (2) |
Degradation of om-CMS is accompanied by an alkalization of the corrosive media due to the production of hydroxide ions (OH−). A pH increase during the first few days of immersion has been reported by several in vitro studies.26,27 The alkalization of the om-CMS and the acidification of the PBSu solutions reach equilibrium after 2 weeks while the pH of the SBF solution containing om-CMS/PBSu stabilizes. This finding demonstrates that the om-CMS/PBSu scaffold is a superior biological material than the other two scaffolds. This stable neutral pH may promote cell proliferation and high biological safety.28
Figure 6B represents the percentage of weight remaining of the om-CMS/PBSu and PBSu scaffolds compared with day 0 values as a function of the incubation time. The weight of PBSu was 91.3%±2.1% of the initial weight after 8 weeks of degradation in SBF solution and then decreased dramatically to 57.1%±3.2% at the end of 12 weeks. The om-CMS/PBSu scaffold showed steady degradation over time and the weight loss rate was approximately 3% per week. Biomaterials used for bone regeneration should be degradable and gradually replaced by new bone when implanted in vivo. The constant degradation rate of the om-CMS/PBSu scaffold can be attributed to its mesoporous structure and large surface area and this could improve the osteocompatibility in vivo, ultimately benefiting the physicochemical interaction between the cells and the material.29
Bioactivity of om-CMS/PBSu in vitro
The X-ray diffraction patterns of om-CMS/PBSu after soaking into SBF for 5 days are shown in Figure 7A. A new crystal peak was observed between 10° and 20° after immersion. This could be the signal of phosphorus indicating apatite formation on the om-CMS/PBSu surface. The inductively coupled plasma results (Figure 7B) show changes in Mg and Ca ion concentration of the solution after immersing om-CMS/PBSu into SBF for different time durations. The Mg and Ca ion concentrations of om-CMS/PBSu in SBF increased at different rates with soaking time and reached 47 and 261 mg/L at 96 hours, respectively. It has been reported that ion-dissolution products containing Ca, Si, and Mg from bioactive glasses and ceramics could stimulate the nucleation of bioactive minerals.30 In addition, SBF is a super saturated calcium phosphate solution and its chemical stimulus may activate the nucleation of bioactive minerals including phosphate and carbonate groups and the induction of bioactivity can be carried out by negatively charged groups. In particular, the formation of silanol (-Si-OH) on the surface of a scaffold is known to be beneficial for nucleation of bioactive products. The positively charged Ca2+ and Mg2+ attract negatively charged groups PO43− and CO32− in the SBF and eventually lead to the formation of a phosphate layer on the surface of the scaffold.31 This may in turn be beneficial in enhancing the chances of formation of an osseointegrated interface and cell proliferation after implantation.
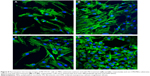
Cell adhesion
The effect of adsorbed FN on adhesion of MC3T3-E1 cells to the om-CMS/PBSu and PBSu substrates after 6 hours of seeding is shown in Figure 8. The cells reach an equilibrium adherence level after 6 hours and then spread on the surface. Figure 8C and D clearly shows that the presence of FN increases cell attachment to either substrate. More MC3T3-E1 cells were observed to attach to the om-CMS/PBSu composite than to the PBSu indicating that the use of om-CMS/PBSu could promote the attachment of MC3T3-E1 cells. It has been shown that materials with a mesoporous structure promote protein unfolding and adsorbed protein density.32 Thus, the om-CMS/PBSu substrate could promote increased FN binding by providing a larger surface area and pore volume thereby enhancing cell adhesion significantly. In addition, the hydrophilic surface of the om-CMS/PBSu is beneficial for cell attachment, spreading, and cytoskeletal organization.33 The silanol groups generated by om-CMS can bind to various functional groups of proteins via hydrogen bonds or long-range electrostatic ionic amine bonds (Si-O− +H3N), thus producing a favorable surface environment for cell attachment.34 Further, Ca2+ and Mg2+ ions on the surface of the om-CMS/PBSu act as ligands for osteoblast favored protein adsorption due to positive electrostatic attraction.35 Therefore, the om-CMS/PBSu composite could exert a positive effect on cell attachment and spreading.
Cell proliferation
The proliferation of MC3T3-E1 cells cultured on the om-CMS/PBSu and PBSu surfaces was analyzed using a Cell Counting Kit-8 assay as shown in Figure 9. Cells grown on the composites displayed a steady continuous increase in proliferation from day 1 to day 7, suggesting no negative effects on cell growth. Cells grown on PBSu exhibited no significant increase in proliferation from day 4 to day 7. The relative proliferation rate of cells grown on the om-CMS/PBSu was significantly higher than those grown on PBSu, indicating that om-CMS/PBSu promotes the proliferation of MC3T3-E1 cells. Studies have shown that the release of appropriate quantities of Si ions from silicate-containing materials plays an important role in stimulating the proliferation of osteoblasts and bone marrow stromal cells.36 Further, appropriate levels of Ca2+ and Mg2+ are known to stimulate osteoblast proliferation by modulating the expression of specific ion channel isoforms in osteoblasts. In addition, the formation of an apatite layer on the composite surface is known to be beneficial for osteoblast proliferation.
Conclusion
In this study, a bioactive scaffold was fabricated by incorporating om-CMS into PBSu using a process of compounding and molding. The resulting om-CMS/PBSu scaffold had a large surface area of 891 m2/g and highly ordered channels of pores of approximately 13 nm in size. The om-CMS/PBSu is degradable with a weight loss ratio of 38% after immersion in SBF solution for 12 weeks with a relatively constant pH value of the solution. The om-CMS/PBSu showed good bioactivity in vitro, which could induce apatite formation on its surfaces after immersion in SBF. Further, the use of om-CMS/PBSu significantly increased attachment, proliferation, and spreading of MC3T3-E1 cells compared to PBSu. The positive effects of om-CMS/PBSu on cellular responses are most likely due to the mesoporous structure of this surface, formation of apatite-layer, and the Si, Ca, and Mg ions released from the composite. Due to its increased bioactivity and biocompatibility the om-CMS/PBSu scaffold is believed to be a promising orthopedic implant material for application in bone repair.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Nature Science Foundation of China (grant number: 81201177 and 81571796), Youth Innovation Promotion Association, CAS, and the SA-SIBS Scholarship Program. The authors would like to thank Dr S Liu and Dr Shangchun Guo for their excellent technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
Ingavle GC, Leach JK. Advancements in electrospinning of polymeric nanofibrous scaffolds for tissue engineering. Tissue Eng Part B Rev. 2014;20(4):277–293. | ||
Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21(24):2521–2527. | ||
Serra IR, Fradique R, Vallejo MCS, Correia TR, Miguel SP, Correia IJ. Production and characterization of chitosan/gelatin/β-TCP scaffolds for improved bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2015;55:592–604. | ||
Lv YM, Yu QS. Repair of articular osteochondral defects of the knee joint using a composite lamellar scaffold. Bone Joint Res. 2015;4(4):56–64. | ||
Zhou P, Xia Y, Cheng X, Wang P, Xie Y, Xu S. Enhanced bone tissue regeneration by antibacterial and osteoinductive silica-HACC-zein composite scaffolds loaded with rhBMP-2. Biomaterials. 2014;35(38):10033–10045. | ||
Lu W, Ji K, Kirkham J, et al. Bone tissue engineering by using a combination of polymer/Bioglass composites with human adipose-derived stem cells. Cell Tissue Res. 2014;356(1):97–107. | ||
Van Rie J, Declercq H, Van Hoorick J, et al. Cryogel-PCL combination scaffolds for bone tissue repair. J Mater Sci Mater Med. 2015;26(3):123. | ||
Stoyanova N, Paneva D, Mincheva R, et al. Poly(L-lactide) and poly(butylene succinate) immiscible blends: from electrospinning to biologically active materials. Mater Sci Eng C Mater Biol Appl. 2014;41:119–126. | ||
Wang H, Xu M, Wu Z, Zhang W, Ji J, Chu PK. Biodegradable poly(butylene succinate) modified by gas plasmas and their in vitro functions as bone implants. ACS Appl Mater Interfaces. 2012;4(8):4380–4386. | ||
Félix Lanao RP, Sariibrahimoglu K, Wang H, Wolke JG, Jansen JA, Leeuwenburgh SC. Accelerated calcium phosphate cement degradation due to incorporation of glucono-delta-lactone microparticles. Tissue Eng Part A. 2014;20(1–2):378–388. | ||
Arphavasin S, Singhatanadgit W, Ngamviriyavong P, Janvikul W, Meesap P, Patntirapong S. Enhanced osteogenic activity of a poly(butylene succinate)/calcium phosphate composite by simple alkaline hydrolysis. Biomed Mater. 2013;8(5):055008. | ||
Wang H, Ji J, Zhang W, et al. Biocompatibility and bioactivity of plasma-treated biodegradable poly(butylene succinate). Acta Biomater. 2009;5(1):279–287. | ||
Abdollahi S, Ma AC, Cerruti M. Surface transformations of Bioglass 45S5 during scaffold synthesis for bone tissue engineering. Langmuir. 2013;29(5):1466–1474. | ||
Shah Mohammadi M, Chicatun F, Stähli C, Muja N, Bureau MN, Nazhat SN. Osteoblastic differentiation under controlled bioactive ion release by silica and titania doped sodium-free calcium phosphate-based glass. Colloids Surf B Biointerfaces. 2014;121:82–91. | ||
Fernández JM, Molinuevo MS, McCarthy AD, Cortizo AM. Strontium ranelate stimulates the activity of bone-specific alkaline phosphatase: interaction with Zn(2+) and Mg(2+). Biometals. 2014;27(3):601–607. | ||
Singh LP, Bhattacharyya SK, Kumar R, et al. Sol-Gel processing of silica nanoparticles and their applications. Adv Colloid Interface Sci. 2014;214C:17–37. | ||
Chen B, Wang Z, Quan G, et al. In vitro and in vivo evaluation of ordered mesoporous silica as a novel adsorbent in liquisolid formulation. Int J Nanomedicine. 2012;7:199–209. | ||
Wanyika H. Controlled release of agrochemicals intercalated into montmorillonite interlayer space. ScientificWorldJournal. 2014;2014:656287. | ||
Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27(15):2907–2915. | ||
Lee HN, Kim CH, Song GG, Cho SW. Effects of IL-3 and SCF on histamine production kinetics and cell phenotype in rat bone marrow-derived mast cells. Immune Netw. 2010;10(1):15–25. | ||
Zheng BJ, Qi B, Hu YL, Zhang F, Liu HF. Electro-catalytic activity of palladium modified nitrogen doped titania nanoparticles. J Nanosci Nanotechnol. 2014;14(5):3527–3531. | ||
Zhang JH, Zhao SC, Zhu YF, et al. Three-dimensional printing of strontium-containing mesoporous bioactive glass scaffolds for bone regeneration. Acta Biomater. 2014;10(5):2269–2281. | ||
Zhu YF, Wu CT, Ramaswamy Y, et al. Preparation, characterization and in vitro bioactivity of mesoporous bioactive glasses (MBGs) scaffolds for bone tissue engineering. Micropor Mesopor Mater. 2008;112(1–3):494–503. | ||
Wang H, Ji J, Zhang W, et al. Biocompatibility and bioactivity of plasma-treated biodegradable poly(butylene succinate). Acta Biomater. 2009;5(1):279–287. | ||
Yazdimamaghani M, Razavi M, Vashaee D, Tayebi L. Surface modification of biodegradable porous Mg bone scaffold using polycaprolactone/bioactive glass composite. Mater Sci Eng C Mater Biol Appl. 2015;49(4):436–444. | ||
Wong HM, Yeung KW, Lam KO, et al. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials. 2010;31(8):2084–2096. | ||
Yazdimamaghani M, Razavi M, Vashaee D, Tayebi L. Development and degradation behavior of magnesium scaffolds coated with polycaprolactone for bone tissue engineering. Mater Lett. 2014;132:106–110. | ||
Maĭborodin IV, Kuznetsova IV, Shevela AI, Barannik MI, Manaev AA, Maĭborodina VA. Tissue reactions to the use of implants manufactured from lactic acid polymers. Morfologiia. 2014;146(4):78–89. | ||
Baino F, Fiorilli S, Mortera R, et al. Mesoporous bioactive glass as a multifunctional system for bone regeneration and controlled drug release. J Appl Biomater Funct Mater. 2012;10(1):12–21. | ||
Pan Y, Chen C, Wang D, Huang D. Dissolution and precipitation behaviors of silicon-containing ceramic coating on Mg-Zn-Ca alloy in simulated body fluid. Colloids Surf B Biointerfaces. 2014;122:746–751. | ||
Jones JR. Review of bioactive glass: from Hench to hybrids. Acta Biomater. 2013;9(1):4457–4486. | ||
Lee CH, Lang J, Yen CW, Shih PC, Lin TS, Mou CY. Enhancing stability and oxidation activity of cytochrome C by immobilization in the nanochannels of mesoporous aluminosilicates. J Phys Chem B. 2005;109(25):12277–12286. | ||
Allen LT, Tosetto M, Miller IS, et al. Surface-induced changes in protein adsorption and implications for cellular phenotypic responses to surface interaction. Biomaterials. 2006;27(16):3096–3108. | ||
Wu CT, Chang J, Ni SY, Wang JY. In vitro bioactivity of akermanite ceramics, J Biomed Mater Res A. 2006;76(1):73–80. | ||
Walmsley GG, McArdle A, Tevlin R, et al. Nanotechnology in bone tissue engineering. Nanomedicine. 2015;11(5):1253–1263. | ||
Shi M, Zhou Y, Shao J, et al. Stimulation of osteogenesis and angiogenesis of hBMSCs by delivering Si ions and functional drug from mesoporous silica nanospheres. Acta Biomater. 2015;21:178–189. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.