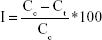Back to Journals » International Journal of Nanomedicine » Volume 10 » Supplement 1 Challenges in biomaterials research
Biobased silver nanocolloid coating on silk fibers for prevention of post-surgical wound infections
Authors Dhas S, Anbarasan S, Mukherjee A, Chandrasekaran N
Received 31 March 2015
Accepted for publication 26 May 2015
Published 1 October 2015 Volume 2015:10(Supplement 1 Challenges in biomaterials research) Pages 159—170
DOI https://doi.org/10.2147/IJN.S82211
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Sindhu Priya Dhas, Suruthi Anbarasan, Amitava Mukherjee, Natarajan Chandrasekaran
Center for Nanobiotechnology, VIT University, Vellore, India
Abstract: Bombyx mori silk fibers are an important biomaterial and are used in surgical sutures due to their remarkable biocompatibility. The major drawback to the application of biomaterials is the risk of bacterial invasion, leading to clinical complications. We have developed an easy and cost-effective method for fabrication of antibacterial silk fibers loaded with silver nanoparticles (AgNPs) by an in situ and ex situ process using an aqueous extract of Rhizophora apiculata leaf. Scanning electron microscopy revealed that well dispersed nanoparticles impregnated the silk fibers both in situ and ex situ. The crystalline nature of the AgNPs in the silk fibers was demonstrated by X-ray diffraction. The thermal and mechanical properties of the silk fibers were enhanced after they were impregnated with AgNPs. The silver-coated silk fibers fabricated by the in situ and ex situ method exhibited more than 90% inhibition against Pseudomonas aeruginosa and Staphylococcus aureus. Silk fibers doped with AgNPs were found to be biocompatible with 3T3 fibroblasts. The results obtained represent an important advance towards the clinical application of biocompatible AgNP-loaded silk fibers for prevention of surgical wound infections.
Keywords: silk fibers, silver nanoparticles, antibacterial activity, wound infections, cytotoxicity, 3T3 fibroblast cells
Introduction
Silk is a high molecular weight biopolymer spun in the form of fibers by the Lepidoptera larvae of silkworms, mites, spiders, and scorpions,1,2 and is approved by the US Food and Drug Administration as a biomaterial.3 The silk obtained from Bombyx mori has been extensively characterized and is used in the textile and biomedical fields.2,4 B. mori is a commercially important silkworm and is extremely productive under the prevailing climatic conditions in India.5 B. mori silk fibers have excellent mechanical properties owing to their antiparallel β-pleated structure.6 The unique properties of silk, ie, biocompatibility, biodegradability, high tensile strength, lack of toxicity, and bioresorbability, make it an important biomaterial for clinical application.7 Silk fibers have been used as nonabsorbable surgical sutures to close wounds.8 However, the presence of sutures at the wound site may increase the susceptibility to postoperative wound infections. Hence, functionalization of silk fibers with antimicrobial agents is essential for prevention of such infections.
Silver nanoparticles (AgNPs) have been recognized as new generation antibacterial agents due to their enhanced antibacterial properties.9 The green approach to creating AgNPs using biological resources is gaining importance because of the ease of synthesis and ability to create biocompatible and stable nanoparticles.10 The good bactericidal activity of AgNPs is attributed to their high surface to volume ratio. Use of nanosilver is increasing, with these products being incorporated into wound dressings, clothing, food containers, ointments, and implant coatings.11,12 The development of AgNP-coated silk fibers as surgical sutures is an innovative and promising strategy.
In the present study, silk fibers were functionalized with AgNPs using an aqueous extract of Rhizophora apiculata. Polymers (polyacrylic acid, polyvinyl alcohol, polymethyl methacrylate, polyacrylonitrile, and polyimide) along with reducing agents (sodium borohydride, sodium hydrazine, and sodium citrate) have been used to functionalize the surface of silk with AgNPs.13–16 Use of such polymers increases the density of AgNPs in silk fibers, but also increases the production costs and may lead to release of contaminants into the environment. Recently, microwave and gamma irradiation techniques have replaced the use of chemical reductants for functionalization of AgNPs on silk fibers. Use of expensive instruments as irradiation sources limits its scalability.17,18 Further, such methods are tedious to perform and expensive. Hence, the green route using a plant extract for in situ and ex situ impregnation of silk fibers by AgNPs would be simple and cost-effective. The biomimetic approach reduces the need for costly instrumentation, and the properties of the nanomaterials used can be tuned according to the intended biological application. To the best of our knowledge, this is the first report of silk fibers being coated with AgNPs by an in situ and ex situ approach using a biological source for clinical application.
The antibacterial silk fibers were tested against the bacterial pathogens Pseudomonas aeruginosa and Staphylococcus aureus, which are common sources of nosocomial and postoperative wound infections.19 We also tested the biocompatibility of silver-impregnated silk fibers with 3T3 fibroblasts.
Materials and methods
Chemicals
Silver nitrate (AgNO3) was obtained from Merck Chemicals (Mumbai, India). Nutrient broth and nutrient agar and were purchased from HiMedia (Mumbai, India). The silk fibers were obtained from the Sericulture Department, Vaniyambadi (Vellore District, Tamil Nadu, India).
Synthesis and characterization of AgNPs
Fresh R. apiculata leaves were collected from the Pichavaram mangrove forest in Tamil Nadu, India, washed with distilled water, shade-dried, and powdered. An aqueous extract was prepared and the nanoparticles were synthesized as in our previous report.20
The synthesized AgNPs were characterized using a double-beam ultraviolet-visible spectrophotometer (2201, Systronics, Hyderabad, India). The particle size and zeta potential of the colloidal suspension were determined using a particle size analyzer (NanoBrook 90 Plus, Brookhaven Instruments, Holtsville, NY, USA). The morphology of the AgNPs was determined using a transmission electron microscope at 80 kV (Technai-10, Philips, Eindhoven, the Netherlands).
Impregnation of silk fibers with AgNPs
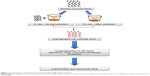
The silk fibers were impregnated with AgNPs by a simple adsorption (dipping) method using in situ and ex situ methods. For in situ impregnation, the silk fibers were treated with 10 mL of aqueous extract and 40 mL of 4 mM silver nitrate for 6 hours. The ex situ impregnation was done by treating the fibers with colloidal nanosilver for 6 hours. A schematic representation of the in situ and ex situ approaches used for impregnation of the silk fibers is shown in Figure 1.
Characterization of AgNP-impregnated silk fibers
Scanning electron microscopy (SEM)/energy-dispersive X-ray spectroscopy
The surface morphology of the silk fibers and its elemental composition were observed by SEM combined with energy-dispersive X-ray spectroscopy (S-400, Hitachi, Tokyo, Japan).
X-ray diffraction
The crystalline nature of the silk and the AgNP-impregnated silk fibers was determined using an X-ray diffractometer (D8 Advance, Bruker, Ettlingen, Germany) with a target of Cu Kα-ray (λ=1.54 A°).
Thermogravimetric analysis
Thermogravimetric analysis of the control and silver-impregnated silk fibers was done using an STA 449 F3 Jupiter instrument, Netzsch, Selb, Germany). The instrument was operated at a heating rate of 25°C per minute and under constant nitrogen flow (20 mL per minute).
Mechanical properties
The breaking load was determined using an H5KS universal testing machine (Tinius Olsen Ltd, Redhill, UK) running at a crosshead speed of 5 mm per minute. The sample fibers were cut into samples with a cross-sectional area of 1 cm ×2 cm.
Analysis of initial silver content in silk fibers
The total content of silver in the fibers was determined using an atomic absorption spectrophotometer (AA240, Varian Inc, Palo Alto, CA, USA). The silk fibers were digested with 70% nitric acid and then analyzed by atomic absorption spectrophotometer.
Bactericidal activity
Test organisms
S. aureus (MTCC 96, pathogenic strain) and P. aeruginosa (MTCC 741, clinical isolate) were purchased from the Microbial Type Culture Collection (Institute of Microbial Technology, Chandigarh, India).
Zone of inhibition method
The antibacterial activity of the control and AgNP-impregnated silk fibers was evaluated using the zone of inhibition method. Bacterial lawns were prepared on nutrient agar using sterile cotton swabs at a concentration of 108 colony-forming units (CFU)/mL.11 The silk fibers were cut into small pieces (1 cm length and 2 mm thickness) and placed on nutrient agar. The plates were examined for zone of inhibition after incubation at 37°C for 24 hours.
Bacterial reduction assay
P. aeruginosa and S. aureus (~108 CFU/mL) bacteria suspended in phosphate-buffered saline were added to sterilized conical flasks. The fabricated silk fibers were put into the flasks. Untreated silk fibers were used as the control. The flasks were placed on a shaker and incubated for 24 hours at 37°C. After incubation, 100 μL bacterial aliquots were taken and spread on nutrient agar plates. The bacterial colonies were counted, and percentage inhibition of bacterial growth was determined using the following equation:
|
where, I is the percentage inhibition, Cc is CFU of control silk fibers, and Ct is CFU of viable colonies of AgNP-coated silk fibers. The statistical significance of the bactericidal activity of the in situ and ex situ silk fibers was analyzed by Student’s t-test at the P<0.05 level.
Assessment of membrane integrity by fluorescence microscopy
Live and dead S. aureus and P. aeruginosa cells were discriminated by the differential staining technique described by Jakopec et al using a combination of acridine orange and ethidium bromide dyes.21 Fluorescence microscopy was used to identify the live and dead bacterial cells. The double staining method was used. First, the treated and untreated bacterial cells were centrifuged at 6,000 rpm for 10 minutes. Next, 10 μL each of acridine orange 15 μg/mL and ethidium bromide 50 μg/mL were added to the bacterial suspension, followed by incubation in the dark for 5 minutes. The bacterial suspension was then centrifuged to remove the unbound dyes, and the pellet obtained was mixed with phosphate-buffered saline. The bacterial suspension was next placed on slides and observed using a fluorescent microscope (DM-2500, Leica Microsystems, Chennai, India).
SEM
Morphological changes in the bacterial cells after interaction with unmodified and modified silk fibers were observed using SEM. The bacterial cultures were centrifuged for 10 minutes at 6,000 rpm and the pellets were washed twice with phosphate-buffered saline. The bacterial suspensions were coated onto a piece of glass (1 cm ×1 cm) and air-dried. The bacterial cells were then fixed using 2.5% glutaraldehyde (HiMedia) for 4 hours, then dehydrated in a series of graded ethanol (30%, 50%, 70%) washes for 2 minutes each. The samples were then coated with gold by sputtering under vacuum in an argon atmosphere. SEM was used to observe the surface morphology of the bacterial cells.
Cytotoxicity test for biocompatibility
3T3 fibroblast cell lines were cultured in Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum and 1% penicillin-streptomycin. The cells were maintained at 37°C, 5% CO2, and 95% relative humidity in a CO2 incubator.
The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide] cytotoxicity assay was carried out on the control and treated silk fibers. Cell viability was measured by MTT assay.22 The 3T3 fibroblasts were seeded at a density of 1×105 cells per well in a 24-well plate containing silk fibers. After 24 and 120 hours of incubation, the medium was removed and the cells were washed with phosphate-buffered saline. The wells were then replaced with MTT (stock concentration 5 mg/mL). The plate was then incubated for 4 hours at 37°C. After incubation, the formazan reaction product was dissolved in solubilizing buffer and absorbance was measured at 570 nm using a microtiter plate reader (Powerwave X2, BioTek Instruments, Winooski, VT, USA). The morphology of cells treated with the silk fibers was viewed under an inverted phase contrast optical microscope (Axiostar II, Carl Zeiss, Oberkochen, Germany).
Results and discussion
Synthesis and characterization of AgNPs
The AgNPs were synthesized using an aqueous extract of R. apiculata leaf. Synthesis and characterization of the AgNPs have been reported in our previous study.20 The results are summarized in Table 1. Polyphenols present in the aqueous extract acted as reducing and stabilizing agents for synthesis and stabilization of AgNPs.
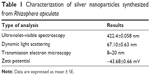  | Table 1 Characterization of silver nanoparticles synthesized from Rhizophora apiculata |
Characterization of functionalized silk fibers
Scanning micrographs of control and AgNP-coated silk fibers are shown in Figure 2. In Figure 2A and D, it can be seen that the control silk fibers have a smooth surface at both lower and higher magnification. Surface scanning of the AgNP-impregnated silk fibers showed the presence of AgNPs on the fibers (Figure 2B, C, E, and F). At higher magnification (Figure 2E and F), a uniform coating of AgNPs is observed on both the in situ and ex situ fibers, with no aggregation of nanoparticles found. The SEM-energy dispersive X-ray spectrum (Figure 2G and H) further confirmed the presence of silver on the silk fibers. The AgNPs impregnated on the silk fibers were spherical in shape and the average particle size was less than 25 nm. The elemental composition (Figure 2G and H) of the silk fibers impregnated in situ was more than that of the silk fibers impregnated ex situ in terms of both weight and atomic percent of silver.
The bioorganic components of R. apiculata, a mangrove plant, would have helped in the binding of AgNPs to the silk fibers. The mangrove plant is rich in polyphenols, with tannin as the major constituent.23 The main property of tannin is its ability to form metal-chelating complexes, which has led to its use in the textile-dyeing industry.24 The hydroxyl group on the polyphenols in the extract served as a binder for chemisorption of AgNPs onto the silk fibers.
X-ray diffraction
X-ray diffraction patterns for the control and silver-impregnated silk fibers are shown in Figure 3. Diffraction peaks at 2θ =20.45° (β-form, Protein Data Bank ID 2SLK) and 2θ =28.8° (α-form, Protein Data Bank ID 1SLK) were observed in both the control and impregnated silk fibers.25 The peaks correspond to the silk fibroin conformations of the β-form (anti-parallel β-pleated sheet) and the α-form (β-turn), respectively.26 The crystalline phase of the silk fibers was not altered by the coating of AgNPs. The silk fibers functionalized with AgNPs displayed a characteristic peak at 2θ =38.15°, which corresponds to the (111) plane of the face-centered cubic structure of the AgNPs.20
  | Figure 3 X-ray diffraction patterns of control, ex situ, and in situ silk fibers. |
Thermogravimetric analysis
Thermal decomposition graphs for the control and impregnated silk fibers are shown in Figure 4. The control silk fiber decomposed in two stages. The initial weight loss was 6.89% at an inflection point of 60.5°C, and was due to evaporation of water. The second stage of decomposition was marked at 250°C–465°C, with a maximum at 313°C and a total mass change of 52.21% observed. The weight loss was due to thermal decomposition of the antiparallel β-sheet structure of fibroin which forms the structural core of silk.6 At 1,000°C, a total mass change of 82.56% was observed with a residual mass of 17.44%. The AgNP-coated silk fibers processed using both ex situ and in situ methods showed increased thermal stability with a mass change of around 35% from 250°C to 465°C. At 1,000°C, the total mass change was only 41%, with a residual mass of 59%. The increased thermal stability of the functionalized silk fibers could be due to the bioorganic component present in the aqueous extract of R. apiculata.
  | Figure 4 Thermogravimetric analysis of control, in situ, and ex situ silk fibers. |
Mechanical properties
The breaking strength of the control and impregnated silk fibers is shown in Table 2. The breaking strength of the control and in situ and ex situ silk fibers was 43.5 N, 52.3 N, and 50.10 N, respectively. The breaking strength was higher for the functionalized silk fibers. This is probably due to adsorption of biofunctionalized AgNPs onto the silk fibers. In a similar report, cotton fibers impregnated with AgNPs showed enhanced mechanical properties due to binding of AgNPs onto the hydroxyl groups of the cellulose chains of cotton fibers.27
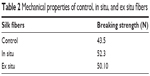  | Table 2 Mechanical properties of control, in situ, and ex situ fibers |
Analysis of initial silver content in the silk fibers
The amount of silver in the silk fibers was determined by atomic absorption spectrophotometry. The silver content in the in situ silk fibers treated with 250 mg/L colloidal solution was 110 mg/kg (Table 3). However, for the ex situ fibers, the silver content decreased to 50 mg/kg. The in situ approach allowed the precursors (the plant extract and AgNO3) to adsorb onto the surface of the silk fibers and facilitated the reduction of Ag+ to Ag0. However, using the ex situ method, the silver colloids are directly adsorbed onto the silk fibers without any involvement of precursors. This indicates that the organic components of the plant extract help in the binding of AgNPs on the silk fibers.
  | Table 3 Initial content of silver in silk fibers |
Bactericidal activity
Table 4 shows the antibacterial activity of AgNP-impregnated silk fibers in P. aeruginosa and S. aureus. The silk fibers impregnated through in situ method showed significantly greater antibacterial activity against both microbial strains than the silk fibers impregnated ex situ. The higher activity of fibers functionalized in situ is due to their greater silver content (Table 4). The control silk fibers did not show any zone of inhibition. According to the standard SNV 195920–1992 antibacterial test, a product is considered to have antibacterial activity when the microbial zone of inhibition is more than 1 mm.27 The in situ and ex situ silk fibers both exhibited a zone of inhibition >2 mm. These findings suggest that functionalized silk fibers can be used for antibacterial purposes.
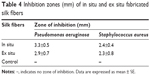  | Table 4 Inhibition zones (mm) of in situ and ex situ fabricated silk fibers |
The bacterial reduction assay was used to investigate the microbicidal activity of the modified silk fibers. Figure 5 shows that the AgNP-functionalized silk fibers had substantial antibacterial activity against P. aeruginosa and S. aureus. The in situ silk fibers demonstrated 99.9% and 95% inhibition against P. aeruginosa and S. aureus, respectively, with the ex situ fibers showing 92% and 90% inhibition against P. aeruginosa and S. aureus, respectively. The antibacterial activity of the in situ silk fibers was significantly higher than that of the ex situ fibers, which is consistent with the zone of inhibition results. In a study reported by De Simone et al silver-coated silk sutures showed 81% inhibition against S. aureus.28 In another report by Dubas et al silver-coated polyamide surgical sutures achieved bacterial reduction of 76%.29 Hence, the antibacterial activity of our silk fibers impregnated with AgNP using in situ and ex situ methods is comparatively greater than that in the previous reports.
The effect of nanoparticles on the permeability of the bacterial cell membrane was studied by fluorescent analysis. The silk fibers modified in situ showed increased antibacterial activity when compared with the silk fibers modified ex situ. Therefore, the silk fibers modified in situ were used for the subsequent antibacterial studies. Control cells appeared green and cells treated with silk fibers in situ appeared orange (Figure 6). Acridine orange dye stains the intact cell and causes the nucleus to appear green (Figure 6A and B), whereas a cell that has lost its membrane integrity takes up ethidium bromide and the nucleus appears to be red.30 P. aeruginosa and S. aureus cells lost their membrane permeability (Figure 6C and D) on treatment with silver-coated silk fibers, resulting in their death.
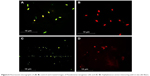  | Figure 6 Fluorescent micrographs of (A, B) control and treated images of Pseudomonas aeruginosa cells and (C, D) Staphylococcus aureus interacting with in situ silk fibers. |
The mode of interaction between AgNP-coated silk fibers and bacteria was studied further by SEM. SEM images of P. aeruginosa and S. aureus treated with unmodified and modified silk fibers are shown in Figure 7. P. aeruginosa and S. aureus cells exposed to unmodified silk fibers showed an intact rod and cocci-shaped morphology (Figure 7A and C). However, the P. aeruginosa cells demonstrated significant membrane damage on treatment with in situ silk fibers (Figure 7B). The damage is indicated by white arrows, which show the components of the bacterial cell seeping out due to complete loss of membrane integrity. Distortion and alteration in cocci-shaped S. aureus cells exposed to in situ silk fibers is seen in Figure 7D.
The antibacterial mechanism postulated by Feng et al,31 ie, the close interaction between AgNPs and the cell membrane alters the membrane permeability, was apparent in our study. SEM (Figure 7) showed that Gram-negative P. aeruginosa cells suffered significantly worse membrane damage on exposure to AgNP-modified silk fibers when compared with Gram-positive S. aureus cells. The greater susceptibility of P. aeruginosa to damage is due to the difference between Gram-negative and Gram-positive bacteria with regard to the peptidoglycan layer of the cell wall.32 The primary function of the peptidoglycan layer is to provide mechanical strength and shape for the bacterium.33 The cell wall of a Gram-negative bacterium contains a thin peptidoglycan layer (5–10 nm) and a lipopolysaccharide layer, both of which lack rigidity and strength,34 whereas the cell wall of a Gram-positive bacterium is primarily composed of a thick peptidoglycan layer (20–80 nm). This layer consists of polymers including teichoic acids, peptidoglycolipids, and polysaccharides that are covalently attached to the peptidoglycan to form a rigid three-dimensional structure.35 This rigidity and cross-linking enhances the structural integrity of the cell, thereby increasing its resistance to the toxicity of the AgNP-impregnated silk fibers. Kim et al reported that the membrane damage caused by AgNPs was more prominent in Escherichia coli than in S. aureus.36 In a similar report by Taglietti et al, glutathione-capped AgNPs had a more limited antibacterial effect on S. aureus than on E. coli. The thick peptidoglycan layer of S. aureus prevented penetration of nanoparticles into the cytoplasm. The cell wall of E. coli was adversely affected due to penetration of the nanoparticles into the cytoplasm, and subsequent interaction with cell wall components further damaged the cell.37 Hence, the difference in toxicity of AgNPs seen between different bacterial species is attributed to the composition of the bacterial cell wall.
Our antibacterial results suggest that silk fibers coated with AgNPs represent a promising biomaterial that can be used in sutures to prevent postoperative wound infections. These infections are a major problem in hospitals, resulting in prolonged in-hospital stays, substantial morbidity, and higher treatment costs. Use of antibacterial biomaterials in health care centers is strongly recommended.
MTT assay for cytotoxicity
The MTT assay was used to investigate the cytotoxicity of the fibers in 3T3 fibroblasts over periods of 24 and 120 hours. The viability of control cells and those treated with AgNP-impregnated silk fibers is shown in Figure 8. Cell viability after 24 hours of incubation with untreated (control) silk fibers and in situ and ex situ silk fibers was found to be 100%, 95%, and 97%, respectively. The fibroblasts demonstrated excellent viability (~90%) even after 5 days of incubation with the AgNP-impregnated silk fibers. The AgNP-impregnated silk fibers did not cause any toxicity to 3T3 fibroblasts. Further, optical micrographs (Figure 9) confirmed that the AgNP-impregnated silk fibers did not cause any distinct morphological changes in the cells after 24 and 120 hours of incubation.
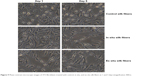  | Figure 9 Phase contrast microscopic images of 3T3 fibroblasts treated with control, in situ, and ex situ silk fibers at 1 and 5 days (magnification 400×). |
The cytotoxicity results demonstrate that both control and AgNP-impregnated silk fibers are biocompatible and do not affect proliferation of fibroblasts. Luangbudnark et al and Liu et al reported that silk fibroin films were not toxic to human dermal and L929 mouse fibroblast cell lines.38,39 In a report by De Simone et al cell viability of 3T3 fibroblasts was 82% on exposure to silver coated silk sutures.28 Silk fibroin coated with AgNP-ciprofloxacin-doped poly-2-hydroxyethyl methacrylate exhibited better cell proliferation for 3T3 fibroblasts,40 and silk nanofibers modified with hydroxyapatite and a lower concentration of AgNPs were not toxic to 3T3 cells.41 The above reports are consistent with the results of our present study. Thus, AgNP-impregnated silk fibers are compatible biomaterials and suitable for clinical application.
Conclusion
The silver nanocolloids produced by simple one-pot green method was used to develop functionalized silk fibers. SEM indicated that AgNPs in the size range of 25 nm were uniformly coated onto in situ and ex situ silk fibers. X-ray diffraction analysis confirmed the crystalline nature of the AgNPs deposited on the silk fibers. Incorporation of AgNPs in the silk fibers enhanced their thermal and mechanical properties. Silver-doped silk fibers showed antibacterial activity against P. aeruginosa and S. aureus. In situ silk fibers had significantly increased antibacterial activity against both microbial strains when compared with the ex situ silk fibers due to their higher silver content. The AgNP-coated silk fibers did not have any toxic effects on fibroblasts, even after 5 days of exposure. The above results suggest that functionalized silk fibers could be useful in the development of novel antibacterial sutures for the prevention of postoperative wound infections.
Acknowledgments
We thank Johnson & Johnson for sponsoring this paper and management at VIT University, Vellore, for providing laboratory facilities to carry out this research. We would also like to acknowledge the Sophisticated Analytical Instrument Facility, Indian Institute of Technology, Chennai, for the SEM-energy-dispersive X-ray spectroscopy and thermogravimetric analysis.
Disclosure
The authors report no conflicts of interest in this work, and declare that they alone are responsible for the content and writing of this paper.
References
Jin H-J, Fridrikh SV, Rutledge GC, Kaplan DL. Electrospinning Bombyx mori silk with poly(ethylene oxide). Biomacromolecules. 2002;3(6): 1233–1239. | ||
Altman GH, Diaz F, Jakuba C, et al. Silk-based biomaterials. Biomaterials. 2003;24(3):401–416. | ||
Cao Y, Wang B. Biodegradation of silk biomaterials. Int J Mol Sci. 2009;10(4):1514–1524. | ||
Zhang H, Magoshi J, Becker M, Chen JY, Matsunaga R. Thermal properties of Bombyx mori silk fibers. J Appl Polym Sci. 2002;86(8): 1817–1820. | ||
Govindaraju K, Tamilselvan S, Kiruthiga V, Singaravelu G. Silver nanotherapy on the viral borne disease of silkworm Bombyx mori L. J Nanopart Res. 2011;13(12):6377–6388. | ||
Elakkiya T, Malarvizhi G, Rajiv S, Natarajan TS. Curcumin loaded electrospun Bombyx mori silk nanofibers for drug delivery. Polym Int. 2014;63(1):100–105. | ||
Bettinger CJ, Cyr KM, Matsumoto A, Langer R, Borenstein JT, Kaplan DL. Silk fibroin microfluidic devices. Adv Mater. 2007;19(19):2847–2850. | ||
Kardestuncer T, McCarthy MB, Karageorgiou V, Kaplan D, Gronowicz G. RGD-tethered silk substrate stimulates the differentiation of human tendon cells. Clin Orthop Relat Res. 2006;448:234–239. | ||
Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1):76–83. | ||
Narayanan KB, Sakthivel N. Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv Colloid Interface Sci. 2011; 169(2):59–79. | ||
Ghosh S, Kaushik R, Nagalakshmi K, et al. Antimicrobial activity of highly stable silver nanoparticles embedded in agar-agar matrix as a thin film. Carbohydr Res. 2010;345(15):2220–2227. | ||
Lara HH, Ayala-Núñez NV, Ixtepan Turrent LdC, Rodríguez Padilla C. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J Microbiol Biotechnol. 2009;26(4):615–621. | ||
Lee HK, Jeong EH, Baek CK, Youk JH. One-step preparation of ultrafine poly(acrylonitrile) fibers containing silver nanoparticles. Mater Lett. 2005;59(23):2977–2970. | ||
Hong KH, Park JL, Sul IH, Youk JH, Kang TJ. Preparation of antimicrobial poly(vinyl alcohol) nanofibers containing silver nanoparticles. J Polym Sci B Polym Phys. 2006;44(17):2468–2474. | ||
Kong H, Jang J. Antibacterial properties of novel poly(methyl methacrylate) nanofiber containing silver nanoparticles. Langmuir. 2008;24 (5):2051–2056. | ||
Zhang Q, Wu D, Qi S, Wu Z, Yang X, Jin R. Preparation of ultrafine polyimide fibers containing silver nanoparticles via in situ technique. Mater Lett. 2007;61(19):4027–4030. | ||
Chang S, Kang B, Dai Y, Chen D. Synthesis of antimicrobial silver nanoparticles on silk fibers via γ-radiation. J Appl Polym Sci. 2009; 112(4):2511–2515. | ||
Fuku K, Hayashi R, Takakura S, Kamegawa T, Mori K, Yamashita H. The synthesis of size- and color-controlled silver nanoparticles by using microwave heating and their enhanced catalytic activity by localized surface plasmon resonance. Angew Chem Int Ed. 2013;52(29):7446–7450. | ||
Hendricks KJ, Burd TA, Anglen JO, Simpson AW, Christensen GD, Gainor BJ. Synergy between Staphylococcus aureus and Pseudomonas aeruginosa in a rat model of complex orthopedic wounds. J Bone Joint Surg Am. 2001;83-A(6):855–861. | ||
Dhas SP, John SP, Mukherjee A, Chandrasekaran N. Autocatalytic growth of biofunctionalized antibacterial silver nanoparticles. Biotechnol Appl Biochem. 2014;61(3):322–332. | ||
Jakopec S, Dubravcic K, Polanc S, Kosmrlj J, Osmak M. Diazene JK-279 induces apoptosis-like cell death in human cervical carcinoma cells. Toxicol In Vitro. 2006;20(2):217–226. | ||
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods.1983;65(1–2):55–63. | ||
Bandaranayake WM. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetlands Ecology and Management. 2002;10(6):421–452. | ||
Higazy A, Hashem M, ElShafei A, Shaker N, Hady MA. Development of anti-microbial jute fabrics via in situ formation of cellulose-tannic acid-metal ion complex. Carbohydr Polym. 2010;79(4): 890–897. | ||
Ma M, Zhong J, Li W, et al. Comparison of four synthetic model peptides to understand the role of modular motifs in the self-assembly of silk fibroin. Soft Matter. 2013;9(47):11325–11333. | ||
Lu Q, Hu X, Wang X, et al. Water-insoluble silk films with silk I structure. Acta Biomater. 2010;6(4):1380–1387. | ||
Ravindra S, Murali Mohan Y, Narayana Reddy N, Mohana Raju K. Fabrication of antibacterial cotton fibres loaded with silver nanoparticles via “green approach”. Colloids Surf A Physicochem Eng Asp. 2010; 367(1–3):31–40. | ||
De Simone S, Gallo AL, Paladini F, Sannino A, Pollini M. Development of silver nano-coatings on silk sutures as a novel approach against surgical infections. J Mater Sci Mater Med. 2014;25(9):2205–2214. | ||
Dubas ST, Wacharanad S, Potiyaraj P. Tuning of the antimicrobial activity of surgical sutures coated with silver nanoparticles. Colloids Surf A Physicochem Eng Asp. 2011;380(1):25–28. | ||
Sugumar S, Nirmala J, Ghosh V, Anjali H, Mukherjee A, Chandrasekaran N. Bio-based nanoemulsion formulation, characterization and antibacterial activity against food-borne pathogens. J Basic Microbiol. 2013;53(8):677–685. | ||
Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52(4):662–668. | ||
Daima HK, Selvakannan PR, Kandjani AE, Shukla R, Bhargava SK, Bansal V. Synergistic influence of polyoxometalate surface corona towards enhancing the antibacterial performance of tyrosine-capped Ag nanoparticles. Nanoscale. 2014;6(2):758–765. | ||
Denyer SP, Maillard JY. Cellular impermeability and uptake of biocides and antibiotics in Gram-negative bacteria. J Appl Microbiol. 2002;92 Suppl 1:35S–45S. | ||
Dror-Ehre A, Mamane H, Belenkova T, Markovich G, Adin A. Silver nanoparticle-E. coli colloidal interaction in water and effect on E. coli survival. J Colloid Interface Sci. 2009;339(2):521–526. | ||
Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. 2007;18(22):225103. | ||
Kim SH, Lee HS, Ryu DS, Choi SJ, Lee DS. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. J Microbiol Biotechnol. 2011;39(1):77–85. | ||
Taglietti A, Diaz Fernandez YA, Amato E, et al. Antibacterial activity of glutathione-coated silver nanoparticles against Gram positive and Gram negative bacteria. Langmuir. 2012;28(21):8140–8148. | ||
Luangbudnark W, Viyoch J, Laupattarakasem W, Surakunprapha P, Laupattarakasem P. Properties and biocompatibility of chitosan and silk fibroin blend films for application in skin tissue engineering. Scientific World Journal. 2012;2012:697201. | ||
Liu TL, Miao JC, Sheng WH, et al. Cytocompatibility of regenerated silk fibroin film: a medical biomaterial applicable to wound healing. J Zhejiang Univ Sci B. 2010;11(1):10–16. | ||
Sastry TP. Silk fibroin coated with poly 2-hydroxyethyl methacrylate impregnated with silver nanoparticles coupled with ciprofloxacin as a biomaterial for biomedical applications. Trends Biomater Artif Organs. 2014;28(4). | ||
Sheikh FA, Woo Ju H, Mi Moon B, et al. Facile and highly efficient approach for the fabrication of multifunctional silk nanofibers containing hydroxyapatite and silver nanoparticles. J Biomed Mater Res A. 2014;102(10):3459–3469. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.