Back to Journals » Vascular Health and Risk Management » Volume 18
Bidirectional Ventricular Tachycardia: Challenges and Solutions
Authors Almarzuqi A, Kimber S, Quadros K, Senaratne J
Received 4 March 2022
Accepted for publication 14 May 2022
Published 7 June 2022 Volume 2022:18 Pages 397—406
DOI https://doi.org/10.2147/VHRM.S274857
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Ahmed Almarzuqi,1 Shane Kimber,1 Kenneth Quadros,1 Janek Senaratne1,2
1Division of Cardiology, Department of Medicine, Mazankowski Alberta Heart Institute, University of Alberta, Edmonton, Canada; 2Department of Critical Care Medicine, University of Alberta, Edmonton, Canada
Correspondence: Janek Senaratne, Tel +1 (780) 463-2184, Fax +1 (780) 450-8359, Email [email protected]
Abstract: Bidirectional ventricular tachycardia (BiVT) is a rare form of ventricular tachycardia that manifests on surface electrocardiogram by dual QRS morphologies alternating on a beat-to-beat basis. It was first reported in the 1920s as a complication of digoxin, and since then, it has been reported in other conditions including fulminant myocarditis, sarcoidosis, catecholaminergic polymorphic ventricular tachycardia, and Andersen-Tawil syndrome. The mechanism for BiVT is not as well known as other forms of ventricular tachycardia but appears to include typical mechanisms including triggered activity from afterdepolarizations, abnormal automaticity, or reentry. This review will go beyond the definition, surface electrocardiogram, mechanisms, causes, and treatment of BiVT as per our current understanding.
Keywords: bidirectional ventricular tachycardia, dual reentry, ventricular arrhythmia, digoxin toxicity, catecholaminergic polymorphic ventricular tachycardia
Introduction
Bidirectional ventricular tachycardia (BiVT) is a rare ventricular tachycardia (VT) where dual QRS morphologies alternate on a beat-to-beat basis. Given its rarity, it is much less well described in the literature compared to other arrhythmias in terms of its typical surface electrocardiogram (ECG) features and mechanisms, as well as defining etiologies. However, though rare, BiVT, unlike other ventricular tachycardias, has a much more limited number of causes, which allows the knowledgeable clinician to tailor both diagnostic investigations and treatment to specific mechanisms. The present review article will go beyond the history and current knowledge of BiVT, including the mechanisms that likely result in its formation as well as the etiologies that provide the substrate to produce BiVT. We will also discuss both general and disease specific targeted therapies for BiVT.
History, Definition and Surface ECG
BiVT is characterized by dual QRS morphologies that alternate on a beat-to-beat basis on the surface ECG (Figures 1–4). It is typically classified as a polymorphic ventricular tachycardia given that there is more than one QRS morphology, but sometimes it is defined as its own entity as a dual morphology ventricular tachycardia. It was first described in 1922 by Schwensen as secondary to digitalis toxicity.1 In 1928, it was described by Palmer and White, who described BiVT as every other beat alternating in cycle length and direction. They attributed BiVT mostly to digitalis toxicity (Figure 2) and severe heart failure.2 The most common type described in the literature is the right bundle branch block (RBBB) morphology with alternating axis.3 However, an alternating RBBB with left bundle branch block (LBBB) morphology and a narrow complex QRS with alternating axis have also been described.4,5
 |
Figure 1 BiVT secondary to fulminant myocarditis. LBBB, ventricular axis is alternating between left axis at −45 degrees and right axis at +90 degrees. |
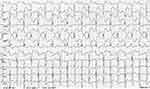 |
Figure 2 From open access figures from Life in the Fast Lane, (CC BY-NC-SA 4.0 license). Digoxin toxicity leading to BiVT with alternating axis between right and left axis. Reproduced from Burns E, Buttner R. Bidirectional Ventricular Tachycardia (BVT). Available from: https://litfl.com/bidirectional-ventricular-tachycardia-bvt-ecg-library/. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.34 |
 |
Figure 3 BiVT, QRS axis is alternating between right and left axis with each beat. Reproduced from Burns E, Buttner R. Bidirectional Ventricular Tachycardia (BVT). Available from: https://litfl.com/bidirectional-ventricular-tachycardia-bvt-ecg-library/. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.34 |
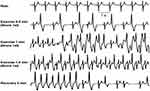 |
Figure 4 BiVT developing following 1 min of exercise in a patient with CPVT, arrhythmia resolved following resting. Reproduced from Burns E, Buttner R. Bidirectional Ventricular Tachycardia (BVT). Available from: https://litfl.com/bidirectional-ventricular-tachycardia-bvt-ecg-library/. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.34 |
BiVT Mechanism
The mechanism of tachyarrhythmias is typically one of the three mechanisms: 1) enhanced automaticity; 2) triggered activity; 3) re-entry. It is thought that more than one mechanism can likely lead to both the generation and perpetuation of BiVT with different mechanisms involved in different etiologies. Notably, BiVT by definition requires two morphologically distinct foci or circuits that alternate and are stable – if they degenerate into more foci or circuits, the BiVT will also degenerate into polymorphic VT or ventricular fibrillation (VF). Enhanced automaticity, triggered activity, and re-entry appear to all be possible primary initiating mechanisms for BiVT. Re-entry appears to be critical in some circumstances for the propagation/continuation of BiVT.
Enhanced automaticity occurs when either a normally firing focus fires at a faster than baseline rate (eg increased sympathetic influence on the sinoatrial node leading to sinus tachycardia), which is known as enhanced normal automaticity or a focus that normally does not activate now depolarizes (eg an idiopathic premature ventricular contraction – PVC) which is known as abnormal automaticity. One mechanism of BiVT, called the “Ping Pong” theory, relates to abnormal automaticity. Baher et al constructed a 2D model of rabbit ventricles and induced premature ventricular contractions at the His fiber by pacing at rates exceeding the action potential threshold thus generating a PVC. This PVC then induced another PVC downstream in the Purkinje system. The PVC at the Purkinje fiber subsequently reciprocated generating another PVC in the His system, thus forming BiVT.6 In another study, optical mapping of a mouse ventricle with catecholaminergic polymorphic VT revealed two foci in the right and left ventricle, which with septal pacing generated a BiVT that originated from a ventricular bigeminy.7 Other studies suggest that dual parasystolic foci due to abnormal automaticity may be possible. Two separate foci fire completely independently with each focus protected by an area of depressed excitability providing a shield of the entrance block to the focus but allowing for the impulse to exit.8 Finally, another study in 1972 by Cohen and his group reported a case of BiVT secondary to digoxin toxicity where they found an ectopic focus in the left-bundle branch that fired with signal alternating exit points through the left anterior fascicle and left posterior fascicle, respectively, yielding bidirectional axis. The underlying mechanism was variation in the refractory period between the two branches.9
Triggered activity occurs when electrical tissue develops a new action potential during the repolarization phase due to electrical instability, therefore creating a new potential right after a depolarization. The triggered activity can occur early during Phase 2 or 3 of the action potential (most commonly seen with torsades de pointes in the setting of prolonged QT syndromes) or delayed during Phase 4 of the action potential. In digitalis toxicity, cytoplasmic calcium overload lengthens the action potential duration, increasing excitability, which can then lead to delayed afterdepolarization (DAD). In catecholaminergic polymorphic VT, the primary electrical issue is an abnormal ryanodine receptor, which also leads to increased cytoplasmic calcium, which again can lead to DADs. This has been modelled in rats by Zhang et al who used dobutamine and caffeine to cause cytoplasmic calcium overload, which then triggered afterdepolarizations leading to PVCs. The study postulated that both dobutamine and caffeine lead to a lower threshold for the ryanodine receptor to release stored calcium from the sarcoplasmic reticulum, leading to further calcium-induced calcium release.10 This mechanism is similar to the ryanodine receptor dysfunction that occurs in catecholaminergic polymorphic VT that causes a calcium overloaded state. Many instances of BiVT occurred in the study with the use of dobutamine and caffeine. The effects of dobutamine and caffeine were attenuated by pre-treatment with dantrolene.
Although triggered activity through DADs can initiate BiVT, some studies suggest that BiVT also requires the presence of re-entry in order to sustain the arrhythmia given the need for two different morphologies of QRS complexes. Re-entry causes tachyarrhythmia by the formation of a circular electrical circuit and typically requires 3 conditions to be met: (a) an area of unidirectional block must exist; (b) the excitation wave propagates along a distinct pathway, returns to its point of origin, and starts again; and (c) interruption of the circuit at any point would terminate this circus movement.11 In the case of BiVT, the DAD can form the initial PVC that initiates the rhythm, but this first PVC may go through a re-entrant circuit, which eventually leads to a secondary excitable focus, which is triggered forming a second PVC, which then conducts back to the site of the primary PVC. This is typical of scar mediated circuits.12
Rarely, re-entry appears to be the primary initiating mechanism of BiVT. Ueda-Tatsumoto et al described a patient with arrhythmogenic right ventricular cardiomyopathy where a macro-reentrant circuit with one critical/common pathway utilized two different exit pathways resulting in BiVT.13 This theory requires a critical/common pathway, two exiting pathways, and a macro-reentrant circuit. The circuit has two exits with different refractorinesses. The impulse first exits at one site giving rise to one VT morphology followed by the electrical activity exiting at a second site leading to the second VT morphology. The electrical activity then returns to the beginning of the critical/common pathway leading to BiVT.
Reported Causes of BiVT
BiVT has been described in the literature in the context of different underlying etiologies including but not limited to digitalis toxicity, catecholaminergic polymorphic ventricular tachycardia (CPVT), acute myocardial ischemia, chronically healed infarcted myocardial tissue, familial hypokalemic periodic paralysis, Andersen-Tawil syndrome, fulminant myocarditis, and exercise induced BiVT. We will discuss each of these underlying etiologies below. Table 1 summarizes the different etiologies, postulated mechanisms, and specific treatments.
 |
Table 1 Summarizing Different Bidirectional Ventricular Tachycardia (BiVT) Causes and Their Proposed Mechanisms and Treatment |
Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT)
CPVT is a rare, but potentially lethal inherited arrhythmia that can present as sudden cardiac death (SCD). It is mostly present in childhood but can also extend into adulthood and requires careful familial genetic assessment to prevent SCD. The classic presentation is exercise-related BiVT, but CPVT can also be presented with other arrhythmias. In a study by Priori et al, 30 patients with known CPVT were studied and had the following arrhythmias: 14 patients had BiVT, 12 had polymorphic VT, and 4 had idiopathic VF.14 Figure 4 illustrates a surface ECG induced by exercise in CPVT.
The classic presentation is arrhythmia evoked by exercise or physical or emotional stress. The genetic mutation that is classically linked to this inherited arrhythmia is a ryanodine receptor mutation. The ryanodine receptor is responsible for calcium-induced calcium release, which releases calcium from the sarcoplasmic reticulum. Malfunction of this receptor leads to calcium overload in the cytoplasm, which can then prolong the action potential and cause triggered activity through DADs, which are the source of the arrhythmias including BiVT.
Treatment of BiVT in CPVT patients is tailored toward the underlying mechanism of CPVT, the use of beta-blockers, flecainide, and calcium channel blockers (eg verapamil) can help reduce the rate of DAD occurrence. Dantrolene (by its effect on preventing calcium leak from the sarcoplasmic reticulum) can also reduce arrhythmia occurrence. Exercise can sometimes provoke arrhythmias in CPVT and so these patients should ideally be counselled on the risks of exercise.
Digoxin Toxicity
Digoxin is well associated with atrial, and ventricular arrhythmias. It can induce atrial and junctional tachyarrhythmias, atrioventricular block, and/or ventricular premature beats. The electrical effect of digoxin stems from two major mechanisms. One is the enhancement of vagal tone at the atrioventricular node leading to slower conduction, which is the basis for its use in supraventricular tachyarrhythmias, such as atrial fibrillation. Secondly, digoxin acts on the Na/Ca pump exchanger in myocytes, which lead to increased calcium influx into the cytoplasm, and Na efflux to the extracellular space. This effect stimulates the sarcoplasmic reticulum to release more Ca into the cytoplasm thus enhancing the contractility of the myocardial cell (increased inotropy). However, in excessive amounts with digoxin toxicity, intracellular calcium overload can potentiate triggered activity through DADs, which can lead to both polymorphic VT and BiVT. Multiple case reports have linked BiVT to digoxin toxicity.1,2,15,16
Digoxin toxicity can be life threatening and should be immediately recognized. Increased PVCs can be a marker of digoxin side effect and potentially evolving arrhythmia.17 If digoxin toxicity is recognized, Digifab should be given. Indications to give Digifab include any life-threatening ventricular arrhythmia, hemodynamically unstable bradyarrhythmia, and ingestion of more than 10 milligrams of digoxin in healthy adults. Digitalis originates from the foxglove plant and rare cases of eating foxglove can cause similar toxicity.18
Acute Myocardial Ischemia
In acute ischemia, multiple electrical and neurohormonal abnormalities occur. Rarely, this can lead to BiVT. In acute ischemia, hypoxia promotes an acidic environment in which K+ will shift extracellularly in the infarcted zone, depleting intracellular K+. This results in net Na+ influx, which then activates the Ca-Na exchanger, which promotes more Ca2+ inside the cell. This increase in intracellular calcium can increase DADs, which can increase the onset of polymorphic VT including BiVT. For BiVT to occur, it is postulated that on top of the DADs, a re-entrant circuit is also required. This is likely due to heterogeneity in the infarcted zone, with both areas of depressed electrical signal propagation but also areas of normal viable electrical tissue that can conduct. El-Sherif et al evaluated a canine model 3–7 days post left anterior descending artery (LAD) ligation where they found that conduction velocities and recovery times differed in different areas of the infarcted zone. The authors found that a reentrant ventricular beat arising from the infarcted zone could travel through different reentrant pathways with different exit points to the normal zone, leading to a ventricular beat with altered QRS morphology, that if sustained, would lead to BiVT, suggesting that subacute ischemia can induce BiVT.19 Wase also reported BiVT in the context of non-ST elevation myocardial infarction (NSTEMI), but no mechanism was postulated.20
In acute myocardial ischemia, the treatment of BIVT is very similar to other forms of ventricular arrhythmia. If the patient is unstable, the Advanced Cardiac Life Support (ACLS) algorithm should be followed by early cardioversion and the consideration of anti-arrhythmic agents, such as amiodarone, lidocaine, and beta-blockers. Early consideration of coronary angiography with revascularization would be expected to reduce the incidence of BiVT due to this mechanism.
Ischemic Cardiomyopathy
Scar formation following myocardial infarction is a well-known cause of ventricular arrhythmia through remodeling, dilation, and eccentric hypertrophy. BiVT has been described by Yeo et al as mediated by scar when a de novo BiVT developed following scar mediated monomorphic VT ablation. They found that following an exit point ablation of RBBB monomorphic VT, a BiVT developed. Their explanation was that the monomorphic VT led to concealed conduction of the exit points of the BiVT generating focus. After ablation of the monomorphic VT, the second focus was able to generate the BiVT by conducting through two different pathways each with different cycle lengths which leads to alternation in the pathways from beat to beat, thus leading to alternating QRS morphology with fixed tachycardia cycle length through re-entry.12
The treatment of BiVT in ischemic heart disease is similar to other forms of ventricular arrhythmia. For stable patients, the use of a beta-blocker or amiodarone may alter the re-entrant circuit refractoriness, thus reducing BiVT incidence. With regard to ablation for BiVT, the only reported case was from the same study by Yeo et al where ablation of the exit point for the de novo BiVT was performed successfully.12
Cardiac Sarcoidosis
BiVT has been implicated in case reports of cardiac sarcoidosis. The postulated mechanism is that the severely inflamed myocardium in sarcoidosis acts as an irritable focus with ionic imbalances leading to generation of arrhythmogenic disturbances including triggered activity from DADs, which can lead to BiVT if it is from two different foci.21 Furthermore, parasystolic foci surrounded by entrance block from inflamed myocardium in sarcoidosis may also form BiVT.8
Durocher et al reported a case of BiVT in a patient with fulminant cardiac sarcoidosis that was diagnosed based on pathology of the explanted heart post-heart transplantation. This patient was initially misdiagnosed as Giant Cell Myocarditis (GCM) based on findings from an endomyocardial biopsy. They concluded that DADs in two different foci were the likely driving factor in the scarred myocardium, which triggered the BiVT. They proposed that the presence of BiVT can be a strong differentiating tool between GCM and sarcoidosis.21
The treatment of BiVT in this special etiology is focused on the treatment of sarcoidosis with the importance of involving a sarcoidosis specialist. The use of immunosuppressants
is the mainstay of treatment. Treatment of sarcoidosis can often alter the substrate that can lead to BiVT reducing its risk for recurrence.
Andersen-Tawil Syndrome (ATS)
ATS is associated with prolongation of the QT interval. It is also known as long QT syndrome (LQTS) type 7 though there is some controversy on this classification.
Zhang et al argued against the classification of ATS as LQTS and described it as a separate channelopathy entity. The argument was that some studies have included the U wave in the QT measurement. Based on their study, the median QTc interval in ATS patients was 440 milliseconds which would preclude it being classified as a long QT.22
ATS includes dysmorphic phenotypic features, ventricular arrhythmias, and periodic paralysis.22,23
ATS is associated with a genetic defect in a protein encoding the inward rectifier current of potassium, which is responsible for the late stage of cellular repolarization. This defect leads to prolonged Phase 3 repolarization, creating an arrhythmogenic substrate that may lead to ventricular arrhythmias. Morita et al conducted a canine model mimicking ATS by injecting canines with Cesium Chloride (CsCl) at different concentrations to block the inward rectifier current of K creating prolonged QT and U development. They found that the transmembrane dispersion was more profound in the context of low serum K with high CsCl concentration. Prolonging this late repolarization phase led to substantial development of triggered activity through DADs. The addition of isoproterenol promoted DADs and VT, whereas verapamil was found to shorten the action potential duration and eliminate the risk of DADs.24
Ventricular arrhythmia in ATS ranges from monomorphic VT to polymorphic VT. Studies found that almost 60% of the ventricular arrhythmias in ATS are polymorphic in nature, and almost one fourth of those actually have BiVT. In the above mentioned study by Zhang et al, 22 out of 96 ATS patients had polymorphic VT and 68% of them had BiVT.22
Treatment of BiVT in the context of ATS is focused on prevention of arrhythmia development, which involves the avoidance of QT prolonging medications or metabolic causes mainly hypokalemia. If the arrhythmia develops, QT shortening medications can be used such as lidocaine. Verapamil can also shorten the QT duration in ATS patients. Exercise can sometimes provoke arrhythmias in ATS and as such these patients should be counselled on the risks associated with exercise.25
Myocarditis
Myocarditis has been associated with BiVT. A patient suffering from prostate cancer, treated with the immune checkpoint inhibitor (ICI) pembrolizumab developed myocarditis related to the ICI. This patient was found to have BiVT. Figure 1 illustrates the surface ECG of this BiVT. Myocarditis can lead to instability of the membrane leading to calcium overload in the cytoplasm leading to triggered activity of DADs.26
The therapeutic approach for BiVT in this specific subtype is targeting the underlying cause of the myocarditis with the consideration of immunosuppression and in this case report, plasmapheresis was used successfully to remove the ICI that was causing the myocarditis.
Familial Hypokalemic Periodic Paralysis
Familial Hypokalemic Periodic Paralysis (FHPP) is an inherited disorder with two third of cases inherited as an autosomal dominant disorder, and the other one third being sporadic. At the cellular level, it involves ionic imbalances with alteration in the intracellular and extracellular potassium levels that can lead to paralysis/weakness. The cardiac involvement with this syndrome is mostly in the form of arrhythmia related to ionic imbalances.27
One case report has described BiVT associated with FHPP. A young patient with FHPP was reported to have an episode of BiVT evident on the surface of ECG. An electrophysiology study was conducted which showed sinoatrial node and atrioventricular node dysfunction with alternating parasystolic ventricular foci behaving as the underlying mechanism of the BiVT.28 The timing of arrhythmias in FHPP is often unrelated to the onset of paralysis.
In FHPP, the primary treatment is oral potassium replacement in awake patients and intravenous replacement in those who cannot take oral medications.
Primary Cardiac Tumors and Cardiac Metastasis
Primary cardiac tumors or cardiac metastasis can alter the cardiac electrical tissue structure and function. BiVT has been described in a male patient who had cardiac metastasis with a 4 mm nodule in the interventricular septum. An electrophysiology study found two ectopic foci in the right bundle branch and the left bundle branch with the proposed mechanism being a single irritable focus in the interventricular septum that had two exit points in the right bundle branch and left bundle branch yielding alternating QRS morphology. Presumably, tumors that are closer to or involve multiple parts of the conduction system are more likely to be able to create BiVT.5
The treatment of the BiVT in this case required surgical treatment of the primary cancer. Drugs such as amiodarone and beta-blockers could be used for medical stabilization while awaiting surgery.
Drug Overdose
Caffeine poisoning was reported by Toya et al as the cause of BiVT in a young patient who ingested caffeine tablets exceeding a total dose of 5 grams of caffeine. The reported mechanism was calcium overload.29 Caffeine is a ryanodine receptor agonist mimicking the proposed mechanism of CPVT inducing BiVT.
Aconitine poisoning has also been reported to cause different ventricular arrhythmias, of which BiVT was one of them, according to Kitamura et al, in which they reported VF, VT, and BiVT in a patient with aconitine poisoning.30 The proposed mechanism was enhanced sodium inward current during the plateau phase of the ventricular depolarization leading to afterdepolarizations.31 Aconitine is still used in some traditional Chinese medicine and herbal products.
The management of both of these overdoses is supportive. Co-ingestions should always be considered.
Coronary Allograft Vasculopathy
Coronary Allograft Vasculopathy (CAV) is a complication following heart transplantation due to rejection and can lead to diffuse coronary artery disease.32 BiVT can be associated with CAV. Unfortunately, the mechanism is not well understood, but it has been proposed that two competing parasystolic foci can lead to alternating LBBB and RBBB.33 Treatment may include alteration of immunosuppression or coronary revascularization.
General Diagnostics and Therapy for the Patient Presenting with BiVT
Although there is much still to be learned about BiVT, the recognition of BiVT on a surface ECG is critical as it informs both diagnostic and therapeutic considerations. BiVT typically is due to a very limited number of etiologies and thus once diagnosed, every attempt should be made to look for the causes listed above. Specifically, digoxin toxicity, CPVT, and ATS are the most common causes of BiVT by far and there should be a low index of suspicion for these conditions in any patient presenting with BiVT. Features that should be elucidated during history taking include a history of physical/emotional/exertional stress for CPVT and weakness/paralysis for FHPP. A thorough medication/toxin history and extensive family history of sudden cardiac death for the inherited causes of BiVT should be completed. Physical examination features are not particularly specific other than the dysmorphic features of ATS. Basic investigations should include both baseline and current 12-lead electrocardiograms and an echocardiogram to look for features of left ventricular dysfunction. After the basic history, physical, and investigations are completed, some patients may need to move on to specialized testings such as cardiac MRI and electrophysiology studies depending on suspected cause.
From a treatment perspective, as with any tachyarrhythmia, the first step in treatment is deciding whether a patient is stable or unstable. In the unstable patient, standard ACLS algorithms should be immediately followed. Once stable, given the short differential diagnosis for etiologies associated with BiVT, therapies targeted at the primary cause are critical in ensuring a good patient outcome. Thus, though rare, BiVT presents an opportunity to tailor therapy to the underlying cause more so than more non-specific rhythms such as VF.
Conclusion
BiVT represents a rare but important type of arrhythmia. Recognition of this rare rhythm is important as it is typically due to a limited set of etiologies, which can help with tailoring diagnostic testing and informing therapy. Given its rarity, it has been a challenge to understand the underlying mechanisms that lead to BiVT. However, several possible mechanisms that cause BiVT include triggered activity from DADs, abnormal automaticity, and dual re-entry. Further study of BiVT will be important going forward for us to fully understand this arrhythmia and further direct therapy. BiVT is an important area for clinicians to be aware of given its association with several clinical conditions including acute ischemia, ischemic cardiomyopathy, digoxin toxicity, myocarditis, CPVT, and myocarditis amongst others. When encountering a patient with BiVT, the aforementioned diagnostic and therapeutic algorithms often allow the clinician to rapidly get to a diagnosis and tailor therapy, which can then lead to better patient outcomes.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Schwensen C. Ventricular tachycardia as the result of the administration of digitalis. Heart. 1922; 9:199–204.
2. Palmer RS, White PD. Paroxysmal ventricular tachycardia with rhythmic alternation in the direction of the ventricular complexes of the electrocardiogram. Am Hear J. 1928;3(4):454. doi:10.1016/S0002-8703(28)90391-9
3. Leenhardt A, Lucet V, Denjoy I, et al. Catecholaminergic polymorphic ventricular tachycardia in children: a 7-year follow-up of 21 patients. Circulation. 1995;91(5):1512–1519. doi:10.1161/01.CIR.91.5.1512
4. Rothfeld EL. Bidirectional tachycardia with normal QRS duration. Am Heart J. 1976;92(2):231–233. doi:10.1016/S0002-8703(76)80259-1
5. Dorfman FK, Mesas CE, Cirenza C, et al. Bidirectional ventricular tachycardia with alternating right and left bundle branch block morphology in a patient with metastatic cardiac tumors. J Cardiovasc Electrophysiol. 2006;17(7):784–785. doi:10.1111/j.1540-8167.2006.00409.x
6. Baher AA, Uy M, Xie F, et al. Bidirectional ventricular tachycardia: ping pong in the His-Purkinje system. Heart Rhythm. 2011;8(4):599–605. doi:10.1016/j.hrthm.2010.11.038
7. Cerrone M, Noujaim SF, Tolkacheva EG, et al. Arrhythmogenic mechanisms in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2007;101(10):1039–1048. doi:10.1161/CIRCRESAHA.107.148064
8. Unusual Incessant SJ. Ventricular tachycardia, what is the underlying cause and the possible mechanism? Circ Arrhythm Electrophysiol. 2015;8(6):1507–1511. doi:10.1161/CIRCEP.115.002886
9. Cohen SI, Deisseroth A, Hecht HS. Infra-His bundle origin of bidirectional tachycardia. Circulation. 1973;47(6):1260–1266. doi:10.1161/01.CIR.47.6.1260
10. Zhang C, Zhang Y. Caffeine and dobutamine challenge induces bidirectional ventricular tachycardia in normal rats. Heart Rhythm O2. 2020;1(5):359–367. doi:10.1016/j.hroo.2020.08.005
11. Mines GR. On dynamic equilibrium in the heart. J Physiol. 1913;46(4-5):349-383. doi:10.1113/jphysiol.1913.sp001596.
12. Yeo C, Green MS, Nair GM, et al. Bidirectional ventricular tachycardia in ischemic cardiomyopathy during ablation. Heart Rhythm Case Rep. 2017;3(11):527–530. doi:10.1016/j.hrcr.2017.08.005
13. Ueda-Tatsumoto A, Sakurada H, Nishizaki M, et al. Bidirectional ventricular tachycardia caused by a reentrant mechanism with left bundle branch block configuration on electrocardiography. Circ J. 2008;72(8):1373–1377. doi:10.1253/circj.72.1373
14. Priori SG, Napolitano C, Memmi M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106(1):69–74. doi:10.1161/01.cir.0000020013.73106.d8
15. Kummer JL, Nair R, Krishnan SC. Images in cardiovascular medicine. Bidirectional ventricular tachycardia caused by digitalis toxicity. Circulation. 2006;113(7):e156–e157. doi:10.1161/CIRCULATIONAHA.105.557561
16. Valent S, Kelly P. Images in clinical medicine. Digoxin-induced bidirectional ventricular tachycardia. N Engl J Med. 1997;336(8):550. doi:10.1056/NEJM199702203360805
17. Bennette AJ, Carter S, Joglar JA, New A. Tachyarrhythmia in a 60-year-old woman. What is the mechanism? Circulation. 2019;140(11):965–968. doi:10.1161/CIRCULATIONAHA.119.042642
18. Norn S, Kruse PR. Hjerteglykosider: fra oldtiden over Witherings digitalis til endogen glykosider [Cardiac glycosides: from ancient history through Withering’s foxglove to endogeneous cardiac glycosides]. Dan Medicinhist Arbog. 2004;2004:119–132.
19. El-Sherif N, Scherlag BJ, Lazzara R, et al. Re-entrant ventricular arrhythmias in the late myocardial infarction period. 1. Conduction characteristics in the infarction zone. Circulation. 1977;55(5):686–702. doi:10.1161/01.cir.55.5.686
20. Wase A, Masood AM, Garikipati NV, Mufti O, Kabir A. Bidirectional ventricular tachycardia with myocardial infarction: a case report with insight on mechanism and treatment. Indian Heart J. 2014;66(4):466–469. doi:10.1016/j.ihj.2014.05.024
21. Durocher D, El-Hajjaji I, Gilani SO, et al. Bidirectional ventricular tachycardia in a patient with fulminant myocarditis secondary to cardiac sarcoidosis mimicking giant cell myocarditis. CJC Open. 2021;3(12):1509–1512. doi:10.1016/j.cjco.2021.07.007
22. Zhang L, Benson DW, Tristani-Firouzi M, et al. Electrocardiographic features in Andersen-Tawil syndrome patients with KCNJ2 mutations: characteristic T-U-wave patterns predict the KCNJ2 genotype. Circulation. 2005;111(21):2720–2726. doi:10.1161/CIRCULATIONAHA.104.472498
23. Tawil R, Ptacek LJ, Pavlakis SG, et al. Andersen’s syndrome: potassium-sensitive periodic paralysis, ventricular ectopy, and dysmorphic features. Ann Neurol. 1994;35(3):326–330. doi:10.1002/ana.410350313
24. Morita H, Zipes DP, Morita ST, et al. Mechanism of U wave and polymorphic ventricular tachycardia in a canine tissue model of Andersen-Tawil syndrome. Cardiovasc Res. 2007;75(3):510–518. doi:10.1016/j.cardiores.2007.04.028
25. Chakraborty P, Kaul B, Mandal K, et al. Bidirectional ventricular tachycardia of unusual etiology. Indian Pacing Electrophysiol J. 2016;15(6):296–299. doi:10.1016/j.ipej.2016.02.007
26. Alhumaid W, Yogasundaram H, Senaratne JM. Slow bidirectional ventricular tachycardia as a manifestation of immune checkpoint inhibitor myocarditis. Eur Heart J. 2021;42(29):2868. doi:10.1093/eurheartj/ehab219
27. Ahlawat SK, Sachdev A. Hypokalemic paralysis. Postgrad Med J. 1999;75(882):193–197. doi:10.1136/pgmj.75.882.193
28. Stubbs WA. Bidirectional ventricular tachycardia in familial hypokalemic periodic paralysis. Proc R Soc Med. 1976;69(3):223–224.
29. Toya N, Isokawa S, Suzuki A, Otani N, Ishimatsu S. Bidirectional ventricular tachycardia induced by caffeine poisoning. Am J Emerg Med. 2019;37(11):
30. Kitamura T, Fukamizu S, Hojo R, et al. Various morphologies of bidirectional ventricular tachycardia caused by aconite “Torikabuto” poisoning. J Cardiol Cases. 2012;7(2):e42–e44. doi:10.1016/j.jccase.2012.10.004
31. Douglas P. Zipes cardiomyopathies induced by drugs or toxins, braunwald’s heart disease: a textbook of cardiovascular medicine. Cardiovasc Med. 2019;80:1631–1640.
32. Pavri BB, O’Nunain SS, Newell JB, et al. Prevalence and prognostic significance of atrial arrhythmias after orthotopic cardiac transplantation. J Am Coll Cardiol. 1995;25(7):1673–1680. doi:10.1016/0735-1097(95)00047-8
33. Bhavnani SP, Clyne CA. Bidirectional ventricular tachycardia due to coronary allograft vasculopathy a unique presentation. Ann Noninvasive Electrocardiol. 2012;17(4):405–408. doi:10.1111/j.1542-474X.2012.00520.x
34. Burns E, Buttner R. Bidirectional Ventricular Tachycardia (BVT). Available from: https://litfl.com/bidirectional-ventricular-tachycardia-bvt-ecg-library/ Accessed May 23, 2022.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
