Back to Journals » OncoTargets and Therapy » Volume 12
β-elemene inhibits radiation and hypoxia-induced macrophages infiltration via Prx-1/NF-κB/HIF-1α signaling pathway
Authors Yu X , Li Z, Zhang Y, Xu M, Che Y, Tian X, Wang R, Zou K, Zou L
Received 4 December 2018
Accepted for publication 4 April 2019
Published 28 May 2019 Volume 2019:12 Pages 4203—4211
DOI https://doi.org/10.2147/OTT.S196910
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Xiaomu Yu,1,* Zongjuan Li,1,* Yang Zhang,2,* Maoyi Xu,1 Yilin Che,1 Xiaoyuan Tian,1 Ruonan Wang,1 Kun Zou,1 Lijuan Zou1
1Department of Radiation Oncology, The Second Affiliated Hospital, Institute of Cancer Stem Cell & The First Affiliated Hospital, Dalian Medical University, Dalian, People’s Republic of China; 2Department of Radiation Oncology, Qingdao University Medical College Affiliated Yantai Yuhuangding Hospital, Yantai, People’s Republic of China
*These authors contributed equally to this work
Background: In cancers, tumor-associated macrophages (TAMs) play an important role in the progression, evasion of immunity and sensitivity to therapy. Unfortunately, radiation and hypoxia could induce the M2 macrophages infiltration and polarization.
Materials and methods: In this study, we investigated the relevance of macrophage recruitment with radiation and hypoxia by transwell. We also evaluated the effect of β-elemene on the infiltration of M2 macrophages and explored its underlying molecular mechanism by a series of in vitro and in vivo experiments.
Results: Irradiated or hypoxia lung cancer cells recruit macrophages, and the recruitment is MCP-1 dependent. We also found that radiation and hypoxia-induced MCP-1 secretion follows upregulation of Prx-1, which leads to nuclear accumulation of NF-κB and HIF-1α expression. In addition, β-elemene could effectively suppress this recruitment phenomenon through Prx-1/NF-κB/HIF-1α signaling.
Conclusion: Our study showed that radiation and hypoxia significantly promoted the macrophages recruitment. β-elemene could effectively suppress this recruitment phenomenon and MCP-1 expression via inhibiting Prx-1/NF-κB/HIF-1α pathways.
Keywords: β-elemene, radiation, hypoxia, macrophages infiltration, MCP-1, lung cancer
Corrigendum for this paper has been published
Introduction
Lung cancer is one of the most common cancers in the world. There were an estimated more than 1.8 million new cases, with almost 1.6 million deaths in 2012.1 Though tumor therapy has been developing rapidly these years, the 5-year survival in patients with lung cancer is still poor in many countries and representing little global variation.2 Currently, therapeutic strategies of lung cancer are inadequacy, and patients often present with locally advanced or metastatic disease.
Macrophages are an important component of innate immunity and exert a crucial role in inflammation and the body’s defense system by acting as the first line of resistance against microorganisms.3 Two distinct TAM phenotypes, M1 or M2, have been described with the abilities to inhibit or promote tumor growth, respectively. The M1 phenotype is proinflammatory and has high levels of iNOS production; the M2 phenotype is anti-inflammatory, pro-angiogenic, metastasis-promoting, and has high levels of Arg I production.4,5 Many studies have found that TAMs frequently exhibit an M2 phenotype, they support angiogenesis and tumor invasion, suppress antitumor immune responses, and may result in resistance to tumor therapies.6 Macrophages infiltration into the tumor microenvironment is chemokines and cytokines driven. Among these many chemokines and cytokines, monocyte chemoattractant protein-1/chemokine (C-C motif) ligand 2 (MCP-1/CCL2), a member of the CC chemokines subfamily, is the most important that regulates the migration and infiltration of macrophages. Its expression positively correlates with TAMs abundance in various types of cancer.7–9 Hypoxia is an important prognostic indicator of poor tumor treatment outcome after radio- and chemotherapy. Hypoxia-induced release of chemoattractants results in enhanced TAM recruitment, which further accumulates the macrophages and amplifies the protumoral response.10 Radiation therapy is one of the major therapeutic modalities for most solid tumors and is one of the major treatments for lung cancer. Radiation also disrupts the tumor vasculature aggravated tumor hypoxia and accelerated the infiltration of macrophages.11
β-elemene (1-methyl-1-vinyl-2,4-diisopropenyl-cyclohexane C15H24) is extracted from the Chinese medicine herb Curcuma Wenyujin. Its anti-tumor activity has been proven in broad range of solid tumors with little toxicity.12–14 β-elemene is also confirmed as a radiosensitizer in recent years. It sensitized the lung cancer cell by enhancing DNA damage and inhibiting DNA repair, or by increasing apoptosis in radiation.15,16 In our study, we detected the influence of radiation and hypoxia on the secretion of MCP-1 and M2 macrophages recruitment. In addition, we investigated the immunization sensitization effect of β-elemene and its underlying molecular mechanisms. The findings from this study suggested that irradiation and hypoxia induced the macrophages infiltration by promoting the secretion of MCP-1 via Prx-1/NF-κB/HIF-1α signaling pathway. β-elemene as a radiosensitizer could effectively inhibit the activation of Prx-1/NF-κB/HIF-1α pathway which stimulated by radiation and hypoxia. The macrophages infiltration after radiation and hypoxia could be an attractive target for anti-cancer therapy, and β-elemene may play a more important role in immune sensitization.
Materials and methods
Reagents and antibodies
β-elemene was obtained from the National Institutes for Food and Drug Control (NIFDC; Beijing, China). Polyclonal antibodies Prx-1, monoclonal antibodies HIF-1α were obtained from Abcam (Cambridge, MA, USA). Monoclonal antibody p65 was obtained from Cell Signaling Technology (Danvers, MA, USA). Monoclonal antibody F/80 was obtained from Santa Cruz (Santa Cruz, CA, USA). Polyclonal antibody MCP-1 was obtained from R&D systems (Minneapolis, MN, USA). Polyclonal antibody Histone-H3, monoclonal antibodies β-actin, MCP-1 were obtained from ProteinTech (Chicago, IL, USA). Fluorescein (FITC)–conjugated Goat Anti-Mouse IgG(H+L) antibody was obtained from ProteinTech (Chicago, IL, USA).
Cell culture
Mouse Lewis lung carcinoma cells were obtained from American Type Culture Collection. Mouse RAW264.7 macrophages were obtained from Cell Bank of Chinese Academy of Sciences (Shanghai, China). Lewis cells and RAW264.7 cells were cultured in DMEM medium, supplemented with and 10% fetal bovine serum (Gibco, USA). Cells were incubated at 37°C in a humidified incubator containing 5% CO2. For hypoxia exposure, cells were placed in a hypoxia chamber that maintained a low oxygen tension (1% O2, 5% CO2, and 94% N2). Cell irradiation was performed using X-RAD320ix (Precision X-Ray, North Branford, CT, USA) with a dose of 4 Gy at room temperature.
Macrophage chemotaxis assay
The culture supernatants of Lewis cells (conditioned medium, CM) were harvested after different treatments. CM was centrifuged to remove any cell debris and stored in −20°C until used in assays. RAW264.7 cells were suspended in serum-free medium and 2×104 cells were added to the upper chambers of Transwell system (24-wells, Corning Costar). The lower chamber contained 600 μL CM. After 12-hr incubation at 37°C, cells migrated through the polycarbonate membrane were fixed with methanol and stained with Crystal Violet. The stained cells were subsequently photographed and counted (magnification, ×100, Olympus, Tokyo, Japan).
Western blot
Cells were collected and lysed with mammalian protein extraction buffer (CWBIO, China) supplemented with a protease inhibitor cocktail (Hoffman-La Roche, Basel, Switzerland) and then centrifuged at 12,000 g, 4°C for 15 mins to obtain the supernatant. Cell lysates containing 30 µg protein were heated for 10 mins at 100°C, separated by 10–12% SDS-PAGE, and transferred onto polyvinylidene difluoride membranes (Immobilon-P; Millipore, Billerica, MA, USA). After incubation in 5% BSA–0.1% Tween 20 in TBS (TBST) at room temperature for 2 hrs to block aspecific binding, the membranes were incubated with primary antibodies (1:1,000) in 0.1% TBST at 4°C overnight. Next, these membranes were washed three times with TBST and incubated with the secondary antibody (1:5,000) for 2 hrs at room temperature. The membranes were washed three times with TBST and visualized by enhanced chemiluminescence using a SuperSignal West Pico trial kit (Thermo Fisher Scientific).
RT-qPCR assays
Total RNA was extracted by Trizol reagent (Invitrogen) and the cDNA was synthesized by PrimeScript RT Reagent Kit (TaKaRa Bio) according to the manufacturer’s instructions. The primers were synthesized by Invitrogen (Carlsbad). The forward and reverse primers for HIF-1α are 5ʹ-ACCTTCATCGGAAACTCCAAAG-3ʹ and 5ʹ-CT-GTTAGGCTGGGAAAAGTTAGG-3ʹ, respectively. The forward and reverse primers for p65 are 5ʹ-ACTGCCGGGATGGCTACTAT-3ʹ and 5ʹ-TCTGGATTC-GCTGGCTAATGG-3ʹ, respectively. The forward and reverse primers for Prx-1 are 5ʹ-AATGCAAAAATT-GGGTATCCTGC-3ʹ and 5ʹ-CGTGGGACACACAAAA-GTAAAGT-3ʹ, respectively. The forward and reverse primers for MCP-1 are 5ʹ-TAAAAACCTGGATCGGAA-CCAAA-3ʹ and 5ʹ-GCATTAGCTTCAGATTTACGGGT-3ʹ, respectively. The forward and reverse primers forβ-actin are 5ʹ-GGCTGTATTCCCCTCCATCG-3ʹ and 5ʹ-CC-AGTTGGTAACAATGCCATGT-3ʹ, respectively.
Transfection of siRNA
Transient transfection was performed by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturers’ protocols. Prx-1 siRNA (5ʹ-CCAGUUCACUGACAAACAUdTdT-3ʹ), p65 siRNA (5ʹ-GGAGUACCCUGAAGCUAUAdTdT-3ʹ), HIF-1α siRNA (5ʹ-CUGAUAACGUGAACAAAUAdTdT-3ʹ) and control siRNA (5ʹ-UUCUCCGAACGUGUCACGUdTdT-3ʹ) were synthesized by Gemagene (Suzhou, Jiangsu, China).
Immunohistochemistry analysis
Tumors were fixed in formalin overnight before paraffin embedding. Small tissues were embedded in paraffin for sectioning, incised to 6 μm thick, and stained with hematoxylin and eosin (H&E). Immunohistochemistry (IHC) was performed using the DAB (3,3ʹ-diaminobenzidine) Kit (Origene, China).
Ethics statements
The approval for use of animals in research was performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA) and approved by the Animal Care and Use Committee for Health Sciences of Dalian Medical University. All animal experiments were carried out according to the Animal Care Guidelines of Dalian Medical University. The animals were housed under specified-pathogen-free conditions that meet international standards; they were regularly checked by the certified veterinarian responsible for health monitoring, animal welfare supervision, experimental protocols, and procedure revision. At the time of sacrifice, they were euthanized by cervical dislocation. All efforts were made to reduce the number of animals used and to minimize the animals’ suffering.
Animal studies
Female C57BL/6 mice were purchased from SPF Laboratory Animal Center at Dalian Medical University. Lewis cells (1×106) were inoculated subcutaneously into the flank of the mice. When the tumor diameters reached 4 mm to 5 mm, mice were randomly distributed into 4 groups: Control group, Irradiation group, β-elemene group, irradiation plus β-elemene group. Mice were irradiated using an X-RAD 320 irradiator (Precision X-ray, CT, USA) once every two days for four times in irradiation group. A total of 45 mg/kg β-elemene was injected intraperitoneally once every two days for four times in β-elemene group. In irradiation plus β-elemene group, the mice were irradiated at 12 hrs after injecting intraperitoneally with β-elemene once every 2 days for 4 times. Mice received 4 Gy X-ray irradiation at a rate of 1.32 Gy/min with 320 keV (peak), 6 mA, filtered with 2 mm Al. Tumor volume was calculated as V=1/2 (width2×length).
Statistical analysis
All experiments were repeated three times. Data are presented as the mean±standard deviation (SD). Statistical analysis was performed by using ANOVA following by a multiple comparison test for multiple experimental groups. P<0.05 was considered statistically significant.
Results
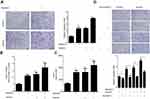
Radiation and hypoxia recruit macrophages accumulation through inducing MCP-1 expression
To determine the influence of irradiation and hypoxia on macrophage recruitment, we examined the effect of conditioned medium from Lewis cells treated with or without radiation and hypoxia on the migration of murine macrophage RAW 264.7 cells. As shown in Figure 1A, conditioned medium from radiation, hypoxia or radiation combine with hypoxia group significantly promote RAW 264.7 cell migration compared to that the medium from untreated cells. Monocyte chemoattractant protein-1 (MCP-1) is considered to be one important chemokine regulating migration and infiltration of macrophages. We found an up-regulation of MCP-1 mRNA level in Lewis cells that treated with irradiation (4 Gy) and hypoxia (Figure 1B). Furthermore, we analyzed the supernatant obtained from Lewis cells treated with radiation and hypoxia. Similarly, MCP-1 protein secretion was significantly enhanced after treatment with both radiation and hypoxia (Figure 1C). To further confirm the effect of MCP-1, MCP-1 neutralizing antibody was used. As shown in Figure 1D, the migration of RAW 264.7 cells induced by radiation, hypoxia, or combination were all significantly decreased by MCP-1 neutralizing antibody. These results suggested radiation and hypoxia promote lung cancer cells to recruit more macrophages accumulation by inducing MCP-1 secretion from lung cancer cells.
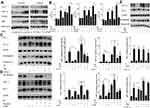
Prx-1, NF-κB, and HIF-1α are involved in radiation and hypoxia-induced expression of MCP-1
Prx-1 is a member of the peroxidases family and involved in cellular response to oxidative stress, it also involved in the activation of various signaling pathways. Elevated Prx-1 expression is associated with diminished overall survival and poor clinical outcome. Induction of Prx-1 upon radiation treatment has been reported earlier and is considered to be one of the resistance mechanisms for radiotherapy. In our study, we found that Prx-1protein levels are increased within 12 hrs of radiation treatment and continue to rise up to 24 hrs of treatment. However, the Prx-1 expression is not changed after hypoxia only. In hypoxia and radiation environment, Prx-1 expression increased with time again (Figure 2A). When treated with siPrx-1 to radiation cells, the expression of MCP-1 was abolished which was induced by radiation, but no obvious suppression which was induced by hypoxia (Figure 2B). Radiation and hypoxia all have been reported to modulate the activity of transcription factor NF-κB and HIF-1α. These transcription factors have also been reported in MCP-1 regulation in different cancer types. To investigate whether NF-κB and HIF-1α mediate radiation and hypoxia induced up-regulation of MCP-1, we monitored the change in their expression following radiation and hypoxia. The data demonstrate that radiation and hypoxia all cause a remarkable and time-dependent increase in nuclear levels of NF-κB/p65 and HIF-1α expression (Figure 2A). Then, we silenced the expression of p65 or HIF-1α through RNA interference in Lewis cells prior to the treatment with radiation and hypoxia. Our data demonstrate that suppression of NF-κB/p65 or HIF-1α led to the abrogation of radiation and hypoxia-induced MCP-1 expression (Figure 2B). These data suggested that Prx-1 participates in the regulation of MCP-1 expression under radiation, not hypoxia condition. NF-κB and HIF-1α were involved in MCP-1 expression induced by radiation and hypoxia both.
The regulation between Prx-1, NF-κB, and HIF-1α in radiation and hypoxia
Prx-1 is reported to regulate NF-κB activation in lung cancer cells and bladder cancer cells.17 Moreover, numerous studies suggested a direct relationship between NF-κB and HIF-1α. To understand whether there was a hierarchy or cross talk among Prx-1, NF-κB, and HIF-1α, we treated the Lewis cells with specific siRNA prior to stimulation with radiation or hypoxia, and examined the effect of radiation or hypoxia on Prx-1, NF-κB, and HIF-1 expression. The data clearly demonstrate that pretreatment with Prx-1 siRNA abrogates radiation-induced activation of both NF-κB/p65 and HIF-1α; however, hypoxia-induced activation of NF-κB/p65 and HIF-1α is not affected (Figure 2C). We then observed that the inhibition of NF-κB could significantly reduce the expression of HIF-1α which induced by radiation but had no effect on the expression of Prx-1 (Figure 2D). And hypoxia-induced HIF-1α expression is also abrogated by NF-κB/p65siRNA. Furthermore, HIF-1α siRNA decreased its own protein level, but none of the other proteins was affected in radiation or hypoxia (Figure 2E). These results show that radiation activates Prx-1-NF-κB-HIF-1α sequentially in hierarchy, hypoxia also activates NF-κB-HIF-1α by some other factors.
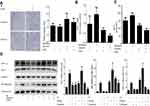
β-elemene inhibits radiation and hypoxia-induced macrophages infiltration via inhibiting Prx-1/NF-κB/HIF-1α pathway
β-elemene was clinically used as a radiosensitizer or chemosensitizer. In our study, we found that β-elemene could significantly inhibit the macrophages infiltration induced by radiation and hypoxia (Figure 3A). Furthermore, we analyzed the levels of MCP-1 mRNA and protein in the Lewis cells and found that β-elemene could block the transcription and expression of recruitment factor MCP-1 (Figure 3B and C). Next, we further examine the effect of β-elemene on Prx-1, NF-κB/p65, and HIF-1α expression induced by radiation and hypoxia in the Lewis cells. Our data showed that β-elemene inhibited the expression of Prx-1 and HIF-1α which induced by radiation. And it also inhibits the NF-κB/p65 translocation induced by radiation and hypoxia (Figure 3D). All the results above collectively proved that β-elemene significantly inhibited macrophages infiltration and MCP-1 secretion induced by radiation and hypoxia via inhibiting Prx-1/NF-κB/HIF-1α pathway.
β-elemene enhanced the radiosensitivity of lung cancer and inhibit the infiltration of macrophages in vivo
To further examine the effect of β-elemene on lung cancer in vivo, we formed tumor xenografts by injecting Lewis cells into C57BL/6 mice. The mice bearing tumors were treated with β-elemene, radiation or β-elemene combined radiation. As shown in Figure 4A and B, the growth of tumor xenografts was slower in the β-elemene group and radiation group compared to the control group. It suggested that β-elemene and radiation could suppress the growth of lung cancer. The growth of tumor xenografts in β-elemene combined radiation group was the slowest of the four groups, and it was slower than that of radiation group. The similar trends were also seen in the tumor weight (Figure 4C). Moreover, the tumor volume doubling times of each group were obtained to calculate the enhancement factor (EF), and the EF is 1.65. It confirms β-elemene had a radiosensitive effect on lung cancer in vivo. F4/80 is thought to be a marker of macrophages.18 Then we further analysis the tumor samples by IHC, confirmed that macrophages infiltration increased in radiation group, and it could be reduction after combing with β-elemene.19 The expression of MCP-1 was also increased in radiation group, and β-elemene could inhibit the expression of MCP-1 induced by radiation (Figure 4D). Collectively, our data strongly suggest that β-elemene enhanced the radiosensitivity of lung cancer, inhibit the MCP-1 expression and macrophage infiltration induced by radiation in vivo.
Discussion
Radiotherapy (RT) is currently used in more than 50% of the cancer patients during the course of their disease in the curative, adjuvant, or palliative setting. Improvements in long-term radiotherapy success could be made not only through further enhancing tumor cell kill and local control but also through preventing any radiotherapy-induced metastatic mechanisms.20,21 Many researchers also reported that IR and hypoxia could promote the infiltration of M2 macrophages, which was reported to promote tumor growth, survival, and may result in resistance to tumor therapies.22,23 Most of these studies are about the effect of stromal cells on recruiting macrophages, but few parts of the studies focus on the recruitment effect of tumor cells, and there is no research about the effect of irradiated or hypoxia lung cancer cells on macrophages infiltration.
In our study, we found that hypoxia and radiation could activate the NF-κB/HIF-1α pathway leading to the secretion of the recruitment factor MCP-1 by lung cancer cells. M2 macrophage infiltration increased the metastasis ability of lung cancer cells. Previous studies showed that Prx-1 is elevated in some cancers and it is correlated with the tumor prognosis. It protects cancer from cancer therapy, such as cancer drugs, ionizing radiation.24–26 Many researchers have suggested that Prx-1 can serve as a target molecule for the improvement of anticancer activity.27 We further demonstrated that radiation could promote the secretion of recruitment factor MCP-1 through activation of Prx-1/NF-κB/HIF-1α pathway, but hypoxia was not Prx-1 depended. It deserves our further investigation whether there are any other factors participated in the M2 macrophage infiltration in hypoxic environment. Our results provide additional support for earlier findings where researchers showed a negative role of Prx-1 in tumor therapy.
Previous studies have suggested that β-elemene has antitumor effects in many types of tumors with low toxicity and has been widely used in China. In our study, we discovered β-elemene could inhibit MCP-1 secretion and macrophages recruitment which induced by radiation and hypoxia in lung cancer. Our results showed a new anti-tumor target of β-elemene. And the molecular mechanism of β-elemene acts on MCP-1 expression is probably by inhibiting Prx-1/NF-κB/HIF-1α axis (Figure 4E). If combine β-elemene with radiotherapy, it will improve the therapeutic outcomes by affecting both cancer cells and macrophages. The study also implies the importance of tumor microenvironment. We should focus on both cancer cells and the whole environment they stay in, and take synergistic therapy strategies.
In summary, our research has demonstrated that radiation or hypoxia activated the Prx-1-NF-κB-HIF-1α axis to promote MCP-1 secretion and macrophages recruitment, β-elemene could inhibit the axis so that inhibit the infiltration of macrophages.
Acknowledgments
This work was supported by the funds from the National Natural Science Foundation of China (81703904 KZ, 81473452 LZ).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
2. Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385(9972):977–1010. doi:10.1016/S0140-6736(14)62038-9
3. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi:10.1038/nri3175
4. Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33(3):119–126. doi:10.1016/j.it.2011.12.001
5. De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23(3):277–286. doi:10.1016/j.ccr.2013.02.013
6. Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196(3):254–265. doi:10.1002/path.1027
7. Arenberg DA, Keane MP, DiGiovine B, et al. Macrophage infiltration in human non-small-cell lung cancer: the role of CC chemokines. Cancer Immunol Immunother. 2000;49(2):63–70.
8. Negus RP, Stamp GW, Relf MG, et al. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest. 1995;95(5):2391–2396. doi:10.1172/JCI117933
9. Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi:10.1038/nature10138
10. Murdoch C, Lewis CE. Macrophage migration and gene expression in response to tumor hypoxia. Int J Cancer. 2005;117(5):701–708. doi:10.1002/ijc.21422
11. Chiang CS, Fu SY, Wang SC, et al. Irradiation promotes an m2 macrophage phenotype in tumor hypoxia. Front Oncol. 2012;2:89. doi:10.3389/fonc.2012.00089
12. Zhao S, Wu J, Zheng F, et al. Beta-elemene inhibited expression of DNA methyltransferase 1 through activation of ERK1/2 and AMPKalpha signalling pathways in human lung cancer cells: the role of Sp1. J Cell Mol Med. 2015;19(3):630–641. doi:10.1111/jcmm.12476
13. Dai ZJ, Tang W, Lu WF, et al. Antiproliferative and apoptotic effects of beta-elemene on human hepatoma HepG2 cells. Cancer Cell Int. 2013;13(1):27. doi:10.1186/1475-2867-13-27
14. Zhang H, Xu F, Xie T, Jin H, Shi L. beta-elemene induces glioma cell apoptosis by downregulating survivin and its interaction with hepatitis B X-interacting protein. Oncol Rep. 2012;28(6):2083–2090. doi:10.3892/or.2012.2022
15. Li LJ, Zhong LF, Jiang LP, Geng CY, Zou LJ. Beta-Elemene radiosensitizes lung cancer A549 cells by enhancing DNA damage and inhibiting DNA repair. Phytother Res. 2011;25(7):1095–1097. doi:10.1002/ptr.3367
16. Liu JS, Che XM, Chang S, et al. Beta-elemene enhances the radiosensitivity of gastric cancer cells by inhibiting Pak1 activation. World J Gastroenterol. 2015;21(34):9945–9956. doi:10.3748/wjg.v21.i34.9945
17. Liu DL, Zhao LX, Zhang S, Du JR. Peroxiredoxin 1-mediated activation of TLR4/NF-kappaB pathway contributes to neuroinflammatory injury in intracerebral hemorrhage. Int Immunopharmacol. 2016;41:82–89. doi:10.1016/j.intimp.2016.10.025
18. Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11(10):805–815. doi:10.1002/eji.1830111013
19. van den Berg TK, Kraal G. A function for the macrophage F4/80 molecule in tolerance induction. Trends Immunol. 2005;26(10):506–509. doi:10.1016/j.it.2005.07.008
20. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16(13):e498–e509. doi:10.1016/S1470-2045(15)00007-8
21. Blyth BJ, Cole AJ, MacManus MP, Martin OA. Radiation therapy-induced metastasis: radiobiology and clinical implications. Clin Exp Metastasis. 2018;35(4):223–236. doi:10.1007/s10585-017-9867-5
22. Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–237. doi:10.1016/j.coi.2010.01.009
23. Wu Q, Allouch A, Martins I, Modjtahedi N, Deutsch E, Perfettini JL. Macrophage biology plays a central role during ionizing radiation-elicited tumor response. Biomed J. 2017;40(4):200–211. doi:10.1016/j.bj.2017.06.003
24. Lehtonen ST, Svensk AM, Soini Y, et al. Peroxiredoxins, a novel protein family in lung cancer. Int J Cancer. 2004;111(4):514–521. doi:10.1002/ijc.20294
25. Joshi G, Aluise CD, Cole MP, et al. Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: implications for oxidative stress-mediated chemobrain. Neuroscience. 2010;166(3):796–807. doi:10.1016/j.neuroscience.2010.01.021
26. Kim YJ, Lee WS, Ip C, Chae HZ, Park EM, Park YM. Prx1 suppresses radiation-induced c-Jun NH2-terminal kinase signaling in lung cancer cells through interaction with the glutathione S-transferase Pi/c-Jun NH2-terminal kinase complex. Cancer Res. 2006;66(14):7136–7142. doi:10.1158/0008-5472.CAN-05-4446
27. Moore LB, Sawyer AJ, Charokopos A, Skokos EA, Kyriakides TR. Loss of monocyte chemoattractant protein-1 alters macrophage polarization and reduces NFkappaB activation in the foreign body response. Acta Biomater. 2015;11:37–47. doi:10.1016/j.actbio.2014.09.022
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.