Back to Journals » Clinical Interventions in Aging » Volume 11
Benefits of use, and tolerance of, medium-chain triglyceride medical food in the management of Japanese patients with Alzheimer’s disease: a prospective, open-label pilot study
Authors Ohnuma T, Toda A, Kimoto A, Takebayashi Y, Higashiyama R, Tagata Y, Ito M, Ota T, Shibata N, Arai H
Received 28 August 2015
Accepted for publication 6 October 2015
Published 8 January 2016 Volume 2016:11 Pages 29—36
DOI https://doi.org/10.2147/CIA.S95362
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Tohru Ohnuma, Aiko Toda, Ayako Kimoto, Yuto Takebayashi, Ryoko Higashiyama, Yuko Tagata, Masanobu Ito, Tsuneyoshi Ota, Nobuto Shibata, Heii Arai
Department of Psychiatry, Juntendo University Alzheimer’s Disease Project, Faculty of Medicine, Juntendo University, Tokyo, Japan
Objectives: This is the first clinical trial of this type in Japan, designed to analyze two important aspects of Alzheimer’s disease (AD) management using medium-chain triglycerides. Axona was administered for 3 months (40 g of powder containing 20 g of caprylic triglycerides). We used an indurating, four-step dose-titration method (from 10 to 40 g per day) for 7 days before the trial, and examined the tolerance and adverse effects of this intervention. We also investigated its effect on cognitive function in mild-to-moderate AD patients.
Patients and methods: This was a clinical intervention in 22 Japanese patients with sporadic AD at a mild-to-moderate stage (ten females, 12 males), mean age (± standard deviation) 63.9 (±8.5) years, Mini-Mental State Examination (MMSE) score, 10–25, seven patients were ApoE4-positive. During Axona administration, we examined changes in cognitive function by obtaining MMSE and AD assessment-scale scores. Intolerance and serum ketone concentrations were also examined.
Results: The tolerance of Axona was good, without severe gastrointestinal adverse effects. Axona did not improve cognitive function in our sample of AD patients, even in those patients without the ApoE4 allele. However, some ApoE4-negative patients with baseline MMSE score ≥14 showed improvement in their cognitive functions.
Conclusion: The modified dose-titration method, starting with a low dose of Axona, decreased gastrointestinal adverse effects in Japanese patients. Axona might be effective for some relatively mildly affected patients with AD (with cognitive function MMSE score of ≥14 and lacking the ApoE4 allele).
Keywords: Alzheimer’s disease, medium-chain triglycerides, ketone, cognitive function, apolipoprotein E epsilon 4
Introduction
Alzheimer’s disease (AD) is a multifactorial disease, and its development is associated with accumulation of multiple risk factors, such as genetic, nutritional, and lifestyle factors and physical health status.1,2 Elimination of these risk factors might help to prevent the onset and progression of AD. Ingesting such food-containing substances as omega-3 fatty acids, medium-chain triglycerides (MCTs), polyphenol, curcumin, antioxidants, or vitamin B6 might be beneficial.3–8
Three types of medical foods potentially benefiting the pathophysiology and symptoms of AD have been marketed in the US and Europe: Axona®,9 Souvenaid®,10 and CerefolinNAC®.11 Axona is a medical food containing ingredients potentially active against AD. MCTs, particularly caprylic acid (C8) (40 g of Axona contains 20 g of C8), are metabolized to ketone bodies (eg, β-hydroxybutyrate). Ketones, similar to glucose, are an energy source for the neurons. Therefore, the administration of Axona as medical food might be one of the alternative strategies, replacing glucose as an energy source. Reduced cerebral glucose utilization is one of the pathophysiological factors in AD.12 A decrease in glucose utilization has been shown in patients with AD, particularly in those with a genetic risk factor – the ApoE4 allele.13–15 Administration of Axona to patients without ApoE4 in the mild-to-moderate stages of AD and/or memory-impaired patients results in improvements in cognitive function.7–9,16,17 A large study of 152 patients with mild-to-moderate AD has shown such improvements. In that study, the patients were divided into four groups: those with and those without the ApoE4 allele and taking Axona (with an intake rate over 80%) or placebo. The patients without ApoE4, with an intake rate of Axona for more than 80% per day, showed the largest improvement in their cognitive function (over 5 points) in comparison with similar patients taking placebo. Two weeks after Axona was stopped, cognitive scores began to decrease in these patients. This phenomenon was likely caused by the ketones derived from the MCT in Axona. These ketones might provide the necessary daily energy for the neurons.16 However, the high content of MCT in Axona is associated with relatively high frequencies of gastrointestinal intolerance, such as diarrhea (24.4%), bloating (17.4%), and indigestion (9.3%).16 Because the Japanese diet differs from the typical Western diet, it is important to investigate gastrointestinal intolerance in Japanese patients. The Japanese are not normally exposed to a high-fat diet; therefore, administration of Axona often causes diarrhea.
The objectives of the present study were to investigate tolerance of the usual dose of Axona and its effect on cognitive function in Japanese patients with mild-to-moderate AD.
Patients and methods
Study participants
Japanese patients in mild-to-moderate stage of sporadic AD were enrolled at the Department of Psychiatry, Juntendo University Hospital, Tokyo, Japan. The severity of AD was estimated by the Mini-Mental State Examination (MMSE),18 and scores from 10 to 26 were obtained. All the AD cases were diagnosed according to the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria,19 and none had a familial history of AD. None of the patients was diagnosed with mixed, atypical, or other types of dementia. Exclusion criteria for the present study were 1) severe diabetes mellitus or ketoacidosis and 2) diarrhea before taking Axona.
Written informed consent was obtained from all participants after the procedures had been fully explained to each patient and his or her family. The study was carried out in compliance with the World Medical Association’s Declaration of Helsinki, and was approved by the ethics committee of Juntendo Hospital (25577) and the ethics committee of Juntendo University School of Medicine (22042).
Study design
The study was a prospective, open-label, observational study to examine the effects of 90-day administration of Axona in patients with AD. The subjects were allowed to continue Axona use for an additional 90 days, on request. The trial was registered with the number UMIN000013591 and registered at the UMIN-CTR.
Administration of Axona
Considering the high frequency of diarrhea reported previously,16 we used a four-step dose-titration method recommended by Accera Inc: the “Axona Graduated Dosing Plan” (Table S1). This procedure was designed to accustom the patients to Axona. Instead of starting with 40 g Axona per day (20 g of C8), the initial daily dose was 10 g for 2 days. It was then increased to 20 g daily for 2 days, 30 g daily for 2 days, and finally 40 g per day (equivalent to 5, 10, 15, and 20 g of C8, respectively). Then, the 3-month clinical trial study commenced.
Examinations
The types and timing of the physical and mental examinations are summarized in Table S1. We examined intolerance levels before and during Axona treatment, stool condition graded on the Bristol scale,20 and the top ten reported adverse effects.16 Blood tests were performed to check the physical condition of the patients before taking Axona (month 0 [M0; baseline]) and at M1, M2, and M3. The blood tests included a hemogram, liver function (alanine aminotransferase and aspartate aminotransferase), renal function (creatinine and urea nitrogen), nutrition status (glucose, glycohemoglobin A1C, low-density lipoprotein and high-density lipoprotein, triacylglycerol, albumin, and total protein), and electrolyte levels (sodium, chloride, and potassium). Serum total ketone bodies (acetoacetic acid and β-hydroxybutyric acid) were measured using the enzyme-linked immunosorbent assay using buffer solution and reaction reagent for total ketone bodies (Kainos Laboratories Inc, Tokyo, Japan). The procedure was performed according to the manufacturer’s protocol using the BioMajesty™ system (JCA-BM8000; JEOL, Tokyo, Japan) at SRL Inc (Tokyo, Japan). The normal range of acetoacetic and β-hydroxybutyric acid was <55 and <85 μM, respectively. The detailed protocol can be provided upon request.
The effect of Axona on cognitive function was assessed using the MMSE and Alzheimer’s Disease Assessment Scale (ADAS) cognitive subscale, Japanese version (ADAS-Jcog).21
Genomic DNA was extracted from peripheral white blood cells using a QIAamp® DNA Blood Maxi Kit (Qiagen NV, Venlo, the Netherlands). ApoE genotypes were determined as previously reported.22
Statistics
Differences in mean age, onset age, duration of illness, duration of untreated dementia, and cognitive function scores were identified using the Mann–Whitney U-test. The difference between the sex ratios in healthy controls and patients was identified using χ2 tests, employing SPSS version 21 (IBM, Chicago, IL, USA). The differences between the values of ΔMMSE ([M3 MMSE – M0 MMSE]/M0 MMSE) and ΔADAS-Jcog at each time point during the study (M0, M1, M2, and M3) were examined using Friedman’s test. The same test was used for assessing differences between the groups with and without the ApoE4 allele after Axona administration. To find the confounding factors that might affect the cognitive function test, the correlations between cognitive function and various clinical variables were analyzed using Spearman’s correlation test.
Results
Study participants
A total of 26 patients were assessed for eligibility; from this group, 24 patients with sporadic mild-to-moderate AD were enrolled (13 males and eleven females), of which two patients dropped out of the study. One patient dropped out due to the intolerance of Axona, and the other because of a procedural accident (small brain infarction). The remaining 22 patients completed the study. Of the 22 enrolled subjects, six had MMSE scores below 14 and were classified as severe-AD cases, eight had MMSE scores between 14 and 20 and were considered moderate-AD cases, and eight had MMSE scores greater than 20 and were considered mild-AD cases.
Baseline clinical variables
As the basic treatment, 21 patients were given antidementia anticholinesterase drugs: donepezil (5 or 10 mg), six patients; rivastigmine (18 mg), five patients; and galantamine, three patients (8, 16, and 24 mg). Among these patients, nine were concomitantly treated with an anti-N-methyl-D-aspartate receptor agent – memantine (5, 10, 15, and 20 mg). One patient was treated with 10 mg of memantine only. The doses of these antidementia medicines were fixed throughout the study.
Clinical variables of the patients are shown in Table 1. Sex distribution, mean age, and other clinical variables, including scores from cognitive function tests, did not differ significantly between the patients with and without the ApoE4 allele (Table 1).
Compliance
Compliance throughout the study was reasonably good. The frequencies of patients with 100% intake rate of Axona were 86.4% at M1, 90.0% at M2, and 77.3% at M3. Almost all patients (90%) showed >80% intake rate at any time point, and no patients showed <60% intake rate. The reasons given for the lack of compliance were forgetfulness, going out, and inconvenience.
Adverse effects
Table 2 shows adverse events in this study. The most common events were flatulence and abdominal pain. Diarrhea occurred in only one patient at M3. These rates were lower than those observed in US patients (Table S1).16
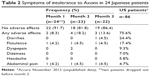  | Table 2 Symptoms of intolerance to Axona in 24 Japanese patients |
Physiological effects
The concentration of serum total ketone bodies (acetoacetic acid and β-hydroxybutyric acid) at M0 was 114.5±105.4 μM (34.7±26.7 and 81.1±79.9 μM, respectively). It rapidly increased during the 1st month (approximately threefold) to 322.6±240.2 μM (79.3±48.7 and 250.0±205.6 μM). Then, at M2 and M3, the levels remained almost the same. The results of blood tests did not change significantly; they were within normal ranges.
Effects on cognitive function
To find the confounding factors that could affect the cognitive function scores, the correlations between these scores (obtained at M3) and clinical variables, such as age, duration of illness, and serum ketone concentrations, were examined. The results failed to show any significant correlations between the cognitive function scores and other clinical variables (r=−0.26 to 2.59, all P>0.05). Naturally, the MMSE and ADAS scores showed strong significant correlations at M0 and M3 (r=−0.86 and −0.94, both P<0.001).
As expected, changes in cognitive function, expressed as ΔMMSE (M3 score - M0 score]/M0 score) and ΔADAS, showed a significant negative correlation (r=−0.498, P=0.019). ΔMMSE and ΔADAS scores during Axona administration are shown in Figure 1. ΔMMSE in both genotype groups (ApoE4− and ApoE4+) decreased at M2 and increased at M3. However, these differences (in comparison with the baseline) were not statistically significant at any time point in either group (Figure 1A). The ΔADAS score showed more improvement in the patients with the ApoE4 allele than in those without. However, this improvement was not statistically significant either (Figure 1B).
The levels of serum total ketone bodies at M3 did not show significant correlation with ΔMMSE (r=0.18, P=0.42) or ΔADAS (r=−0.06, P=0.81) in any of the 22 subjects, not even in the 15 subjects without the ApoE4 allele (r=−0.06, P=0.82, and r=0.29, P=0.28, respectively).
Among the patients with baseline MMSE scores indicating severe AD and mild-to-moderate AD, five of six (83%) showed a decline in the MMSE during Axona treatment, while only two of 16 (12.5%) with mild-to-moderate AD showed such decline. Further careful investigation of data for each patient resulted in an interesting finding. We focused on the patients with the greatest and second-greatest increases or decreases in ΔMMSE and ΔADAS scores (Figure 2). For example, cases A and B in Figure 2A (without the ApoE4 allele), with baseline MMSE score of ≥14, increased this score by 5 and 7 points, respectively, by M3. However, case C, homozygous for the ApoE4 allele (baseline MMSE score of ≥14), showed a decrease in MMSE score at the same time. Case D, lacking the ApoE4 allele, also showed a decrease in MMSE score by M3. However, case D started with a baseline MMSE score <14. Four patients with the largest (and second-largest) changes in their ΔADAS followed the same trend as the patients with the largest changes in ΔMMSE; ΔMMSE and ΔADAS were correlated. Two patients with the largest and second-largest improvements showed relatively low ADAS scores (smaller impairment in cognitive function) at M0. Interestingly, case B was heterozygous for the ApoE4 allele, but the ADAS score of this case improved. The patients showing the largest and second-largest decrease in their ADAS scores had relatively high scores (high level of impairment) at M0 (Figure 2B); their ADAS scores worsened, even though they did not carry the ApoE4 allele.
Discussion
We performed the first clinical trial study in Japan investigating the adverse effects and benefits of a medical food containing an MCT – Axona – in Japanese patients with AD. First, we have to mention the patient who did not complete the study due to a small brain infarction in the pons. The ethics committee of Juntendo Hospital categorized this brain infarction as a “procedural accident”. The criteria for this decision were as follows: 1) There were no such brain infarctions during the preliminary clinical study of MCT and Axona among 238 enrolled patients (Table S1); 2) the incidence of brain infarction during the 5-year postmarketing surveillance for Axona was four cases in 40,000 (one of 10,000; unpublished data 2009–2013, 5 years post marketing safety surveillance); and 3) the incidence of brain infarction in Japanese over 40 years of age is 3.87 (male) and 2.57 (female) cases per 1,000 individuals.23 Therefore, the incidence of brain infarction in patients taking Axona is lower than in Japanese patients not taking Axona. Moreover, the MCT usually prevents brain infarction.24
Our study demonstrated that Japanese patients with AD could be managed with Axona without experiencing severe gastrointestinal adverse effects. The reasons are not entirely clear. However, it is likely that implementing the Axona Graduated Dosing Plan (four-step dose-titration method starting with a low dose of Axona) is largely responsible for this result. A prior study using a two-step dose-titration method (10 g of C8 daily for 7 days and then 20 g for 90 days) reported a high incidence of adverse abdominal effects.16 The gradual introduction of a high-lipid diet before administering the required daily dose of Axona seems necessary in the case of Japanese patients.
However, Axona did not improve cognitive function in Japanese patients with AD, even in patients without the ApoE4 allele. The small number of patients, the major limitation of the present study, might explain the discrepancy between the results of our study and a previous report by Henderson et al.16 That report investigated the effect of Axona on 40 genotyped patients (16 ApoE4− patients and 24 ApoE4+ patients). Only approximately half that number, 22 patients, participated in the present study (15 ApoE4− patients and seven ApoE4+ patients). In addition, no significant correlations were found between serum total ketone bodies at M3 and ΔMMSE and ΔADAS for any of 22 subjects, not even the 15 subjects without the ApoE4 allele. This result might have also been due to the small number of patients. Theoretically, these factors should be significantly correlated, as an increase in levels of ketone bodies increases the availability of brain-energy sources.25,26 However, careful investigation of individual patients showed some interesting features, especially when we focused on changes in the cognitive function scores during Axona administration. A large proportion (83%) of patients with severe AD (baseline MMSE <14) showed a decline in the MMSE during Axona treatment. However, only 12.5% of mild-to-moderate patients (baseline MMSE ≥14) showed such decline. Among patients with largest and second-largest improvement or decrease in their MMSE scores, Axona did not prevent the worsening of cognitive functions in individuals with relatively high cognitive function at M0 and a homozygous ApoE4 allele. Indeed, several studies have shown a decrease in cerebral glucose utilization, especially in carriers of the ApoE4 allele (see the detailed review of Cunnane et al).27 In addition, some gene–dose effects of the ApoE4 allele have been reported during examinations of the postmortem brain28 and morphological features of the premorbid brain.29 Therefore, in patients less affected by the ApoE4 allele, Axona could be used as a brain-energy source instead of glucose. One patient heterozygous for the ApoE4 allele (case B in Figure 2B) showed an improvement in ADAS score (−6.3); this might also have been due to a gene–dose effect.
Axona did not affect the cognitive function of patients lacking the ApoE4 allele with relatively low cognitive function at M0. However, Axona affected patients with mild AD. The mean cognitive function of patients without the ApoE4 allele in this study was slightly lower than the cognitive function of similar patients in a previous report.16 The mean ADAS score in the present study was 23.1, and in the previous report it was 21.9. The mean MMSE score in our study was 17.8, and in the previous report it was 20.3. This feature of our patients might have diminished the effect of Axona. This is a limitation demonstrating the necessity for future studies of Axona treatment using a large number of patients, focusing on mild-AD Japanese patients. Uncontrolled type and dose of preceding antidementia medication might have been another drawback of our study.
To summarize, in patients with relatively mild AD lacking the ApoE4 allele, Axona treatment might improve cognitive function (eg, an MMSE score of ≥14).
Conclusion
We found that Japanese patients with AD could be managed with Axona without severe intolerance or gastrointestinal side effects. The modified dose-titration method, starting with a low dose of Axona (10 g, containing 5 g of C8), reduced adverse effects (such as diarrhea) in Japanese patients. It was not possible to demonstrate clearly any cognitive benefits of Axona in a trial with a limited number of patients and without a placebo control. Further randomized, double-blind studies with a large number of patients, focusing on Japanese patients with mild AD, should be performed to obtain a detailed assessment of Axona effect.
Acknowledgment
This study was funded by a donation from Nestle Japan Ltd. Nestle Japan Ltd had no control over the interpretation, writing, or publication of this work.
Disclosure
TO reports receiving an offer of Axona product. The other authors report no conflicts of interest for the present study.
References
Marques SC, Oliveira CR, Outeiro TF, Pereira CM. Alzheimer’s disease: the quest to understand complexity. J Alzheimers Dis. 2010;21(2):373–383. | ||
Marques SC, Oliveira CR, Pereira CM, Outeiro TF. Epigenetics in neurodegeneration: a new layer of complexity. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):348–355. | ||
Mancuso C, Bates TE, Butterfield DA, et al. Natural antioxidants in Alzheimer’s disease. Expert Opin Investig Drugs. 2007;16(12):1921–1931. | ||
Cole GM, Lim GP, Yang F, et al. Prevention of Alzheimer’s disease: omega-3 fatty acid and phenolic anti-oxidant interventions. Neurobiol Aging. 2005;26 Suppl 1:133–136. | ||
Rossi L, Mazzitelli S, Arciello M, Capo CR, Rotilio G. Benefits from dietary polyphenols for brain aging and Alzheimer’s disease. Neurochem Res. 2008;33(12):2390–2400. | ||
Thomas P, Wang YJ, Zhong JH, et al. Grape seed polyphenols and curcumin reduce genomic instability events in a transgenic mouse model for Alzheimer’s disease. Mutat Res. 2009;661(1–2):25–34. | ||
Henderson ST, Poirier J. Pharmacogenetic analysis of the effects of polymorphisms in APOE, IDE and IL1B on a ketone body based therapeutic on cognition in mild to moderate Alzheimer’s disease; a randomized, double-blind, placebo-controlled study. BMC Med Genet. 2011;12:137. | ||
Henderson ST. Ketone bodies as a therapeutic for Alzheimer’s disease. Neurotherapeutics. 2008;5(3):470–480. | ||
Roman MW. Axona (Accera, Inc): a new medical food therapy for persons with Alzheimer’s disease. Issues Ment Health Nurs. 2010;31(6):435–436. | ||
Scheltens P, Kamphuis PJ, Verhey FR, et al. Efficacy of a medical food in mild Alzheimer’s disease: a randomized, controlled trial. Alzheimers Dement. 2010;6(1):1–10.e11. | ||
Thaipisuttikul P, Galvin JE. Use of medical foods and nutritional approaches in the treatment of Alzheimer’s disease. Clin Pract (Lond). 2012;9(2):199–209. | ||
Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137–152. | ||
Small GW, Mazziotta JC, Collins MT, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995;273(12):942–947. | ||
Small GW, La Rue A, Komo S, Kaplan A, Mandelkern MA. Predictors of cognitive change in middle-aged and older adults with memory loss. Am J Psychiatry. 1995;152(12):1757–1764. | ||
Small GW, Kuhl DE, Riege WH, et al. Cerebral glucose metabolic patterns in Alzheimer’s disease. Effect of gender and age at dementia onset. Arch Gen Psychiatry. 1989;46(6):527–532. | ||
Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond). 2009;6:31. | ||
Reger MA, Henderson ST, Hale C, et al. Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25(3):311–314. | ||
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. | ||
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. | ||
Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920–924. | ||
Mohs RC, Rosen WG, Davis KL. The Alzheimer’s disease assessment scale: an instrument for assessing treatment efficacy. Psychopharmacol Bull. 1983;19(3):448–450. | ||
Wenham PR, Price WH, Blandell G. Apolipoprotein E genotyping by one-stage PCR. Lancet. 1991;337(8750):1158–1159. | ||
Kubo M, Hata J, Doi Y, Tanizaki Y, Iida M, Kiyohara Y. Secular trends in the incidence of and risk factors for ischemic stroke and its subtypes in Japanese population. Circulation. 2008;118(25):2672–2678. | ||
St-Onge MP, Bosarge A, Goree LL, Darnell B. Medium chain triglyceride oil consumption as part of a weight loss diet does not lead to an adverse metabolic profile when compared to olive oil. J Am Coll Nutr. 2008;27(5):547–552. | ||
Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF Jr. Brain metabolism during fasting. J Clin Invest. 1967;46(10):1589–1595. | ||
Hasselbalch SG, Madsen PL, Hageman LP, et al. Changes in cerebral blood flow and carbohydrate metabolism during acute hyperketonemia. Am J Physiol. 1996;270(5 Pt 1):E746–E751. | ||
Cunnane S, Nugent S, Roy M, et al. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition. 2011;27(1):3–20. | ||
Gomez-Isla T, West HL, Rebeck GW, et al. Clinical and pathological correlates of apolipoprotein E epsilon 4 in Alzheimer’s disease. Ann Neurol. 1996;39(1):62–70. | ||
Hostage CA, Roy Choudhury K, Doraiswamy PM, Petrella JR. Dissecting the gene dose-effects of the APOE ε4 and ε2 alleles on hippocampal volumes in aging and Alzheimer’s disease. PLoS One. 2013;8(2):e54483. |
Supplementary material
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




