Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Benefits of the Dermocosmetic Mineral 89 Probiotic Fractions Adjunct to Topical Retinoids for Anti-Aging Benefits
Authors Valpaços C, Leclerc-Mercier S, Lopes L, Svoboda D, Miranda D, Correia P , Junior J, Fernandes E, Francois-Newton V, Mandary MB, Gueniche A, Tan J , Kerob D
Received 12 November 2022
Accepted for publication 25 January 2023
Published 10 February 2023 Volume 2023:16 Pages 375—385
DOI https://doi.org/10.2147/CCID.S396952
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Camila Valpaços,1 Stéphanie Leclerc-Mercier,2 Luana Lopes,1 Diego Svoboda,1 Daniele Miranda,1 Priscila Correia,3 José Junior,3 Erika Fernandes,1 Veronique Francois-Newton,4 Madiiha Bibi Mandary,4 Audrey Gueniche,2 Jerry Tan,5 Delphine Kerob6
1Centre International de Développement Pharmaceutique (Research Institute), Rio de Janeiro, Brazil; 2Vichy Laboratoires, Levallois-Perret, France; 3L’Oréal Brasil, Rio de Janeiro, Brazil; 4Centre International de Développement Pharmaceutique (Research Institute), Phoenix, Mauritius; 5Western University, Department of Medicine and Windsor Clinical Research Inc, Windsor, ON, Canada; 6CAI, L’Oréal Paris, Paris, France
Correspondence: Camila Valpaços, Centre International de Développement Pharmaceutique (Research Institute), Rua dos Inválidos, 212, 401/402, Lapa, Rio de Janeiro, 20231-048, Brazil, Tel +55 21 2221-6180, Email [email protected]
Purpose: Tretinoin is a topical gold standard for photoaging treatment. However, patient adherence can be impaired by local tolerability in the first 1– 2 weeks of treatment. Mineral 89 Probiotic Fractions® (M89PF) containing Vichy volcanic mineralizing water®, probiotic fractions, hyaluronic acid, niacinamide and tocopherol was developed to fulfill the need for adjunctive products that can reinforce skin barrier and manage retinoid induced irritation.
Patients and Methods: The study included 38 women, aged 44– 60 years, phototype II–VI, applying 0.025% tretinoin gel once nightly for 84 days. For 28 days, one hemi face was treated with M89PF and sunscreen SPF 50+ while other hemi face received sunscreen only. Then, M89PF application was changed to full face. Evaluations were performed at days 0, 7, 28 and 84. Erythema, dryness, fine lines, skin tone, radiance and pore appearance were assessed by a dermatologist. Tolerability was evaluated through self-assessment questionnaire. Skin hydration levels, inflammatory and oxidative stress biomarkers were analyzed by immunological assay: Interleukin(IL)-8, IL1-alpha, IL1-Receptor Antagonist (IL-1Ra), Prostaglandin E2 (PGE2), Catalase and Superoxide Dismutase (SOD).
Results: Hemiface analysis showed that erythema, fine lines, skin tone, radiance, pore appearance, hydration, tightness, dryness, burning, itching and stinging sensations were improved (p< 0.05) on the M89PF side. At full face analysis on D84, erythema, fine lines, skin tone, radiance and pore appearance were improved compared to D0 (p< 0.001). Tightness, dryness, burning, itching and stinging were reduced when compared to D7 (p< 0.05). Dermatology Life Quality Index (DLQI) and Skindex 16 showed improvement in quality of life (p< 0.05). IL-1RA increased at D28 (p=0.003) and PGE2 decreased at D28 and D84 compared to D0 (p< 0.01).
Conclusion: M89PF reduced retinoid induced irritation with a good tolerability profile and, used as an adjunct to topical tretinoin, significantly improved skin hydration, erythema, fine lines, skin tone, radiance and pore appearance.
Keywords: skin barrier, irritation, Vichy volcanic mineralizing water®, hyaluronic acid, niacinamide, tocopherol
Introduction
Improving skin function and appearance plays a crucial role in the quality of life in people with sensitive skin. The skin is the point of contact between the environment and is subject to various direct external stimuli.1 The primary role of the skin is to protect against harmful and noxious agents in the external environment. As such, an essential precursor for healthy skin is an intact skin barrier.
The intricate relationship between stress and skin conditions has been widely documented.2 Indeed, environmental factors such as sun exposure, air pollution or tobacco smoke are known for damaging the skin and increasing its susceptibility to skin conditions.3 This loss in skin protection has been linked to inflammation, disruption of the skin barrier, decreased ability to repair wounds and an increased risk of skin cancer. The biological impact of stressed skin also extends to loss of elasticity and increased pigmentation.4,5
In recent years, novel therapeutic breakthroughs have been developed to counter skin aging signs. One of these is the use of topical retinoids such as tretinoin to treat various skin pathologies and reduce signs of photo aging.6–8 In acne, topical retinoids have also been shown to be effective by reducing comedone formation, reducing follicular hyperkeratosis and inhibiting innate immune inflammation.8−11
However, despite its positive impact in dermatological therapeutics, topical retinoids can induce cutaneous irritation. This presents as erythema, dryness, itching and burning sensations due to the alteration of skin barrier especially in those with sensitive skin.12 Accordingly, there is an unmet need for adjunctive products that can maintain the natural skin barrier in those embarking on topical retinoids.
Mineral 89 Probiotic Fractions® (M89PF, Laboratoires Vichy, France) contains 80% of Vichy volcanic mineralizing water® (VMW, Laboratoires Vichy, France) highly enriched with Minerals (Sodium, Sulphur, Calcium, Potassium, Magnesium, Silicon, Fluorine, Lithium, Strontium, Boron, Iron, Ammonium, Orthophosphates and Manganese), 0.4% hyaluronic acid (HA), an extracellular matrix component with viscoelastic and hygroscopic properties, probiotic fractions, niacinamide 4% and tocopherol. The properties of HA are linked to the regulation of water balance in the dermis and an amelioration of the extracellular domain of the cell surface.13,14 Niacinamide (vitamin B3) has been reported to reduce signs of skin aging such as hyperpigmentation and redness.15 Niacinamide has anti-inflammatory properties reducing edema and vasodilatation induced by substance P and inhibiting inflammatory markers IL-1b, IL-6, IL-8, TNF-a.16 As an important fat-soluble antioxidant, vitamin E tocopherol protects the skin from consequences of oxidative stress as a free-radical scavenger.17 Thus, it was hypothesized that M89PF could be effective in mitigating topical retinoid induced irritation by maintaining the skin barrier.
Materials and Methods
Patients
Inclusion criteria were women between 44–60 years with self-perceived sensitive skin receiving their first prescription of anti-aging topical retinoid, tretinoin. All patients provided informed written consent and all received the same treatment. Subjects were Fitzpatrick skin phototype II, III, IV, V and VI, and all had self-declared sensitive skin. 38 healthy female volunteers from Brazil were investigated. Exclusion criteria were breastfeeding or pregnant women, changes in contraception procedures, participation in other studies as well as having undergone chemical or invasive dermo-treatment and beauty treatment within the space of a week.
Study Design and Schedule
In this open, randomized, and prospective study all subjects (N=38) applied tretinoin 0.025% gel once at night for 84 days. The outcomes were assessed intra-individually whereby the subjects applied M89PF on a randomized hemi face for 28 days twice daily. Nightly application followed tretinoin gel 0.025% application while morning application was followed by Ideal Soleil Dry Touch® SPF 50+ sunscreen (Vichy Laboratoires, Paris, France) on the entire face. The sunscreen properties consisted of combined chemical and physical protection with no anti-aging active or ingredients common with M89PF.
After 28 days, the subjects applied the M89PF on full face twice (morning and night) daily (the night application was performed after tretinoin 0.025% application) and maintained sunscreen application up to D84. Until D28, comparison was made against the untreated side of the face on each subject. From D28 to D84 comparison was made on full face compared to baseline. At each visit, subjects underwent a clinical dermatological assessment and instrumental assessment of skin hydration. The swab collection of inflammatory and oxidative stress markers was performed on both cheeks at D0 and D28 and only on the side of the face treated with M89PF in D84. In addition, subjects were also required to complete a self-assessment questionnaire regarding discomfort. The subjects were asked not to change their cosmetic and cleansing routines for the duration of this study.
Evaluation Criteria
Clinical Evaluation by Grading of Facial Skin Parameters
The dermatologist performed evaluations on D0, D7, D28 (hemi face analysis) and D84 (full face analysis) to assess erythema and dryness related to skin irritation through a visual analog scale (VAS scale) ranging from 0 to 10, (0=none, 10=severe). Global skin parameters associated with signs of skin aging (fine lines, skin tone, skin radiance and pore appearance) were also assessed by the dermatologist through VAS scales (0=none, 10=severe).
Efficacy of the Product Through Subjective Questionnaires
Subjects were asked to indicate features of tightness, dryness, burning, itching, and stinging at D0, D7, D28 and D84. These parameters were scored on a range of 0–10 and divided into the left and right hemifaces.
Skin Hydration
The hydration level of the stratum corneum was measured using The Corneometer® CM 825 (Courage+ Khazaka electronic GmbH, Koln, Germany). Measurements were carried out in triplicate on both lateral malar regions on the subject’s face at D0, D7, D28, and D84. Three measurements from specific facial regions were obtained at each visit.
Skin Discomfort, Quality of Life, and Subject Evaluation
The Dermatology Life Quality Index Questionnaire (DLQI questionnaire) and Skindex 16 were completed at each visit at D0, D7, D28 and D84. The DLQI consisted of 10 questions designed to measure the health-related quality of life of adult patients while the Skindex 16 consisted of 16 questions on the subjects’ perception of the impact of skin condition on different domains such as symptoms, emotions, and functioning. Higher scores indicate greater adverse impact of skin disease in each domain.18,19
SWAB Collection for Inflammatory Marker Analysis
Swab samples, a noninvasive method for skin surface evaluation, of 29 subjects were collected on both cheeks on D0 and D28 and in D84 only on the side of the face treated with M89PF. After the zone of interest and size were defined, a first swab previously dampened in the cocktail solution was rubbed onto the surface of interest for 45 seconds. Then, the same step was performed with a second swab previously dampened in the cocktail solution. Both swabs were introduced into a sample tube and stored at −20°C.
For sample extraction, each cotton bud was removed from its stick and placed into another 0.5mL sample tube drilled at the bottom itself and introduced into a 2 mL sample tube. The content was centrifuged for 3 min at 18,000 g at 4°C. The liquid obtained was collected for biochemical exploration.
The IL-8, IL-1alpha and IL1Ra assays were performed by Elisa (R&D Systems Inc., Minneapolis, MN, USA). A plate with a monoclonal antibody respectively specific for IL-8, IL-1α or IL1RA was prepared. Then, the sample of interest was deposited in order to bind to the antibody and to add a polyclonal antibody recognizing the antigen. A secondary antibody bound to the enzyme was added to convert it into a detectable entity (450 nm) directly proportional to the amount of IL-8, IL-1α or IL1RA.
The PGE2 assay, to test the COX-2 (Cyclo-oxygenase-2) activity, was carried out by Elisa (R&D Systems Inc., Minneapolis, MN, USA). A plate with anti-mouse IgG antibodies attached was prepared. A mixture of labeled antigens (alkaline phosphatase conjugated-PGE2) and antigens to be assayed (sample) were deposited on the plate. The Prostaglandin E2 antibody and substrate were then added and the enzymatic reactivity converted to a detectable entity (450 nm) inversely proportional to the amount of PGE2.
The Catalase activity was measured by enzyme kit (R&D Systems Inc., Minneapolis, MN, USA).
This method consists of measuring the residual content of hydrogen peroxide (H2O2) following the catalase action, which induced the fluorescence of resorufin. This is an indirect assay of hydrogen peroxide. The resorufin intensity is inversely proportional to the activity of catalase.
SOD activity was tested by enzyme kit (R&D Systems Inc., Minneapolis, MN, USA).
SOD catalyzes the conversion reaction of O2- into hydrogen peroxide and oxygen. These superoxide ions are produced by the conversion of xanthine to hydrogen peroxide and catalyze the WST-1 conversion reaction to produce a coloration detected at 450 nm.
Statistical Methodology
SPSS 19.0 (SPSS. Inc, Chicago, USA) was used for statistical analysis purpose. Qualitative variables were described as number and percentage of the different response modalities; 95% confidence interval (CI) was calculated to visually assess the evolution across time, by treatment (where applicable). Quantitative measurements were summarized using mean, median, maximum, and minimum and measures of dispersion, such as standard deviation. All statistical analyses were performed at 5% significance using 2-sided tests, except normality testing at 1% (Shapiro–Wilk test).
Results
Panel Description
A total of 43 healthy women were recruited but 4 withdrew consent and 1 was lost to follow-up. Thus, 38 subjects were included in the efficacy analysis (Table 1).
 |
Table 1 Characteristics of the Subjects Included in the Study |
Short-Term Effects
Clinical Efficacy
Dermatologist assessments for erythema, skin dryness, fine lines, skin tone, radiance and pore appearance were conducted on D0, D7 and D28 for the hemi face design. These parameters (Table 2) all showed significant improvement after 28 days of M89PF, tretinoin and sunscreen use compared to the contralateral side. More specifically, significant improvement (p=0.003) was observed in erythema with M89PF, tretinoin and sunscreen compared to contralateral side after 28 days of treatment with tretinoin and sunscreen use only. Fine lines, skin tone, radiance and pore appearance presented 13.2–25.5% difference and showed a significant further improvement (p ≤0.001) with M89PF, tretinoin and sunscreen compared to the treatment with tretinoin and sunscreen use only.
 |
Table 2 Descriptive Statistics, Evolution Across Time (D28-D0) and Treatment (Side) Comparison for Parameters Evaluated by the Investigator |
Tolerance Assessment
Through the self-assessment questionnaire (Table 3), the study volunteers provided insight into the functional signs following 28 days (D28) of treatment with M89PF. It was reported by the volunteers that skin tightness, burning and stinging sensation were significantly increased on both sides of the face after 7 days of study, symptoms usually related to tretinoin irritation on the first days. However, after 28 days of treatment, an improvement was reported by the volunteers. Skin tightness, dryness, burning, itching, and stinging sensations were greatly improved on treated side, by 74.8% (p=0.092), 70.1% (p<0.001), 38.8% (p=0.086), 35.8% (p=0.351) and 47.0% (p=0.140), respectively. The comparison between the two zones did show significant improvement difference for stinging sensation (p=0.039) in favor of the side treated with M89PF on D28.
 |
Table 3 Descriptive Statistics, Evolution Across Time (D28-D7) and Treatment (Side) Comparison for the Responses from Self-Assessment Questionnaire |
Levels of Skin Hydration
From the Corneometer® readings (Table 4), it was revealed that there was a significant improvement of 5.60% (p=0.021) in skin hydration detected on the treated zone at D28 compared to the first week of treatment at D7. The comparison between D28 to D0, also showed that the skin hydration levels were further improved +11.46% (p <0.001) on the treated zone while there was no significant difference in the non-treated zone. This confirmed a better improvement of skin hydration on the treated zone after 28 days.
 |
Table 4 Descriptive Statistics, Evolution Across Time (D28-D0) and Treatment (Side) Comparison for Corneometer® Readings |
Long-Term Effects
Clinical Efficacy
The clinical data (Table 5) showed the improvement in all the clinical parameters after 84 days of M89PF, tretinoin and sunscreen use. More specifically, a 70.0% significant improvement (p< 0.001) was denoted in erythema after 84 days of treatment. Fine lines, skin tone, radiance and pore appearance presented between 32.0–47.9% significant difference (p<0.001) which denoted a significant improvement compared to baseline. No significant improvement in skin dryness was noted after 84 days of treatment when compared to D0.
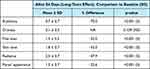 |
Table 5 Descriptive Statistics and Evolution Across Time (D84-D0) for Parameters Evaluated by the Investigator |
Tolerance Assessment
Through the self-assessment questionnaire (Table 6), the study volunteers provided insight into the functional signs following 84 days (D84) of treatment with M89PF. After 84 days of product use, the volunteers presented a significant reduction of 99.4% (p=0.011) in skin tightness, 59.3% (p=0.016) in skin dryness, 63.8% (p=0.002) in burning sensation, 65.7% (p=0.019) in itching sensation, and 80.3% (p=0.003) in stinging sensation when compared to the first 7 days of tretinoin use. No local side effect related to the M89PF was reported.
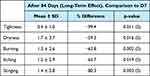 |
Table 6 Descriptive Statistics and Evolution Across Time for the Responses from Self-Assessment Questionnaire |
Levels of Skin Hydration
From the Corneometer® readings (Figure 1), skin hydration levels showed constant improvement (non-significant) in hydration during 84 days of study.
 |
Figure 1 Corneometer® readings evaluated on D0, D7, D28 and D84. Error bars: 95% CI (confidence interval). Abbreviation: AU, arbitrary unit. |
Assessment of Quality of Life
It was observed through the DLQI (Figure 2) that the mean score of volunteers’ assessments of quality of life started to decrease from D7, until D28 and D84. Indeed, a mean change of 41.7% was reported at D28 compared to D0, while a decrease of 47.2% was reported between D84 and baseline. However, these were found not to be significant.
The Skindex 16 evaluated the symptoms, emotions and functioning of the volunteers related to the effects of skin aging on quality of life (Figure 3). Results showed that at D7, the “symptoms” domain manifested was significantly increased by 86.4% (p=0.039) compared to D0. Although an increase was noted at D28 and D84, it was not significant.
The domain “Emotions” decreased indicating less impact on subject’s life as the treatment with M89PF progressed. Indeed, a significant decrease of 77.5% (p=0.002) and 86.6% (p<0.001) was reported at D28 and D84, respectively compared to the baseline D0.
The domain “Function” was significantly decreased by 66.9% (p=0.015) at D7, 89.8% (p=0.001) at D28, and 87.3% (p=0.001) at D84 when compared to D0.
Inflammatory Marker Analysis
As shown in Table 7, IL-1Ra increased significantly (41.21%) at D28 compared to D0 (p=0.003) with no difference between hemi face (M89PF treated vs untreated). Then, at D84 the levels returned to D0 level (Table 8). IL1-alpha decreased significantly (19.31%) at D84 with M89PF compared to D28 (p=0.012) (Table 8). IL8 decreased significantly (38.31%) at D84 with M89PF compared to D28 with no product (p=0.001) (Table 8). PGE2 content decreased significantly (24.28%) at D28 compared to D0 (p=0.006) with no difference between hemi face (Mineral 89PF® versus no additional product) (Table 7) and continued to decrease until D84 (37,01% and p<0.001) (Table 8). SOD content increased (23.52%) at D28 compared to D0 (p=0.070) (Table 7), then returned to baseline level on D84 (Table 8). Catalase content was stable from baseline to D84. (Table 8).
Discussion
The present study aimed to investigate the benefits of Mineral 89 Probiotic Fractions® (M89PF) in mitigating retinoid induced irritation in healthy adult women, and to assess the potential for adjunctive improvement in anti-aging efficacy when combined with topical retinoid treatment and sunscreen.
Within the retinoid family, tretinoin remains the most widely investigated retinoid for use in photoaging.20 It stimulates proliferation and differentiation of keratinocytes, enhances elastic tissue and collagen formation via fibroblast activation and inhibits matrix metalloproteinases. These effects lead to rejuvenation of the epidermis and dermal matrix restoration.9
However, retinoid irritant reactions, typically occurring within the first 1–2 weeks (characterized by burning, scaling, dermatitis, erythema) can limit its acceptability and reduce adherence.9
In this study, where tretinoin 0.025% gel was applied nightly, dermatologist assessment indicated the absence of local side effects on the hemiface receiving M89PF. There was also significant improvement of erythema, skin hydration levels, skin tightness, dryness, burning, itching and stinging sensations. The additional benefit of M89PF as a complement to tretinoin and sunscreen was demonstrated with greater improvement in fine lines, skin tone, radiance, pore appearance and skin hydration levels.
The diminution in clinical manifestations such as skin dryness, erythema, fine lines and the improvement of skin tone and radiance throughout this study confirmed the possible anti-aging potential of M89PF in association with tretinoin. Additionally, a significant increase in skin hydration was observed with M89PF, indicating higher water content in the epidermis. Thus, it can be inferred that M89PF restored the skin barrier and its function within the first week of use. This finding is in accord with a previous study on the clinical effectiveness and tolerability of Vichy volcanic mineralizing water®, where similar findings were reported. Thus, M89PF may restore skin barrier function following onset of dermatoses which otherwise impair the skin barrier.21
In the skin cytokine analysis, the initial levels of inflammatory markers at D0 were consistent with the profile found in healthy skin.22 On both hemifaces, IL-1Ra increased significantly at D28 compared to baseline in the absence of a change in IL1-alpha. This suggests activation of an anti-inflammatory effect attributable to tretinoin and/or sunscreen. SOD content increased at D28 compared to D0 related to boost of antioxidant defense.23 Decrease of PGE2 at D28 and D84 compared to baseline might be related to decreased erythema based on clinical evaluation.24
The current investigation on M89PF in addition to tretinoin also demonstrated improved subject’s perception of quality of life (QOL) based on the DLQI and Skindex 16. The adverse QOL impact of skin aging lessened with the use of M89PF and tretinoin from D0 to D84.
Conclusion
In this study, we assessed the potentiation efficacy of Mineral 89 Probiotic Fractions® (M89PF, Vichy Laboratoires France) to improve the cutaneous tolerability of tretinoin when applied for 84 days. M89PF reduced retinoid induced irritation within the first weeks of use by improving signs of erythema, skin tightness, skin dryness, burning, itching, and stinging sensations as early as Day 7 with a good tolerability profile. M89PF improved skin hydration levels despite the use of tretinoin. M89PF used as an adjunct to topical tretinoin also significantly improved signs of skin aging such as fine lines, skin tone, radiance, and pore appearance. Finally, the use of M89PF in combination with tretinoin and sunscreen improved QOL.
Therefore, it is possible to conclude that M89PF is effective in reducing irritation induced by topical tretinoin treatment and is an effective antiaging adjunct in adult women.
Ethics Statement
The study was conducted in accordance with the principles of Resolution 466/2012 of the National Health Council Brazil, ethical principles stemming from the Declaration of Helsinki (and subsequent modifications) defined by ICH E612 ref. EMA / CHMP / ICH / 135/1995, 2016. All patients provided informed written consent prior to study commencement. This study was conducted in accordance with the Good Clinical Practice guidelines of the International Conference on Harmonization. The protocol was approved by an independent Brazilian Committee of Ethics Hospital Pró Cardiaco (EC approval number 4.508.026).
Acknowledgments
Authors would like to acknowledge Vichy Laboratoires for funding the study and providing the test material. Authors also acknowledge CIDP - Centre International de Développement Pharmaceutique for their valuable input, managing the project and medical writing. CIDP- Centre International de Développement Pharmaceutique conducted the study under sponsorship of Vichy Laboratoires.
Disclosure
The following authors work for Vichy Laboratoires, A L’Oreal company: Stéphanie Leclerc-Mercier, Priscila Correia, José Junior, Audrey Gueniche. Luana Lopes represented the CRO and reports fee for conducting this study from LÓreal France, during the conduct of the study. Dr Jerry Tan reports personal fees from La Roche Posay, during the conduct of the study; personal fees from Vichy, CeraVe, and Boots Walgreens, outside the submitted work. Delphine Kerob is a full time employee of La Roche-Posay, a L’Oreal company. The authors report no other conflicts of interest in this work.
References
1. Lobo RA. Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Elsevier; 2007.
2. Chen Y, Lyga J. Brain-skin connection: stress, inflammation and skin aging. Inflamm Allergy Drug Targets. 2014;13(3):177–190. doi:10.2174/1871528113666140522104422
3. Parrado C, Mercado-Saenz S, Perez-Davo A, Gilaberte Y, Gonzalez S, Juarranz A. Environmental stressors on skin aging. mechanistic insights. Front Pharmacol. 2019;10:759. doi:10.3389/fphar.2019.00759
4. Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci. 2017;85(3):152–161. doi:10.1016/j.jdermsci.2016.09.015
5. Passeron T, Zouboulis CC, Tan J, et al. Adult skin acute stress responses to short-term environmental and internal aggression from exposome factors. J Eur Acad Dermatol Venereol. 2021;35(10):1963–1975. doi:10.1111/jdv.17432
6. Orfanos CE, Zouboulis CC, Almond-Roesler B, Geilen CC. Current use and future potential role of retinoids in dermatology. Drugs. 1997;53(3):358–388. doi:10.2165/00003495-199753030-00003
7. Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1(4):327–348. doi:10.2147/ciia.2006.1.4.327
8. Cosio T, Di Prete M, Gaziano R, et al. Trifarotene: a current review and perspectives in dermatology. Biomedicines. 2021;9(3):237. doi:10.3390/biomedicines9030237
9. Kang S, Kim K, Jun SH, et al. Anti-Irritant strategy against retinol based on the genetic analysis of Korean Population: a genetically guided top-down approach. Pharmaceutics. 2021;13(12):2006. doi:10.3390/pharmaceutics13122006
10. Shalita AR, Myers JA, Krochmal L, Yaroshinsky A; Clindamycin Foam Study G. The safety and efficacy of clindamycin phosphate foam 1% versus clindamycin phosphate topical gel 1% for the treatment of acne vulgaris. J Drugs Dermatol. 2005;4(1):48–56.
11. Schmidt N, Gans EH. Tretinoin: a review of its anti-inflammatory properties in the treatment of acne. J Clin Aesthet Dermatol. 2011;4(11):22–29.
12. Stucker M, Hoffmann M, Altmeyer P. Instrumental evaluation of retinoid-induced skin irritation. Skin Res Technol. 2002;8(2):133–140. doi:10.1034/j.1600-0846.2002.00330.x
13. Stern R, Maibach HI. Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Clin Dermatol. 2008;26(2):106–122. doi:10.1016/j.clindermatol.2007.09.013
14. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4(3):253–258. doi:10.4161/derm.21923
15. Jerajani HR, Mizoguchi H, Li J, Whittenbarger DJ, Marmor MJ. The effects of a daily facial lotion containing vitamins B3 and E and provitamin B5 on the facial skin of Indian women: a randomized, double-blind trial. Indian J Dermatol Venereol Leprol. 2010;76(1):20–26. doi:10.4103/0378-6323.58674
16. Ungerstedt JS, Blomback M, Soderstrom T. Nicotinamide is a potent inhibitor of proinflammatory cytokines. Clin Exp Immunol. 2003;131(1):48–52. doi:10.1046/j.1365-2249.2003.02031.x
17. Keen MA, Hassan I. Vitamin E in dermatology. Indian Dermatol Online J. 2016;7(4):311–315. doi:10.4103/2229-5178.185494
18. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi:10.1111/j.1365-2230.1994.tb01167.x
19. Chren MM, Lasek RJ, Sahay AP, Sands LP. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5(2):105–110. doi:10.1007/BF02737863
20. Noble S, Wagstaff AJ. Tretinoin. A review of its pharmacological properties and clinical efficacy in the topical treatment of photodamaged skin. Drugs Aging. 1995;6(6):479–496. doi:10.2165/00002512-199506060-00008
21. Tan J, Spada J, Orlandi C, et al. Vichy mineralizing water with hyaluronic acid is effective and well tolerated as an adjunct to the management of various dermatoses and after esthetic procedures. J Cosmet Dermatol. 2020;19(3):682–688. doi:10.1111/jocd.13229
22. Lyubchenko T, Collins HK, Goleva E, Leung DY. Skin tape sampling technique identifies proinflammatory cytokines in atopic dermatitis skin. Ann Allergy Asthma Immunol. 2021;126(1):46–53. doi:10.1016/j.anai.2020.08.397
23. Shariev A, Menounos S, Laos AJ, et al. Skin protective and regenerative effects of RM191A, a novel superoxide dismutase mimetic. Redox Biol. 2021;38:101790. doi:10.1016/j.redox.2020.101790
24. Kabashima K, Nagamachi M, Honda T, et al. Prostaglandin E2 is required for ultraviolet B-induced skin inflammation via EP2 and EP4 receptors. Lab Invest. 2006;87(1):49–55. doi:10.1038/labinvest.3700491
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




