Back to Journals » Nature and Science of Sleep » Volume 11
Benefit effect of REM-sleep deprivation on memory impairment induced by intensive exercise in male wistar rats: with respect to hippocampal BDNF and TrkB
Authors Mahboubi S, Nasehi M, Imani A, Sadat-Shirazi MS, Zarrindast MR, Vousooghi N, Noroozian M
Received 2 March 2019
Accepted for publication 29 July 2019
Published 24 September 2019 Volume 2019:11 Pages 179—188
DOI https://doi.org/10.2147/NSS.S207339
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sutapa Mukherjee
Sarah Mahboubi,1 Mohammad Nasehi,2 Alireza Imani,3,4 Mitra-Sadat Sadat-Shirazi,1–5 Mohammad-Reza Zarrindast,5–7 Nasim Vousooghi,1 Maryam Noroozian1–8
1Department of Neuroscience, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran; 2Department of Cognitive and Neuroscience Research Center (CNRC), Amir-Almomenin Hospital, Tehran Medical Sciences Branch, Islamic Azad University, Tehran, Iran; 3Department of Physiology, School of Medicine, Tehran University of Medical Science, Tehran, Iran; 4Department of Occupational Sleep Research Center, Tehran University of Medical Sciences, Tehran, Iran; 5Department of Iranian National Center for Addiction Studies, Tehran University of Medical Sciences, Tehran, Iran; 6Department of Pharmacology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran; 7Department of Endocrinology and Metabolism Research Institute, Tehran University of Medical Science, Tehran, Iran; 8Memory and Behavioral Neurology Division, Department of Psychiatry, Roozbeh Hospital, Tehran University of Medical Sciences, Tehran, Iran
Correspondence: Maryam Noroozian
Memory and Behavioral Neurology Division, Department of Psychiatry, Roozbeh Hospital, Tehran University of Medical Sciences, South Kargar Avenue, P.O. Box 1333795914, Tehran, Iran
Tel +98 21 55 419 1515
Fax +98 21 5 541 9113
Email [email protected]
Background: Many factors affect our learning and memory quality, but according to different studies, having a positive or negative impact pertains to their characteristics like intensity or the amount.
Purpose: The present study was conducted to investigate the effect of 24-hour REM-sleep deprivation on continuous-high intensity forced exercise-induced memory impairment and its effect on Brain-Derived Neurotrophic Factor (BDNF) and Tyrosine kinase B (TrkB) levels in the hippocampus and Prefrontal Cortex area (PFC).
Material and methods: Animals were conditioned to run on treadmills for 5 weeks then, were deprived of sleep for 24 h using the modified multiple platforms. The effect of intensive exercise and/or 24-h REM-SD was studied on behavioral performance using Morris Water Maze protocol for 2 days, and BDNF/TrkB levels were assessed in hippocampus and PFC after behavioral probe test using western blotting.
Results: After 5 weeks of intensive exercise and 24-h REM-SD, spatial memory impairment and reduction of BDNF and TrkB levels were found in hippocampus and PFC. 24-h REM-SD improved memory impairment and intensive exercise-induced downregulation of BDNF and TrkB protein levels.
Conclusion: The results of the study suggested that sleep deprivation might act as a compensatory factor to reduce memory impairment when the animal is under severe stressful condition.
Keywords: intensive exercise, sleep deprivation, spatial memory, hippocampus, prefrontal cortex, BDNF, TrkB
Introduction
Previous studies have confirmed the benefits of regular physical activity on mental function over the whole life course.1 Still, these exercise-induced benefits are dependent on various characteristics of physical exercise such as the differences in type (aerobic vs resistance exercise),2 the rate of recurrence (sessions per week),3 quantity (intensity and volume),4 as well as animal’s health condition and the brain region. These parameters change the intracellular signaling factors and expression of different neurotransmitters mediating the effect of exercise on physical and mental activity.5–8 Neurobiological experiments revealed an inverted U-shaped relation between the exercise intensity and its physical and mental benefits.9,10
Accordingly, emerging evidence has shown that moderate forced or voluntary activity improved cognitive functions, such as memory,11–13 executive function,14 mood in depression disorder15,16 and increased plasticity even in late life.17,18 However, in response to severe exercise, disruption of hippocampal neurogenesis,19 mitochondrial dysfunction in the brain,6 changes in intracellular mechanism involved in neurotrophins expression20 were observed as well as cognitive impairments.21–24 For instance, a high index of oxidative stress in the brain caused by 10-day intensive and exhaustive running program has been shown to impair hippocampus-dependent memory.23
The areas of the brain that are most important in learning are those involved in memory,and it is thought to rely primarily on medial temporal lobe structures, including the hippocampus with its widespread functional connections to the cortex.25 The prefrontal–hippocampal interactions are well known for incorporation of cognitive and emotional information for supporting adaptive actions like spatial information.26
Brain-derived neurotrophic factor (BDNF), the most important neurotrophin in the central nervous system, and its main receptor, TrkB,27,28 influenced by various factors affecting the learning and memory29 such as physical exercise30–32 and sleep duration30–34 have considered widely in animal studies to evaluate the effect of different factors on learning and memory. The binding of BDNF to its receptor tyrosine kinase, TrkB, results in the activation of cytoplasmic signaling pathways including mitogen-activated protein kinase, phospholipase C-ɣ, phosphatidylinositol-3 kinase (PI3-K) and Akt, a serine-threonine protein kinase that demonstrated to phosphorylate the mammalian target of rapamycin (mTOR).28 Previous studies showed activation of PI3-K/Akt/mTOR signaling and protein synthesis signaling pathway induced by BDNF is important for BDNF-dependent long-term potentiation,LTP, in the hippocampus and different types of learning and memory like spatial memory.35,36
Studies suggested that, adequate sleep has a crucial effect on different types of memory particularly hippocampus-dependent.37 Experimental evidence revealed that, two main parts of sleep including non-rapid eye movement and rapid eye movement (REM) modulated the consolidation of declarative and non-declarative memories, respectively, in a different way.35,38 In recent years, the effect of sleep deprivation (SD) on cognitive capacity and performance has received a lot of attention. Not having enough sleep influences negatively on cognitive behaviors such as attention vigilance,39 non-declarative and declarative memory,40–42 executive function,43 decision making44 and emotion perception.45 Although SD may impair cognitive capability and can reduce the learning and memory, recent experiments have shown conflicting results. For instance, different types of SD, either total or partial have a fast and transient antidepressant effect46 and they were considered as a short-term treatment for stress-induced depressive behavior.46–48
The current study was carried out to investigate the effect of REM-SD on the memory impairment induced by high-intensity exercise. In addition, to support the changes of memory performance, the level of BDNF and its receptor TrkB were evaluated in the hippocampus and prefrontal cortex.
Materials and methods
Animals
Thirty-two adult male Wistar rats (Pasteur Institute, Tehran, Iran) were used in this study. The experimental protocols and procedures in this study were approved by the Research and Ethics Committee of Tehran University of Medical Sciences, and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (1996 revision).The animals were housed in groups of four in standard polypropylene cages. The room temperature was kept at 22±1°C. The animals were housed in a 12 hrs:12 hrs light-dark schedule (07:00–19:00) and had free access to food and water. All experimental procedures were performed between 9:00 AM and 14:00 PM.
Experimental procedure
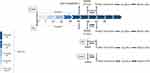
This paradigm consists of 32 animals that distributed in 4 different groups. Animals randomly assigned to control (cont., n=8), 4 weeks physical exercised (Ex, n=8), 24 hrs of REM-sleep deprivation (SD24, n=8) and the interaction of exercise and sleep deprivation (ExSd, n=8). Animals in the control group were placed in the non-running treadmill. After 24 hrs, exercised animals in the ExSd group were gone under 24-hr REM-SD. Ex group received 5 weeks treadmill with increasing intensity. The spatial and then non-spatial behavioral tests were done for control and Ex groups 24 hrs after the last exercise procedure,but it was performed in REM-SD and ExSd groups immediately after the REM-SD procedure. All rats were used for behavioral evaluation and then were sacrificed for brain tissue collecting.
Exercise training protocol
Using a 4-lane animal treadmill (IITC Life Science Inc., USA), rats were handled and trained to familiarize with running 3 days prior to start the exercise (speed: 5 m/min, 5 mins per run). The bars at the end of the treadmill were delivered electrical shocks (1.0 mA) to the hind part of the body when the animal stops running and forced them to run forward during exercise time. After 2 rest days, the procedure was performed, one session daily, 5 days a week for 5 weeks. The main phase of treadmill training was started at a speed of 18 m/min for 30 mins and no tilt for the first week. The duration and treadmill’s tilt was increased progressively, 10 mins and 5 degrees increased in each week. The speed was maintained at 18 m/min for all this period. Sedentary animals were placed on a stationary treadmill daily and were given electrical stimulation in a way identical to that used for the exercise group.49
Induction of REM-SD
REM-SD was induced by placing four to six rats in modified water-filled multiple platforms (125 cm×44 cm) that consisted of 8 small circular platforms (6.5 cm, in diameter) which submerged to about 1–2 cm above the water surface. The platforms were located at a distance so rats could move freely from one to another and balance on the platforms. Due to natural REM’s muscle paralysis, the rats would come into contact with the water and awaken. The rats had free access to clean water and food pellet baskets hanging from the aquarium cover. For the control group, using larger platforms (14 cm in diameter) caused to falling sleep without falling down.49,50 The REM-SD period lasted for 24 hrs.
Apparatus and behavioral procedure
To assess spatial learning and memory, all animals were trained in the Morris water maze (MWM), a most widely used task to assess spatial learning and memory in rodents.51 The apparatus consists of a circular, black-painted tank (pool), 150 cm in diameter and 60 cm deep, containing filled with opaque water at around 30 cm. The sufficient stressful water temperature was maintained at 20±2°C. Several distinctive, distal visual cues were placed on the walls of pool’s laboratory room and their position remained unchanged to aid animals to identify their location in space. The tank was divided into four equal quadrants with four starting locations called north (N), east (E), south (S) and west (W) at equal distances on the rim. A plexiglas escape circular platform (10 cm in diameter) was submerged 1 cm beneath the surface of the water in the center of the target quadrant (north-west quadrant). During the experiments, the images of the swimming animal were captured by a camera placed above the center of the maze, which was connected to a computer. A video tracking system (Etho-Vision XT v 8.5; Noldus Information Technology, the Netherlands) was used to measure the parameters such as the time to find the hidden platform (escape latency), path length to reach the hidden platform (traveled distance), the time spent in quadrants and the velocity of each rat in the training session.52
Eight trials in a single training session with four starting points that positioned equally around the maze. Each trial was started from one of the four starting points. During 60 s of each free-swimming trial, the animal had to find a hidden platform by using distal spatial clues. If the animal stepped on the platform, he was allowed to stay there for 20 s, if not, it was guided to the platform by the experimenter and allowed to remain there for 20 s. The gap time between trials was 20 s. In each trial, escape latency (s) and traveled distance (cm) were measured to assess the development of spatial memory, and then swimming speed (cm/s) was used to evaluate motor function. Probe trial as a retrieval test session was carried out 24 hrs after the training. It applied in a 60 s free-swimming period without a platform and traveled distance and the total time spent in the main quadrant was recorded. On the last day, following probe trial, for evaluating non-spatial visibility test, the platform that covered with a piece of aluminum foil was elevated above the water in the center of the north-east quadrant. This procedure is believed to provide information on the possible non-specific effects involving motor, visual or motivational abilities unrelated to learning and memory.52
Brain tissue collecting and Western blotting
After probe and non-spatial behavioral test, animals were sacrificed under CO2 asphyxiation. Rats were decapitated and the brain was removed. Hippocampus and PFC were dissected and frozen in liquid nitrogen immediately, and stored at −80°C until needed for later analysis. We used five brain samples for each group to perform Western blotting test.
Protein concentration was measured by spectrophotometry at 230 nm using Picodrop instrument (Picodrop, Hinxton, UK), and the results were acquired as microgram per milliliter. Samples were loaded in SDS-8% polyacrylamide gel (PH=8.3) and electrophoresis was carried out at 120 V for 120 mins, then transferred to polyvinylidene fluoride membrane (Chemicon Millipore Co., Temecula, CA, USA). To block non-specific protein binding sites, membranes were incubated in 5% skim milk (PH=8.6) for 90 mins. Then, blots were incubated overnight at 4°C with a primary antibody (Abcam, 1:1000 diluted in skimmed milk). On the next day, Tris-buffered saline and Tween 20 (TBST) were used to wash membranes three times and then blots were incubated for 1 hr, with secondary antibody (Horseradish peroxidase-linked goat anti-rabbit IgG, Abcam, 1:5000). To detect bounds, blots were exposed by enhanced chemiluminescence (ECL; Amersham, UK) and then visualized by exposure to autoradiographic film for adequate time.53
All the experimental procedure part is summarized as a timetable in Figure 1.
Statistical analysis
SPSS (Version 23) was used for this study. For trial sessions, repeated measures ANOVA was used for data analysis. In this test Ex, SD24 and ExSd groups are measured versus the control group. Data were obtained in the probe session and non-spatial test analyzed by one-way ANOVA. To comparison differences between groups, post-hoc (Tukey) test was done. For analyzing Western blotting data, Image J software was used to densitometry of bonds. After that, data obtained with Image J were subjected to one-way ANOVA analysis using SPSS. Post-hoc (Tukey) test was used for within-subject comparisons. In all comparisons, the p -values 0.05 and less were considered as statistically significant. Data were analyzed by two people individually but blinded for one of them. We did it to ensure the accuracy of the analyzing.
Results
Behavioral experiment
Twenty-four-hour REM-SD made better high-intensity exercise-induced spatial memory deficit.
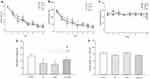
For testing the effect of REM-SD on long-term memory deficit induced by high-intensity exercise, we used a 2-day MWM protocol, where all groups went through eight trials in 1 day. Instantly after the last trial, the SD24 and ExSd24 rat groups were put down in the modified multiple platform aquarium for 24 hrs. Immediately after that, probe test was done for assessing the long-term memory. Figure 2(A–C) shows that there was no significant difference in escape latency [F(21, 196)=0.467, p=0.904], traveled distance [F(21, 196)=0.314, p=0.993] and velocity [F(21, 196)=0.769, p=0.692] between REM-SD, Ex and ExSd groups compared with the control group in the learning stage. As is shown in Figure 2D, one-way ANOVA shows a significant difference between groups in the total time spent in the main quadrant over the probe test [F(28, 3)=7.631, P=0.0008]. Post-hoc Tukey analysis showed differences for Ex (p<0.01), REM-SD (p<0.01) and ExSd (p<0.05) in comparison with the control group. One-Way ANOVA revealed there is no significant difference between groups and control for non-spatial test (Figure 2E), [F(28,3)=2.932, p=0.0508].
The effect of the combination of high-intensity exercise and REM-SD on hippocampal/prefrontal BDNF levels
Figure 3A–B shows that there is a significant difference between groups versus control in BDNF levels in the hippocampus [F(16, 3)=21.06, p<0.0001] and prefrontal cortex [F(16, 3)=18.6, p<0.0001]. Post-hoc Tukey analysis showed that there were significant differences in the level of TrkB in the hippocampus between Ex and SD (p<0.001), and ExSd (p<0.01) groups in the hippocampus as compared with the control group. In addition, ExSd group showed an enhancement in BDNF level in comparison with the Ex (p<0.001) and the SD (p<0.01) groups in the hippocampus. Figure 3B illustrates that BDNF level in PFC is significantly lower in the Ex (p<0.01) and SD (p<0.001) groups when compared with the control group; however there is no significant difference between ExSd and control group. Following the intervention of Ex and SD, BDNF was upregulated when compared with the Ex (p<0.001) and SD (p<0.0001) groups.
The effect of the combination of high-intensity exercise and REM-SD on hippocampal/prefrontal TrkB levels
Figure 3C–D shows that there is a significant difference between groups versus control in TrkB levels in the hippocampus [F(16, 3)=10.806, p<0.0001] and prefrontal cortex [F(16, 3)=6.187, p=0.0023]. Post-hoc Tukey analysis showed that there were significant differences in the level of TrkB in the hippocampus between Ex and SD (p<0.001) and ExSd (p<0.05) groups as compared with the control group. In addition, ExSd group showed no significant difference in TrkB level in comparison to the Ex and the SD groups in the hippocampus. Figure 3D illustrates that the level of TrkB in the PFC was significantly lower in the Ex (p<0.01) and SD (p<0.05) groups when compared with the control group; however, there is no significant difference between ExSd and control group. Following the intervention of Ex and SD, TrkB was upregulated when compared with the Ex (p<0.001) and SD (p<0.0001) groups.
Discussion
There is a complex relationship between the exercise and its effect on the cognitive ability, depending on different characteristics of exercise, such as the intensity, duration and type of exercise. The exercise intensity may influence brain oxygen utilization,54 the formation of mitochondrial ROS,5,6 inducing antioxidant imbalance and disturbance of the cellular signaling,55 spatial memory impairment, object recognition disruption56–58 as well as reduction in arousal level by intensive exercise and reaching to the optimal level after medium intensity.59 Therefore, in relation to the complexity of the exercise biology, inverted U-shaped dose–response curves were defined, where low doses are stimulatory and high doses are inhibitory.10 In our study, the combined effect of SD and intense exercise on hippocampus-dependent learning and memory was investigated. Results displayed that, a 5-week gradual high-intensity training exercise may cause a decrease in the total time in the target zone of MWM test with corresponding reductions in the expression of hippocampal and prefrontal BDNF and TrkB level represented to reduce memory performance. Physical fatigue and exhaustion,60 excessive blood lactate,61 decreased glucose uptake,19,62,63 changes in metabolism64 and oxidative stress,65,66 altered inflammatory cytokine expression67 and increased susceptibility to infection68 might be the explanations regarding the adverse effect of intensive exercise. However, the most important issue in the cognitive impairments induced by intense physical exercise might be exhaustion,69 increasing free radical and ROS levels.66,70 Brain with high mitochondrial energy metabolism and poor antioxidant defenses could be affected by oxidative damage induced by high ROS levels71 which, in turn, results in a decrease in the BDNF levels72 and an increase in the neuron degeneration.73
Moreover, chronic and daily foot shock, not single dose, as a stressful experience has been shown to negatively influence the hippocampal neurogenesis. In our study, changes in the BDNF and TrkB levels in the brain were in agreement with the behavioral data. These molecular reductions in the hippocampus and the PFC were associated with impairment of spatial memory.74,75 In the animal models of stress and depression, production of ROS increased in the PFC and hippocampus.73,74 The enhancement of the ROS level in the PFC might be one of the critical factors in decreasing BDNF expression and induction of depressive-like symptoms.76 Stress could also alter the brains’ chemical components such as BDNF, cAMP response element-binding protein , extracellular signal-regulated kinases (ERK1/2) levels73,77–79 in the hippocampus consequently promoting oxidative stress-induced depression-like behaviors and causing memory impairment in rats.80
The destructive effect of sleep deprivation on the cognitive function is generally accepted.81–83 However, one of the controversial findings in the recent studies is the different action of sleep deprivation on the cognitive capability and expression of some neurochemicals in different parts of the brain and then applying these benefits to help cure some cognitive diseases like depression84–86 and mood disorders.87,88 In our study, disturbance of memory function after intensive exercise was ameliorated by subsequent 24-hr REM-SD. In parallel, results also revealed a rise in the levels of BDNF/TrkB within the hippocampus and PFC.
SD for one night subtracted depressive symptoms in 40–60% of cases;89 furthermore, a higher decrease was observed in depressive symptoms by a reduction in REM sleep phase.90 However, previous researches showed different results regarding total sleep deprivation or REM-SD on memory performance and hippocampal BDNF level. For instance, 8 hrs of total sleep deprivation showed no changes in BDNF level91 but short-term REM-SD caused an increase in the BDNF level in the ratsʼ hippocampus.47 However, Fujihara et al92 showed 1–2 hrs SD induced an increase in the BDNF level but 8 and 48 hrs of SD decreased BDNF level in the hippocampus.93 Some studies showed that, extended wakefulness might result in an increased level of BDNF in the hippocampus.94
However, because of partial non-REM deprivation based on our SD protocol,95 REM deprivation and then an increase in wakefulness state, it is not clear which part is mainly responsible for elevation of BDNF/TrkB expression. Furthermore, the proposed mechanisms related to SD that might elevate the BDNF level in rats’ hippocampus are: 1) sleep homeostatic and circadian mechanisms referring to the two process models of sleep regulation;96 2) monoaminergic mechanisms referring to a significant role of monoamines between SD and antidepressants;97 and 3) glutamatergic mechanisms and synaptic plasticity referring to a suggested role for the glutamate and its interplay with monoamine neurotransmitters in the fast antidepressant consequence of SD.98
In this study, the memory performance and BDNF/TrkB levels significantly increased in ExSd group compared to Ex and 24h SD groups, yet their decrease was still less than the control group. Said differently, REM-SD just caused the BDNF expression notably better and not restored it completely.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vina J, Sanchis‐Gomar F, Martinez‐Bello V, Gomez‐Cabrera M. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012;167(1):1–12. doi:10.1111/j.1476-5381.2012.01970.x
2. Pontifex MB, Hillman CH, Fernhall B, Thompson KM, Valentini TA. The effect of acute aerobic and resistance exercise on working memory. Med Sci Sports Exercise. 2009;41(4):927–934. doi:10.1249/MSS.0b013e3181907d69
3. Berchtold N, Chinn G, Chou M, Kesslak J, Cotman C. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–861. doi:10.1016/j.neuroscience.2005.03.026
4. Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res. 2013;2013:5–6.
5. Aguiar A
6. Aguiar AS, Tuon T, Pinho CA, et al. Intense exercise induces mitochondrial dysfunction in mice brain. Neurochem Res. 2008;33(1):51–58. doi:10.1007/s11064-007-9406-x
7. Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95.
8. Radak Z, Taylor AW, Ohno H, Goto S. Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc Immunol Rev. 2001;7:90–107.
9. Diederich K, Bastl A, Wersching H, et al. Effects of different exercise strategies and intensities on memory performance and neurogenesis. Front Behav Neurosci. 2017;11:47. doi:10.3389/fnbeh.2017.00246
10. Hu S, Ying Z, Gomez-Pinilla F, Frautschy SA. Exercise can increase small heat shock proteins (sHSP) and pre-and post-synaptic proteins in the hippocampus. Brain Res. 2009;1249:191–201. doi:10.1016/j.brainres.2008.10.054
11. Kim S-E, Ko I-G, Kim B-K, et al. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010;45(5):357–365. doi:10.1016/j.exger.2010.02.005
12. Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Paper presented at: Mayo Clinic Proceedings; 2011.
13. Sofi F, Valecchi D, Bacci D, et al. Physical activity and risk of cognitive decline: a meta‐analysis of prospective studies. J Intern Med. 2011;269(1):107–117. doi:10.1111/j.1365-2796.2010.02281.x
14. Verburgh L, Königs M, Scherder EJ, Oosterlaan J. Physical exercise and executive functions in preadolescent children, adolescents and young adults: a meta-analysis. Br J Sports Med. 2014;48(12):973–979. doi:10.1136/bjsports-2012-091441
15. Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. Int J Psychiatry Med. 2011;41(1):15–28. doi:10.2190/PM.41.1.c
16. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Sys Rev. 2013;9:9.
17. Erickson KI, Gildengers AG, Butters MA. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci. 2013;15(1):99.
18. Erickson KI, Miller DL, Weinstein AM, Akl SL, Banducci S. Physical activity and brain plasticity in late adulthood: a conceptual and comprehensive review. Ageing Res. 2012;3(1):e6–e6. doi:10.4081/ar.2012.e6
19. Lou S-J, Liu J-Y, Chang H, Chen P-J. Hippocampal neurogenesis and gene expression depend on exercise intensity in juvenile rats. Brain Res. 2008;1210:48–55. doi:10.1016/j.brainres.2008.02.080
20. Siamilis S, Jakus J, Nyakas C, et al. The effect of exercise and oxidant–antioxidant intervention on the levels of neurotrophins and free radicals in spinal cord of rats. Spinal Cord. 2009;47(6):453. doi:10.1038/sc.2008.104
21. Grebot C, Groslambert A, Pernin J-N, Burtheret A, Rouillon J-D. Effects of exercise on perceptual estimation and short-term recall of shooting performance in a biathlon. Percept Mot Skills. 2003;97(3_suppl):1107–1114. doi:10.2466/pms.2003.97.3f.1107
22. Rhodes JS, Van Praag H, Jeffrey S, et al. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003;117(5):1006. doi:10.1037/0735-7044.117.5.1006
23. Rosa EF, Takahashi S, Aboulafia J, Nouailhetas VL, Oliveira MG. Oxidative stress induced by intense and exhaustive exercise impairs murine cognitive function. J Neurophysiol. 2007;98(3):1820–1826. doi:10.1152/jn.01158.2006
24. Taverniers J, Van Ruysseveldt J, Smeets T, von Grumbkow J. High-intensity stress elicits robust cortisol increases, and impairs working memory and visuo-spatial declarative memory in special forces candidates: a field experiment. Stress. 2010;13(4):324–334. doi:10.3109/10253891003642394
25. Brem A-K, Ran K, Pascual-Leone A. Learning and memory. In: Lozano AM, Hallett M, editors. Handbook of Clinical Neurology. Vol. 116. Elsevier; 2013: 693–737. doi: 10.1016/B978-0-444-53497-2.00055-3
26. Churchwell JC, Morris AM, Musso ND, Kesner RP. Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiol Learn Mem. 2010;93(3):415–421. doi:10.1016/j.nlm.2009.12.008
27. Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24(1):677–736. doi:10.1146/annurev.neuro.24.1.677
28. Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91(4):267–270.
29. Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci. 2010;3:1.
30. Coles K, Tomporowski PD. Effects of acute exercise on executive processing, short-term and long-term memory. J Sports Sci. 2008;26(3):333–344. doi:10.1080/02640410701591417
31. Parkhurst CN, Yang G, Ninan I, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. doi:10.1016/j.cell.2013.11.030
32. Vivar C, Potter MC, van Praag H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. In: Belzung C, Wigmore P, editors. Neurogenesis and Neural Plasticity. Springer; 2012:189–210.
33. Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi:10.1016/j.smrv.2005.05.002
34. Sei H, Saitoh D, Yamamoto K, Morita K, Morita Y. Differential effect of short-term REM sleep deprivation on NGF and BDNF protein levels in the rat brain. Brain Res. 2000;877(2):387–390. doi:10.1016/s0006-8993(00)02708-6
35. Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9(4):534–547. doi:10.1162/jocn.1997.9.4.534
36. Mizuno M, Yamada K, Takei N, et al. Phosphatidylinositol 3-kinase: a molecule mediating BDNF-dependent spatial memory formation. Mol Psychiatry. 2003;8(2):217. doi:10.1038/sj.mp.4001224
37. Gais S, Lucas B, Born J. Sleep after learning aids memory recall. Learn Memory. 2006;13(3):259–262. doi:10.1101/lm.132106
38. Plihal W, Born J. Effects of early and late nocturnal sleep on priming and spatial memory. Psychophysiology. 1999;36(5):571–582.
39. Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129(1):305–322. doi:10.1196/annals.1417.002
40. Chee MW, Chuah LY. Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Curr Opin Neurol. 2008;21(4):417–423. doi:10.1097/WCO.0b013e3283052cf7
41. Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8(4):331–343. doi:10.1016/j.sleep.2007.03.011
42. Maquet P. The role of sleep in learning and memory. science. 2001;294(5544):1048–1052. doi:10.1126/science.1062856
43. Nilsson JP, Söderström M, Karlsson AU, et al. Less effective executive functioning after one night’s sleep deprivation. J Sleep Res. 2005;14(1):1–6. doi:10.1111/j.1365-2869.2005.00442.x
44. Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6(3):236.
45. Lautenbacher S, Kundermann B, Krieg J-C. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10(5):357–369. doi:10.1016/j.smrv.2005.08.001
46. Gillin JC, Buchsbaum M, Wu J, Clark C, Bunney W
47. Jiang Y, Zhu J. Effects of sleep deprivation on behaviors and abnormal hippocampal BDNF/miR-10B expression in rats with chronic stress depression. Int J Clin Exp Pathol. 2015;8(1):586.
48. Hines D, Schmitt L, Hines R, Moss S, Haydon P. Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Transl Psychiatry. 2013;3(1):e212. doi:10.1038/tp.2012.136
49. Alkadhi K, Zagaar M, Alhaider I, Salim S, Aleisa A. Neurobiological consequences of sleep deprivation. Curr Neuropharmacol. 2013;11(3):231–249. doi:10.2174/1570159X11311030001
50. Aghajani M, Faghihi M, Imani A, et al. Post-infarct sleep disruption and its relation to cardiac remodeling in a rat model of myocardial infarction. Chronobiol Int. 2017;34(5):587–600. doi:10.1080/07420528.2017.1281823
51. D’Hooge R, De Deyn PP. Applications of the morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36(1):60–90.
52. Alijanpour S, Tirgar F, Zarrindast MR. Role of dorsal hippocampal orexin-1 receptors in memory restoration induced by morphine sensitization phenomenon. Neuroscience. 2016;312:215–226. doi:10.1016/j.neuroscience.2015.11.023
53. Ashabi G, Sadat-Shirazi M-S, Khalifeh S, Elhampour L, Zarrindast M-R. NMDA receptor adjusted co-administration of ecstasy and cannabinoid receptor-1 agonist in the amygdala via stimulation of BDNF/Trk-B/CREB pathway in adult male rats. Brain Res Bull. 2017;130:221–230. doi:10.1016/j.brainresbull.2017.01.020
54. Ide K, Secher NH. Cerebral blood flow and metabolism during exercise. Prog Neurobiol. 2000;61(4):397–414.
55. Aguiar A
56. Blustein JE, McLaughlin M, Hoffman JR. Exercise effects stress-induced analgesia and spatial learning in rats. Physiol Behav. 2006;89(4):582–586. doi:10.1016/j.physbeh.2006.07.017
57. García-Capdevila S, Portell-Cortés I, Torras-Garcia M, Coll-Andreu M, Costa-Miserachs D. Effects of long-term voluntary exercise on learning and memory processes: dependency of the task and level of exercise. Behav Brain Res. 2009;202(2):162–170. doi:10.1016/j.bbr.2009.03.020
58. Kennard JA, Woodruff-Pak DS. A comparison of low-and high-impact forced exercise: effects of training paradigm on learning and memory. Physiol Behav. 2012;106(4):423–427. doi:10.1016/j.physbeh.2012.02.023
59. Chang Y-K, Chi L, Etnier JL, Wang -C-C, Chu C-H, Zhou C. Effect of acute aerobic exercise on cognitive performance: role of cardiovascular fitness. Psychol Sport Exerc. 2014;15(5):464–470. doi:10.1016/j.psychsport.2014.04.007
60. Kashihara K, Maruyama T, Murota M, Nakahara Y. Positive effects of acute and moderate physical exercise on cognitive function. J Physiol Anthropol. 2009;28(4):155–164.
61. Chmura J, Nazar K, Kaciuba-Uścilko H. Choice reaction time during graded exercise in relation to blood lactate and plasma catecholamine thresholds. Int J Sports Med. 1994;15(04):172–176. doi:10.1055/s-2007-1021042
62. Kemppainen J, Aalto S, Fujimoto T, et al. High intensity exercise decreases global brain glucose uptake in humans. J Physiol. 2005;568(1):323–332. doi:10.1113/jphysiol.2005.091355
63. Secher NH, Quistorff B. Brain glucose and lactate uptake during exhaustive exercise. J Physiol. 2005;568(1):3. doi:10.1113/jphysiol.2005.095786
64. Vaynman S, Ying Z, Wu A, Gomez-Pinilla F. Coupling energy metabolism with a mechanism to support brain-derived neurotrophic factor-mediated synaptic plasticity. Neuroscience. 2006;139(4):1221–1234. doi:10.1016/j.neuroscience.2006.01.062
65. Hoffman-Goetz L, Spagnuolo P, Guan J. Repeated exercise in mice alters expression of IL-10 and TNF-α in intestinal lymphocytes. Brain Behav Immun. 2008;22(2):195–199. doi:10.1016/j.bbi.2007.07.002
66. Aguiló A, Tauler P, Fuentespina E, Tur JA, Córdova A, Pons A. Antioxidant response to oxidative stress induced by exhaustive exercise. Physiol Behav. 2005;84(1):1–7. doi:10.1016/j.physbeh.2004.07.034
67. Coleman MA, Garland T
68. Kohut M, Davis J, Jackson D, et al. Exercise effects on IFN-β expression and viral replication in lung macrophages after HSV-1 infection. Am J Physiol Lung Cell Mol Physiol. 1998;275(6):L1089–L1094.
69. Hansson A, Sommer W, Metsis M, Strömberg I, Agnati L, Fuxe K. Corticosterone actions on the hippocampal brain‐derived neurotrophic factor expression are mediated by exon IV promoter. J Neuroendocrinol. 2006;18(2):104–114.
70. Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639(1):167–170.
71. McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220(5170):911. doi:10.1038/220911a0
72. Gray J, Milner T, McEwen B. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214–227. doi:10.1016/j.neuroscience.2012.08.034
73. Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(3):238. doi:10.1038/nm.4050
74. Schiavone S, Jaquet V, Trabace L, Krause K-H. Severe life stress and oxidative stress in the brain: from animal models to human pathology. Antioxid Redox Signal. 2013;18(12):1475–1490.
75. Jesse C, Donato F, Giacomeli R, et al. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na+, K+-ATPase activity in the hippocampus and prefrontal cortex of mice: antidepressant effect of chrysin. Neuroscience. 2015;289:367–380. doi:10.1016/j.neuroscience.2014.12.048
76. Ibi M, Liu J, Arakawa N, et al. Depressive-like behaviors are regulated by NOX1/NADPH oxidase by redox modification of NMDA receptor 1. J Neurosci. 2017;37(15):4200–4212. doi:10.1523/JNEUROSCI.2988-16.2017
77. Murínová J, Hlaváčová N, Chmelová M, Riečanský I. The evidence for altered BDNF expression in the brain of rats reared or housed in social isolation: a systematic review. Front Behav Neurosci. 2017;11:101. doi:10.3389/fnbeh.2017.00246
78. Ota KT, Liu R-J, Voleti B, et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med. 2014;20(5):531.
79. Zhou W, Wang N, Yang C, Li X-M, Zhou Z-Q, Yang J-J. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry. 2014;29(7):419–423. doi:10.1016/j.eurpsy.2013.10.005
80. Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013;1539:73–86. doi:10.1016/j.brainres.2013.09.033
81. Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Memory. 2003;10(3):168–176. doi:10.1101/lm.48803
82. Fishbein W. Disruptive effects of rapid eye movement sleep deprivation on long-term memory. Physiol Behav. 1971;6(4):279–282. doi:10.1016/0031-9384(71)90155-7
83. Silva R, Abilio V, Takatsu A, et al. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004;46(6):895–903. doi:10.1016/j.neuropharm.2003.11.032
84. Wirz-Justice A, Van Den Hoofdakker RH. Sleep deprivation in depression: what do we know, where do we go? Biol Psychiatry. 1999;46(4):445–453.
85. Walker MP, van Der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135(5):731. doi:10.1037/a0016570
86. Giedke H, Schwärzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6(5):361–377. doi:10.1053/smrv.2002.0235
87. Ibrahim L, Duncan W, Luckenbaugh DA, Yuan P, Machado-Vieira R, Zarate CA
88. Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147(1):14. doi:10.1176/ajp.147.1.14
89. Zucconi GG, Cipriani S, Balgkouranidou I, Scattoni R. ‘One night’sleep deprivation stimulates hippocampal neurogenesis. Brain Res Bull. 2006;69(4):375–381. doi:10.1016/j.brainresbull.2006.01.009
90. Landsness EC, Goldstein MR, Peterson MJ, Tononi G, Benca RM. Antidepressant effects of selective slow wave sleep deprivation in major depression: a high-density EEG investigation. J Psychiatr Res. 2011;45(8):1019–1026. doi:10.1016/j.jpsychires.2011.02.003
91. Taishi P, Sanchez C, Wang Y, Fang J, Harding J, Krueger J. Conditions that affect sleep alter the expression of molecules associated with synaptic plasticity. Am J Physiol Regul Integr Comp Physiol. 2001;281(3):R839–R845. doi:10.1152/ajpregu.2001.281.3.R839
92. Fujihara H, Sei H, Morita Y, Ueta Y, Morita K. Short-term sleep disturbance enhances brain-derived neurotrophic factor gene expression in rat hippocampus by acting as internal stressor. J Mol Neurosci. 2003;21(3):223–231.
93. Guzman‐Marin R, Ying Z, Suntsova N, et al. Suppression of hippocampal plasticity‐related gene expression by sleep deprivation in rats. J Physiol. 2006;575(3):807–819. doi:10.1113/jphysiol.2006.115287
94. Peyron C, Wurts S, Srere H, Heller H, Edgar D, Kilduff T mRNA level of brain-derived neurotrophic factor increases in several brain regions after sleep deprivation. Paper presented at: Society for Neuroscience Abstracts; 1998.
95. Mendelson WB, Guthrie RD, Frederick G, Wyatt RJ. The flower pot technique of rapid eye movement (REM) sleep deprivation. Pharmacol Biochem Behav. 1974;2(4):553–556. doi:10.1016/0091-3057(74)90018-5
96. Clark CP, Brown GG, Archibald SL, et al. Does amygdalar perfusion correlate with antidepressant response to partial sleep deprivation in major depression? Psychiatry Res. 2006;146(1):43–51. doi:10.1016/j.pscychresns.2005.09.007
97. Kavčič P, Rojc B, Dolenc-Grošelj L, Claustrat B, Fujs K. The impact of sleep deprivation and nighttime light exposure on clock gene expression in humans. Croat Med J. 2011;52(5):594–603. doi:10.3325/cmj.2011.52.594
98. Dallaspezia S, Benedetti F. Sleep deprivation therapy for depression. In: Meerlo P, Benca RM, Abel T, editors. Sleep, Neuronal Plasticity and Brain Function. Springer; 2014:483–502.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.