Back to Journals » Drug Design, Development and Therapy » Volume 15
Bempedoic Acid for Heterozygous Familial Hypercholesterolemia: From Bench to Bedside
Authors Agarwala A, Quispe R, Goldberg AC, Michos ED
Received 30 December 2020
Accepted for publication 30 March 2021
Published 10 May 2021 Volume 2021:15 Pages 1955—1963
DOI https://doi.org/10.2147/DDDT.S251865
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Georgios Panos
Anandita Agarwala,1 Renato Quispe,2 Anne C Goldberg,3 Erin D Michos2
1Division of Cardiology, Baylor Scott and White Health, Heart Hospital Baylor Plano, Plano, TX, USA; 2Ciccarone Center for the Prevention of Cardiovascular Disease, Division of Cardiology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA; 3Division of Endocrinology, Metabolism and Lipid Research, Washington University School of Medicine, St. Louis, MO, USA
Correspondence: Erin D Michos
Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins Hospital, 600 N. Wolfe Street, Blalock 524-B, Baltimore, MD, 21287, USA
Tel +410-502-6813
Email [email protected]
Abstract: Bempedoic acid is a first-in-class, oral, inhibitor of cholesterol biosynthesis that is approved for use in patients with atherosclerotic cardiovascular disease (ASCVD) and for primary prevention in individuals with heterozygous familial hypercholesterolemia (HeFH) by the United States Food and Drug Administration. Pooled data from the phase III clinical trials, CLEAR Harmony and CLEAR Wisdom, have demonstrated the safety and efficacy of bempedoic acid with regard to lowering of low-density lipoprotein cholesterol (LDL-C) in patients with HeFH as an adjunct or alternative to currently existing lipid-lowering therapies. CLEAR Outcomes is a cardiovascular outcomes trial that is currently underway that will provide additional insight as to where bempedoic acid will fit into treatment regimens among the non-statin lipid-lowering therapy options. Patients who might particularly benefit from bempedoic acid are those with HeFH and those unable to take adequate doses of statins or take any statin therapy altogether who need additional LDL-C lowering. In this review, we will discuss the profile of bempedoic acid from its design, development, and its place in therapy for the management of LDL-C for the purposes of ASCVD prevention.
Keywords: bempedoic acid, familial heterozygous hypercholesterolemia, lipids, atherosclerotic cardiovascular disease, prevention, low-density lipoprotein cholesterol
Introduction
Heterozygous Familial Hypercholesterolemia (HeFH) is characterized by inherited elevations in low-density lipoprotein cholesterol (LDL-C) levels and is associated with a substantially increased risk for atherosclerotic cardiovascular disease (ASCVD).1 HeFH patients carry mutations in one allele of the LDL receptor (LDLR), apolipoprotein B (ApoB), or proprotein convertase subtilisin/kexin type 9 (PCSK9) genes.
While statins have been the mainstay of therapy in these patients, statins alone are often unable to reduce LDL-C levels to the desired therapeutic threshold, and additional pharmacologic therapy may be needed to reduce ASCVD risk.2 Furthermore, some patients are unable to tolerate sufficient or any statin therapy and need alternative pharmacotherapy for adequate LDL-C lowering. Current adjuncts or alternatives to statin therapy include ezetimibe and PCSK9 inhibitors.3,4 Ezetimibe is oral, affordable, and well tolerated; as such, it is the next recommended LDL-C-lowering agent after statins. However, even with the combination of ezetimibe and maximally tolerated statin dose, many patients with HeFH remain above the LDL-C thresholds of 100 mg/dL (primary prevention) and 70 mg/dL (secondary prevention) where add-on therapy for additional LDL-C lowering would be recommended per recent guidelines.5
The 2018 American Heart Association (AHA)/American College of Cardiology (ACC)/Multi-society guideline for the management of blood cholesterol stated that for patients with severe primary hypercholesterolemia that remain above a threshold LDL-C ≥100 mg/dL despite statin plus ezetimibe therapy, in the setting of other ASCVD risk factors, a PCSK9 inhibitor would be reasonable.5 The guideline also states that PCSK9 inhibitors are recommended for secondary prevention patients with ASCVD who remain at a threshold above 70 mg/dL despite therapy with statin plus ezetimibe.5 Nevertheless, therapy with PCSK9 inhibitors is challenging, with greater costs and burdensome pre-authorization approvals in the United States, as well as subcutaneous injection creating barriers for some patients.6,7 Thus, there is a need for alternative less expensive, effective, oral options for LDL-C lowering.
Bempedoic acid is a first-in-class, oral, small molecule inhibitor of cholesterol biosynthesis that functions in the same pathway as statins to lower LDL-C. Bempedoic acid was approved by the Food and Drug Administration (FDA) in February 2020 for use in the United States (US) as an adjunct to diet and maximally tolerated statin therapy for secondary prevention in patients with ASCVD and for primary prevention in individuals with HeFH, who require additional LDL-C lowering. This FDA approval was after the 2018 AHA/ACC Blood Cholesterol Guideline was published, and as such, bempedoic acid was not specifically addressed in prior guidelines.
Mechanism of Action
Bempedoic acid inhibits adenosine triphosphate citrate lyase (ACL), a cytosolic enzyme that is highly expressed in lipogenic tissues and functions upstream of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCoA reductase), the target of statin therapy (Figure 1). ACL inhibition decreases intracellular cholesterol biosynthesis and results in the upregulation of LDL receptors on hepatocytes, increasing uptake of LDL particles and ultimately reducing circulating LDL-C levels.8 ACL plays a key role in linking energy metabolism from carbohydrates to the production of fatty acids because it catalyzes the cleavage of mitochondrial-derived citrate to cytosolic acetyl-CoA and oxaloacetate. Acetyl-CoA is the fundamental building block for de novo synthesis of cholesterol and fatty acids.9 In a complex metabolism of cholesterol biosynthesis that is outside the scope of this review, two ACL-derived acetyl-CoA units are condensed to HMG-CoA by HMG-CoA synthase, and then reduced to mevalonate by action of HMG-CoA reductase.10 Production of mevalonate is the rate limiting step in cholesterol biosynthesis. Statins are HMG-CoA reductase inhibitors.
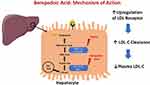 |
Figure 1 Bempedoic acid mechanism of action in the hepatocyte. |
Given its key role in cholesterol synthesis upstream of HMG-CoA reductase, ACL has been a potential target of therapeutic interventions. Prior efforts in in vitro inhibition of ACL have been unsuccessful, possibly due to the compounds’ poor ability to cross cell membranes, reduced affinity for ACL, and nonspecific inhibition of other essential enzymes in vivo.10
Bempedoic acid is an oral, once-daily, small molecule with a half-life of 15–24 hours. It is rapidly absorbed in the small intestine and converted in the liver by an endogenous liver acyl-CoA-synthetase to a coenzyme A derivative. The active metabolite is responsible for the inhibition of ACL. In addition to ACL inhibition, bempedoic acid enhances the activity of 5ʹ adenosine monophosphate-activated protein kinase (AMPK). AMPK activity inhibits the phosphorylation of acetyl-CoA carboxylase and HMG-CoA reductase, reducing both glucose and lipid biosynthesis, thereby contributing to LDL-C reduction via a different mechanism.
A series of genetic, pharmacologic and mouse models have shown that bempedoic acid has ACL and AMPK activities that occur only in cells that are capable of metabolizing bempedoic acid into its active form. In humans, the enzyme responsible for this, very-long-chain acyl-CoA synthetase-1 (ACSVL1) is expressed only by liver cells and not in skeletal muscle.11 Indeed, in vitro studies have shown that bempedoic acid did not have any effect on cholesterol synthesis in muscle cells, nor did it induce muscle-related adverse effects in myotubes, compared with simvastatin or atorvastatin.11 This is a potential mechanism by which bempedoic acid avoids the muscle-related adverse effects that are associated with statins, making bempedoic acid a particularly attractive option for patients with statin-associated muscle symptoms (SAMS).
Preclinical Studies
Bempedoic acid (initially known as ESP-55016 and later as ETC-1002) was initially identified after chemical synthesis as ω-hydroxy-alkanedicarboxylic acid (ESP-55016) and found to have remarkable lipid-lowering activity in female Zucker (fa/fa rats), a model of diabetic dyslipidemia.12 Further investigations on ETC-1002 showed that this molecule acts as an inhibitor of ACL, now known to be its major mode of action.13 In view of its complex mechanism, specific investigations on its properties were performed in primary human monocyte-macrophages and in vivo models of inflammation, which showed that bempedoic acid drives reduction in pro-inflammatory cytokines and chemokines.14 More recent investigations were specifically targeted to animal models with hypercholesterolemia. For instance, bempedoic acid was shown to significantly reduce cholesterol and triglyceride levels as well as improve glucose tolerance in Ldlr-/- mice, in addition to a clear suppression of cholesterol ester accumulation in the aortas from treated animals.15 Finally, the most definitive evidence of ACL inhibition as the mechanism of action was provided by Pinkosky et al.11 Bempedoic acid is activated by very-long chain acyl-CoA synthetase-1 (ACSVL1) to produce its downstream effects of inhibiting ACL, thereby reducing visceral adiposity, proinflammatory cytokines and chemokines, and atherosclerotic plaque size in mouse models.
Phase I and II Clinical Trials
Fifteen phase I clinical trials evaluated the safety and tolerability of single and multiple doses of bempedoic acid in healthy individuals as well as those with mild dyslipidemia.16–18 The results from phase I trials demonstrated on-treatment reductions in LDL-C levels ranging between 17% and 36%. No dose-related side effects were noted in phase I clinical trials.16–18
Ten Phase II clinical trials investigated the safety and efficacy of bempedoic acid in patients with hypercholesterolemia. Specific subpopulations explored in the phase II trials include patients with type 2 diabetes, statin intolerance, hypertension, on a background of either low- or high- intensity statin therapy, on a background of ezetimibe with and without statin therapy, and patients on a PCSK9 inhibitor (evolocumab).19–27 Bempedoic acid was generally well tolerated across these trials and resulted in LDL-C lowering that ranged from 13% to 43%.19–27
Phase III Clinical Trials
The efficacy and safety of bempedoic acid among patients with HeFH was assessed in two placebo-controlled Phase 3 studies: Cholesterol Lowering via Bempedoic Acid, an ACL-Inhibiting Regimen (CLEAR) Wisdom and CLEAR Harmony.28,29
CLEAR Harmony randomized patients with ASCVD with or without the presence of HeFH.28 A total of 2230 patients with LDL-C levels ≥70 mg/dL taking maximally tolerated statin therapy were randomized to receive either bempedoic acid at a dose of 180 mg daily (n = 1487) vs placebo (n = 742) and followed for 52 weeks with a primary endpoint of safety and a secondary endpoint of percent change in LDL-C at week 12. It should be noted in this trial, that nearly 100% of the patients were taking statins and approximately 50% were on a high-intensity statin. At week 12, bempedoic acid demonstrated a 16.5% reduction in LDL-C compared to baseline and an 18.1% reduction compared to the placebo arm (p<0.001). Furthermore, there was an 11.9% reduction in apoB and a 21.5% reduction in high-sensitivity C-reactive protein (hsCRP) at week 12 compared to baseline (p<0.001 for both). These data confirmed the safety and efficacy of bempedoic acid as an add-on to statin therapy regardless of background statin intensity.
CLEAR Wisdom enrolled adults with ASCVD with or without HeFH and LDL-C level ≥70 mg/dL on maximally tolerated lipid-lowering therapy.29 The study randomized 779 patients to either 180 mg bempedoic acid or placebo once daily for a total of 52 weeks. The primary endpoint was percent change in LDL-C from baseline at week 12 of the study. Baseline LDL-C levels were 120 mg/dL, and treatment with bempedoic acid resulted in a 15.1% decrease in LDL-C levels at week 12 compared to baseline levels. Reductions were also noted in total cholesterol (TC) (9.9%), ApoB (9.3%), and hsCRP (median reduction of 18.7%). Patients receiving bempedoic acid vs placebo experienced a reduction of 15.1% vs a 2.4% increase, respectively (difference of 17.4%, p <0.001).
Pooled analyses from CLEAR Harmony and CLEAR Wisdom included a total of 3009 participants with ASCVD, of which 112 (3.7%) had a diagnosis of HeFH. The primary efficacy endpoint was mean percent change in LDL-C compared with baseline at week 12. Characteristics of patients from the pooled analysis with and without HeFH are shown in Table 1.30 Baseline LDL-C levels were higher in individuals with HeFH (n = 112; bempedoic acid, 144.1 [45.1] mg/dL; placebo, 156.5 [69.2] mg/dL) compared to those without HeFH (n = 2897; bempedoic acid, 106.3 [30.9] mg/dL; placebo, 105.6 [30.0] mg/dL). At week 12, patients who received bempedoic acid exhibited a significant reduction in LDL-C levels with placebo-corrected reduction of −22.3% (95% CI −33.3, −11.4) vs −18.3% (95% CI −18.3, −16.6) among individuals with and without HeFH, respectively (P <0.001).
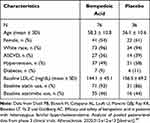 |
Table 1 Baseline Characteristics of Patients with Heterozygous Familial Hypercholesterolemia in Pooled Phase III Analysis |
Secondary efficacy endpoints included percent change at week 12 in non–high-density lipoprotein cholesterol (non-HDL-C), TC, ApoB, hsCRP, triglycerides (TGs), and HDL-C levels. Significant reductions were noted in non-HDL-C, TC, ApoB, and hsCRP in patients with HeFH treated with bempedoic acid vs placebo (Table 2).30 Additionally, no differences were observed in the safety profile among individuals from CLEAR Harmony and CLEAR Wisdom with and without HeFH with regard to adverse events, serious adverse events, and adverse events leading to discontinuation of the drug.
 |
Table 2 Secondary Efficacy Endpoints in Patients with and without Heterozygous Familial Hypercholesterolemia in Pooled Phase III Analysis |
The CLEAR Serenity trial examined bempedoic acid in patients with statin intolerance.31 This trial enrolled 345 patients with hypercholesterolemia and a history of statin intolerance; 93% with a history of SAMS. This trial included 2% (7 out of 345) of patients with HeFH. At 12 weeks, bempedoic acid compared to placebo reduced LDL-C by 21% and hsCRP by 24%. Myalgias occurred less commonly with bempedoic acid than placebo (4.7% vs 7.2%).31 There was no interaction between the primary outcome of change in LDL-C from baseline to week 12 by patient subgroups identified as primary prevention or secondary prevention/HeFH. These data support safety and efficacy of bempedoic acid among patients who cannot tolerate statins.
Bempedoic acid has also been evaluated in combination with ezetimibe in patients with hypercholesterolemia.32 Out of 301 patients in the primary analysis (108 of which were randomized to the bempedoic acid ezetimibe fixed-dose combination), use of the fixed-dose combination of bempedoic acid 180 mg and ezetimibe 10 mg daily reduced LDL-C by 36.2% compared with bempedoic acid alone (−17.2%), ezetimibe alone (−23.2%) or placebo (+1.8%). Thus, the placebo-corrected difference for this fixed-dose combination was −38.0%; P <0.001. Similar LDL-C reductions were seen across varying intensity of statins. The fixed-dose combination regimen also reduced hsCRP by 35.1%, which was greater than ezetimibe alone (−8.2%) or placebo (+21.6%). These data suggest that bempedoic acid and ezetimibe are more effective together in an additive manner, and the fixed-dose combination may be an attractive option for patients to reduce their overall pill burden.33
While LDL-C reduction is the primary target of therapy, there has been a consistently noted reduction in hsCRP with bempedoic acid. The Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) showed that a therapy (canakinumab) that lowered inflammation (hsCRP) without influencing lipid levels also lowered ASCVD events.34 Although canakinumab was ultimately not given FDA label approval for cardiovascular event reduction, CANTOS was a landmark study in proving the concept that targeting inflammation can reduce cardiovascular events in high-risk patients.
Adverse Events/Safety
The most commonly reported treatment-related adverse effects were elevated uric acid levels, gout, myalgias, constipation, muscle spasms, fatigue, urinary tract infections, and oral discomfort across the phase III clinical trials of bempedoic acid. The studies also reported a slight increase in nasopharyngitis.29,32 Myalgias were reported in approximately 1–7% of the patients across the phase III trials but were generally evenly distributed between the bempedoic acid and placebo arm.20–22
Uric acid levels and gout: In CLEAR Harmony, bempedoic acid was found to be associated with a modest increase in uric acid levels and gout cases. The incidence of gout was 1.2% vs 0.3% in the bempedoic acid vs placebo arms, respectively.28 In CLEAR Wisdom, hyperuricemia was also seen more commonly with bempedoic acid (4.2%) than with placebo (1.9%).29 Elevated uric acid levels were also seen with the fixed-dose combination (bempedoic acid plus ezetimibe), although there was not an increase in gout cases in that study.32 Most cases of gout occurred in patients with a prior history of gout, and these data suggest caution, or at least additional monitoring of uric acid levels, in patients with a history of gout or at risk for gout.
Serious adverse events and death: No significant difference was found in serious adverse events, death, and cardiovascular mortality between the treatment and placebo arms across all of the phase III trials. Achilles tendon rupture occurred more frequently in patients treated with bempedoic acid (0.2% treated with bempedoic acid vs 0% of the patients treated with placebo).35
FDA and Drug Development
An Investigational New Drug (IND) application was submitted by Esperion® in October 2009. Bempedoic acid has been evaluated in Phase 1 and Phase 2 clinical studies, and most recently in Phase 3 trials. Two New Drug Applications (NDAs) were submitted for bempedoic acid and the bempedoic acid/ezetimibe combination to the US FDA on February 20, 2019 and February 26, 2019, respectively. Simultaneously, Esperion® submitted two Marketing Authorization Applications (MAAs) to the European Medicines Agency (EMA) for bempedoic acid and bempedoic acid/ezetimibe combination on February 11, 2019.
Both NDAs received their first approval in the US (as monotherapy on February 21, 2020 and as the fixed-dose combination with ezetimibe on February 26, 2020) as an adjunct to diet and maximally tolerated statin therapy in adults with HeFH, or established ASCVD. In the European Union, bempedoic acid was approved for use in adults with primary hypercholesterolemia or mixed dyslipidemia in combination with ezetimibe (March 2020) and as monotherapy (April 2020). A summary of the timeline for this drug development is shown in Figure 2. On April 6, 2020, the European Commission approved the use of bempedoic acid for use in adults with primary hypercholesterolemia, as an adjunct to statin therapy, or as an alternative to statin therapy in those who are statin intolerant or in who statin therapy is contraindicated.
Dosing and Dose Adjustment in Special Populations
The dosing of bempedoic acid is 180 mg orally once daily with or without food. No dose adjustment needs to be done for patients with mild or moderate kidney function impairment defined as having an estimated glomerular filtration (eGFR) ≥30 mL/min/1.73m2.36 However, data are limited about the efficacy and safety of bempedoic acid in patients with eGFR less than 30 mL/min/1.73m2 and the drug has not been studied in patients with end-stage kidney disease on dialysis. No dose adjustment needs to be done for patients with mild or moderate hepatic function impairment, but it has not been studied in patients with more severe liver disease (Child–Pugh score C).36 Finally, in terms of older adults, among 3009 patients included in bempedoic acid clinical trials, 58% were aged ≥65 and 16% were aged ≥75, and there were no significant differences in efficacy or safety between older and younger adults.36 Bempedoic acid should not be administered concomitantly with either a simvastatin dose greater than 20 mg or a pravastatin dose greater than 40 mg due to the concern for statin-related myopathy.37
Current and Potential Role in Therapy
While the efficacy of bempedoic acid appears to be weaker than that of high-intensity statins, it may be a useful adjunct in individuals who achieve less than desirable LDL-C reductions with statins or who cannot tolerate statins, where bempedoic acid alone or in combination with ezetimibe may be useful alternatives. Although the FDA label currently states that bempedoic acid treatment is specifically indicated for patients with HeFH or established ASCVD who need additional lowering of LDL-C, the tolerability and safety profile of bempedoic acid suggest its potential for the use in primary prevention beyond HeFH, such as those with statin intolerance. The effect of bempedoic acid on major adverse cardiovascular events (MACE) is unknown and currently being addressed in an ongoing cardiovascular outcomes trial (described below).
Cardiovascular Outcomes Trial
CLEAR Outcomes is a phase 3 randomized, double-blind, placebo-controlled trial designed to assess the effects of bempedoic acid on major cardiovascular events in patients with or at high risk for cardiovascular disease and statin intolerance.38 The trial began in December 2016 and has completed recruitment,39 with a targeted completion date of March 2022. The estimated enrollment size is 12,600, and participants were randomized to bempedoic acid 180 mg once daily vs placebo. The primary outcome is the time from randomization to the first occurrence of a composite cardiovascular endpoint of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization. Findings from CLEAR Outcomes will provide additional insight on the role of bempedoic acid and how it fits into the ever-growing armamentarium of lipid-lowering therapeutics.
There is overwhelming evidence from genetic studies, observational studies, and randomized clinical trials that support the notion that LDL-C is causally related to ASCVD and that LDL-C-lowering reduces ASCVD risk, particularly in higher risk patients.40,41 Extrapolating from other studies, in a meta-analysis by Silverman et al evaluating other non-statin interventions that work primarily through upregulation of LDL-C receptor (diet, bile acid sequestrants, ileal bypass, and ezetimibe), there was a 23% reduction in major vascular events per 1 mmol/L (39 mg/dL) of LDL-C lowering (HR 0.77).42 This was similar to the degree of reduction (25%) in vascular events per 1 mmol/L (39 mg/dL) of LDL-C lowering conferred by statins (HR 0.75).42 Since bempedoic acid is a non-statin that also similarly works through a mechanism of upregulation of LDL-C receptor, we anticipate similar degrees of reduction in vascular events proportional to that same degree of LDL-C lowering. Thus, while we await confirmation from CLEAR Outcomes to be certain, it is anticipated that given its ability to reduce LDL-C, it will likely also reduce ASCVD.
Conclusion
Bempedoic acid has demonstrated promise as a novel oral agent for LDL-C lowering as an alternative or adjunctive therapy for primary prevention in patients with HeFH and for secondary prevention patients. Pooled analyses from CLEAR Harmony and CLEAR Wisdom demonstrate ample scope for LDL-C lowering in this subgroup of patients even on a background of statin therapy. Furthermore, statistically significant reductions were noted in the secondary endpoints of non-HDL-C, total cholesterol, ApoB, and hsCRP. Overall, bempedoic acid appears to be safe, effective, and well tolerated among patients with HeFH, as well as patients with ASCVD. Bempedoic acid has now been FDA-approved as an adjunct to diet and maximally tolerated statin specifically for these patients – HeFH and ASCVD – who require further LDL-C lowering. However, although not FDA-approved for this purpose, bempedoic acid may also hold promise as another useful agent in the toolkit for reducing LDL-C in primary prevention patients without HeFH who have statin intolerance, but this needs further study. We await the results from the large ongoing cardiovascular outcome trial (CLEAR Outcomes) to confirm the efficacy of bempedoic acid in reducing MACE.
Funding
Dr Michos is supported by the Amato Fund for Women’s Health research at Johns Hopkins University.
Disclosure
Dr Goldberg reports research grants from Amgen, Amarin, AKCEA, Pfizer, Novartis, Regeneron, Sanofi-Regeneron, and Merck; and honoraria from Esperion, Novartis, AKCEA, Regeneron, Merck, and National Lipid Association. The authors report no other conflicts of interest in this work.
References
1. Khera AV, Hegele RA. What is familial hypercholesterolemia, and why does it matter? Circulation. 2020;141(22):1760–1763. doi:10.1161/CIRCULATIONAHA.120.046961
2. Michos ED, McEvoy JW, Blumenthal RS. Lipid management for the prevention of atherosclerotic cardiovascular disease. N Engl J Med. 2019;381(16):1557–1567. doi:10.1056/NEJMra1806939
3. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–2397. doi:10.1056/NEJMoa1410489
4. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–1499. doi:10.1056/NEJMoa1501031
5. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139(25):e1046–e1081. doi:10.1161/CIR.0000000000000624
6. Baum SJ, Toth PP, Underberg JA, Jellinger P, Ross J, Wilemon K. PCSK9 inhibitor access barriers-issues and recommendations: improving the access process for patients, clinicians and payers. Clin Cardiol. 2017;40(4):243–254. doi:10.1002/clc.22713
7. Knowles JW, Howard WB, Karayan L, et al. Access to nonstatin lipid-lowering therapies in patients at high risk of atherosclerotic cardiovascular disease. Circulation. 2017;135(22):2204–2206. doi:10.1161/CIRCULATIONAHA.117.027705
8. Berkhout TA, Havekes LM, Pearce NJ, Groot PH. The effect of (-)-hydroxycitrate on the activity of the low-density-lipoprotein receptor and 3-hydroxy-3-methylglutaryl-CoA reductase levels in the human hepatoma cell line Hep G2. Biochem J. 1990;272(1):181–186. doi:10.1042/bj2720181
9. Pearce NJ, Yates JW, Berkhout TA, et al. The role of ATP citrate-lyase in the metabolic regulation of plasma lipids. Hypolipidaemic effects of SB-204990, a lactone prodrug of the potent ATP citrate-lyase inhibitor SB-201076. Biochem J. 1998;334(Pt 1):113–119. doi:10.1042/bj3340113
10. Burke AC, Telford DE, Huff MW. Bempedoic acid: effects on lipoprotein metabolism and atherosclerosis. Curr Opin Lipidol. 2019;30(1):1–9. doi:10.1097/MOL.0000000000000565
11. Pinkosky SL, Newton RS, Day EA, et al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat Commun. 2016;7(1):13457. doi:10.1038/ncomms13457
12. Cramer CT, Goetz B, Hopson KL, et al. Effects of a novel dual lipid synthesis inhibitor and its potential utility in treating dyslipidemia and metabolic syndrome. J Lipid Res. 2004;45(7):1289–1301. doi:10.1194/jlr.M400018-JLR200
13. Pinkosky SL, Filippov S, Srivastava RA, et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J Lipid Res. 2013;54(1):134–151. doi:10.1194/jlr.M030528
14. Jeong HW, Hsu KC, Lee JW, et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296(4):E955–964. doi:10.1152/ajpendo.90599.2008
15. Samsoondar JP, Burke AC, Sutherland BG, et al. Prevention of diet-induced metabolic dysregulation, inflammation, and atherosclerosis in Ldlr(-/-) mice by treatment with the ATP-citrate lyase inhibitor bempedoic acid. Arterioscler Thromb Vasc Biol. 2017;37(4):647–656. doi:10.1161/ATVBAHA.116.308963
16. A Multiple Ascending Dose Study of ETC-1002 in healthy subjects; 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT01485146.
17. A Multiple Ascending Dose Study of ETC-1002 in subjects with mild dyslipidemia; 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT01105598.
18. Single radiolabeled dose study to investigate the absorption, metabolism and excretion of [14C]-ETC-1002. Available from: https://clinicaltrials.gov/ct2/show/NCT02044627.
19. Gutierrez MJ, Rosenberg NL, Macdougall DE, et al. Efficacy and safety of ETC-1002, a novel investigational low-density lipoprotein-cholesterol-lowering therapy for the treatment of patients with hypercholesterolemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014;34(3):676–683. doi:10.1161/ATVBAHA.113.302677
20. Thompson PD, Rubino J, Janik MJ, et al. Use of ETC-1002 to treat hypercholesterolemia in patients with statin intolerance. J Clin Lipidol. 2015;9(3):295–304. doi:10.1016/j.jacl.2015.03.003
21. Thompson PD, MacDougall DE, Newton RS, et al. Treatment with ETC-1002 alone and in combination with ezetimibe lowers LDL cholesterol in hypercholesterolemic patients with or without statin intolerance. J Clin Lipidol. 2016;10(3):556–567. doi:10.1016/j.jacl.2015.12.025
22. Ballantyne CM, Davidson MH, Macdougall DE, et al. Efficacy and safety of a novel dual modulator of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase in patients with hypercholesterolemia: results of a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. J Am Coll Cardiol. 2013;62(13):1154–1162. doi:10.1016/j.jacc.2013.05.050
23. A study of the safety, pharmacokinetic drug interaction and efficacy of ETC-1002 and atorvastatin in subjects with hypercholesterolemia. Available from: https://clinicaltrials.gov/ct2/show/NCT01779453.
24. Evaluation of ETC-1002 in patients with hypercholesterolemia and hypertension. Available from: https://clinicaltrials.gov/ct2/show/NCT02178098.
25. Evaluation of the efficacy and safety of bempedoic acid (ETC-1002) 180mg when added to PCSK9 inhibitor therapy. Available from: https://clinicaltrials.gov/ct2/show/NCT03193047.
26. Evaluation of the efficacy and safety of bempedoic acid (ETC-1002) 180mg, ezetimibe 10mg, and atorvastatin 20 mg triplet therapy in patients with elevated LDL-C. Available from: https://clinicaltrials.gov/ct2/show/NCT03051100.
27. Lalwani ND, Hanselman JC, MacDougall DE, Sterling LR, Cramer CT. Complementary low-density lipoprotein-cholesterol lowering and pharmacokinetics of adding bempedoic acid (ETC-1002) to high-dose atorvastatin background therapy in hypercholesterolemic patients: a randomized placebo-controlled trial. J Clin Lipidol. 2019;13(4):568–579. doi:10.1016/j.jacl.2019.05.003
28. Ray KK, Bays HE, Catapano AL, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380(11):1022–1032. doi:10.1056/NEJMoa1803917
29. Goldberg AC, Leiter LA, Stroes ESG, et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR wisdom randomized clinical trial. JAMA. 2019;322(18):1780–1788. doi:10.1001/jama.2019.16585
30. Duell PB, Banach M, Catapano AL, Laufs U, Mancini GBJ, Ray KK, Bloedon LT, Ye Z and Goldberg AC. Efficacy and safety of bempedoic acid in patients with heterozygous familial hypercholesterolemia: Analysis of pooled patient-level data from phase 3 clinical trials. Atherosclerosis. 2020;315:e12–e13 [abstract].
31. Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662. doi:10.1161/JAHA.118.011662
32. Ballantyne CM, Laufs U, Ray KK, et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 2020;27(6):593–603. doi:10.1177/2047487319864671
33. Khan SU, Michos ED. Bempedoic acid and ezetimibe - better together. Eur J Prev Cardiol. 2020;27(6):590–592. doi:10.1177/2047487319864672
34. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi:10.1056/NEJMoa1707914
35. Banach M, Duell PB, Gotto AM, et al. Association of bempedoic acid administration with atherogenic lipid levels in phase 3 randomized clinical trials of patients with hypercholesterolemia. JAMA Cardiol. 2020;5(10):1124. doi:10.1001/jamacardio.2020.2314
36. Food and Drug Administration. NEXLETOL (bempedoic acid) prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211616s000lbl.pdf.
37. Food and Drug Administration. Highlights of prescribing information NEXLIZET (bempedoic acid and ezetimibe) tablets, for oral use. Available from: https://www.accessdata.fdagov/drugsatfda_docs/label/2020/211617s000lbl.pdf.
38. Evaluation of major cardiovascular events in patients with, or at high risk for, cardiovascular disease who are statin intolerant treated with bempedoic acid (ETC-1002) or placebo (CLEAR outcomes). Available from: https://clinicaltrials.gov/ct2/show/NCT02993406.
39. Esperion completes patient enrollment in the global CLEAR cardiovascular outcomes trialfor bempedoic acid; 2019. Available from: https://www.esperion.com/investors-media/press-releases/.
40. Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. 2017;38(32):2459–2472.41. doi:10.1093/eurheartj/ehx144
41. Ference BA, Graham I, Tokgozoglu L, Catapano AL. Impact of lipids on cardiovascular health: JACC health promotion series. J Am Coll Cardiol. 2018;72(10):1141–1156. doi:10.1016/j.jacc.2018.06.046
42. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289–1297. doi:10.1001/jama.2016.13985
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

