Back to Journals » Journal of Asthma and Allergy » Volume 16
BDP/FF NEXThaler to Improve Asthma Control Status in the Real World: The NEWTON Study
Authors Braido F , Arnaboldi E, Barile S, Cavalieri L , Ingrassia E , Russo M, Piraino A
Received 26 June 2023
Accepted for publication 18 September 2023
Published 25 October 2023 Volume 2023:16 Pages 1177—1186
DOI https://doi.org/10.2147/JAA.S422832
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Fulvio Braido,1,2 Enrico Arnaboldi,1,2 Sara Barile,3 Luca Cavalieri,4 Eleonora Ingrassia,4 Maria Russo,1,2 Alessio Piraino4
1Respiratory Unit for Continuity of Care, IRCCS, Ospedale Policlinico San Martino, Genova, Italy; 2Department of Internal Medicine (DiMI), University of Genova, Genova, Italy; 3Chiesi Farmaceutici S.p.A., Parma, Italy; 4Chiesi Italia S.p.A ., Parma, Italy
Correspondence: Fulvio Braido, Respiratory Unit for Continuity of Care, IRCCS, Ospedale Policlinico San Martino, Genova, Italy, Email [email protected]
Abstract: In this article, we discuss the importance of real-world data in the treatment of patients with asthma and specifically the role of maintenance and reliever therapy (MART) with beclometasone dipropionate (BDP)/formoterol fumarate dihydrate (FF) delivered through a dry-powder inhaler (DPI) that contains an extrafine formulation. We also present the design of the NEWTON study. This multinational, multicenter, prospective, observational study will evaluate the real-world use of extrafine BDP/FF via a DPI as maintenance therapy and MART in patients with moderate to severe asthma. The study’s primary outcome will be the proportion of patients improving their asthma control. Digitally collected patient-reported outcomes, such as the 5-item Asthma Control Questionnaire, the EuroQol 5-dimension 5-level, and the Test of the Adherence to Inhalers, will be used to assess the patient’s asthma control, quality of life, and treatment adherence. Moreover, a new patient-reported outcome, the “Speed of change in health feeling” questionnaire, will be validated in a subgroup of patients. Overall, the results of this study will provide a real-life assessment of patients who perceived clinical benefits in a large cohort of asthmatics in Europe treated as per current clinical practice.
Keywords: asthma, dry-powder inhaler, beclometasone dipropionate/formoterol fumarate, observational study
Introduction
Asthma is one of the most common chronic inflammatory diseases, with more than 300 million people affected worldwide and increasing prevalence rates.1 It is characterized by variable levels of airflow obstruction, bronchial hyperresponsiveness, and airway inflammation, which can substantially impact a patient’s quality of life and even represent a life-threatening or fatal condition.2 Persistent asthma also causes a substantial burden to the healthcare systems, with estimated mean total costs up to five-times higher for subjects with uncontrolled asthma compared to those with controlled asthma; in detail, the mean cost per patient per year, including all asthmatic patients (intermittent, mild, moderate and severe asthma) is US$1900 in Europe and US$3100 in the USA.3
Asthma can be categorized into controlled, not well controlled, and uncontrolled, depending on the frequency of daytime and nocturnal symptoms, limitation of activities, need for reliever treatment, lung function, and history of asthma exacerbations.2 Several well-validated self-assessment and disease-specific questionnaires, such as the Asthma Control Questionnaire (ACQ)4 and the Asthma Control Test,5 have been developed to assess the patient’s level of asthma control and the need for pharmacological treatment adjustment in case of suboptimal disease control.
Attaining and maintaining optimal asthma control is the primary aim of asthma treatment, which is achieved through long-term management of symptoms, preservation of normal activity levels and pulmonary function, prevention of asthma exacerbations and asthma mortality, and avoidance of adverse effects from asthma medications.2 To reach this goal, the treatment strategy should combine pharmacological treatment, ongoing monitoring of asthma control levels, patient education, and a good partnership between patients and their healthcare providers.2
Asthma Pharmacological Treatment
Inhaled therapy is considered the cornerstone of asthma management, and international guidelines recommend the combination of inhaled corticosteroids (ICS) and long-acting β2-agonists (LABA), either separately or as a fixed-dose formulation in patients who do not reach sufficient asthma control through ICS alone.2,6 The combination of ICS and formoterol, a rapid-onset LABA, is the preferred treatment option according to GINA guidelines, to be used as needed for symptom relief in patients with mild asthma (Steps 1 and 2), and as daily maintenance and reliever therapy (MART) for patients with moderate to severe disease (Steps 3, 4 and 5).2
Two ICS–formoterol combinations are currently recommended, namely beclometasone dipropionate (BDP)/formoterol fumarate dihydrate (FF) and budesonide/FF (BUD/FF), whereas other formulations (eg, mometasone–formoterol, fluticasone propionate–formoterol) have not been studied as anti-inflammatory relievers, either alone or in MART.2 As shown in several clinical trials, the BDP/FF fixed combination has proven safe and effective in treating moderate to severe asthma.7,8 Moreover, BDP/FF would appear to produce superior results compared with either BUD/FF or fluticasone/salmeterol fixed combinations in achieving asthma control without increasing safety concerns.9–11 The extrafine formulation of BDP/FF also helps to increase the deposition of the active principles into the peripheral airways compared with non-extrafine formulations, allowing a greater proportion of the inhaled drug to reach its pharmacological target in the small airways and reducing systemic exposure.12–14
Multiple devices, such as pressurized metered dose inhalers (pMDIs) and dry powder inhalers (DPI), have been developed to administer inhaled therapy. The characteristics of these inhalers, such as the method of powder aerosolization, the minimum and optimum inspiratory flow, the characteristics of the aerosol cloud formed, the method of use, and feedback on the inhalation procedure, play an important role in determining drug delivery and distribution, thus influencing, together with the chosen drug and patient characteristics, the overall effectiveness of the pharmacological treatment.15
Innovative Features for Extrafine BDP/FF Delivery
NEXThaler® is a dry-powder inhaler (DPI) with extrafine BDP/FF that presents several unique, original features and feedback systems that facilitate patient control of the entire inhalation process.
The main innovative technical solutions presented by this DPI are:12,15,16
- The breath-actuated mechanism (BAM) system delays drug delivery until the patient’s inspiratory flow rate is adequate (>35 L/min) to detach drug particles from the lactose carrier. This reduces the emission of large particles (>5 μm), increases the available therapeutic fine particle fraction and favors the deposition of the drug mainly in the lower respiratory tract;
- The inhalation flow-independent performance allows the delivery of a consistent drug dose during inspiration, regardless of inspiratory flow rate (range, 35–90 L/min);
- A triple feedback system ensures correct dosage intake. It comprises a click, which confirms the activation of the BAM system when the inspiratory flow exceeds 35 L/min; a unit dose counter not simply indicating the number of remaining doses in the inhaler but constituting a real “inhalation counter”; and a lactose carrier, whose taste confirms that the inhalation has been completed;
- The extrafine formulation allows the drug particles to be delivered throughout the entire bronchial tree.
Studies conducted on the use of this DPI in patients with asthma have confirmed consistent dose delivery and device activation constantly achieved by patients independently of inhalation flow rates.17 Singh et al have also shown that extrafine BDP/FF DPI is comparable to extrafine Foster® pMDI when administered as reliever therapy after a methacholine challenge, thus supporting the MART approach with Foster® DPI as well.18 In an 8-week randomized, double-blind, parallel-group trial, extrafine BDP/FF DPI was non-inferior to extrafine BDP/FF pMDI in terms of change from baseline in average pre-dose morning peak expiratory flow. Moreover, no significant safety signals were observed, thus proving that extrafine BDP/FF DPI is an effective and well-tolerated delivery device for patients with asthma who need regular treatment.19
BDP/FF DPI has also shown positive results compared with other inhalers regarding usability and patient satisfaction, which can positively influence treatment adherence. The DPI was the easiest to use and most popular device among 106 inhaler-naïve older patients.20 It also showed improved usability and higher satisfaction compared with other DPIs among 66 adult patients.21 It was associated, together with another device, with the lowest number of attempts to achieve error-free use in a prospective study of 105 patients with asthma.22
BDP/FF DPI is also relatively insensitive to improper use as double activation, accidental fall, activation at different degrees of inclination, exhalation inside the device,23 and incorrect exhalation before the inhalation.24
Asthma Maintenance and Reliever Therapy
The MART approach, which consists of using a single inhaler for both maintenance and reliever therapy, is currently considered the “preferred treatment” in the GINA guidelines,2 as well as in the US guidelines.25 Finally, the English NICE guidelines also mention MART in several steps.26
The MART regimen is authorized with either BDP/FF or BUD/FF. It has proven more effective than the combination of ICS and short-acting β2-agonist (SABA) as needed or ICS-LABA plus SABA as needed in reducing risks of severe asthma exacerbations while providing similar levels of daily asthma control.7,27 Extrafine BDP/FF used as maintenance and reliever in a single pMDI significantly prolonged the time to first exacerbation compared with using BDP/FF for maintenance and salbutamol as a reliever (209 days vs 134 days; p=0.0005) while presenting a similar tolerability profile.7 Another advantage of using a single inhaler for MART is that it simplifies asthma management, potentially improving patient adherence to treatment.28 Moreover, the MART regimen conducted with DPI, besides simplifying therapy and reducing exacerbations, can directly reduce greenhouse gas emissions by replacing SABA as a reliever.29
Real-World Evidence for Asthma Management
Despite the positive results in the treatment of asthma reported in the literature, a relevant gap still exists between the results of clinical trials, in which many patients achieve asthma control through pharmacological treatment,30 and the epidemiological data, which suggest that many patients with asthma treated as per standard clinical practice still have poorly controlled disease.31 Thus, real-life studies are needed to better understand asthma treatment in larger and more diverse patient populations and identify what factors in the real-life context are associated with poor asthma control.
Some observational studies have investigated the use of BDP/FF and the effects of MART in clinical practice. The NEXT-Step observational study evaluated the usability/satisfaction of BDP/FF DPI and treatment adherence in asthma patients poorly controlled by low-dose ICS. However, it did not investigate the MART approach.32 The ALFRESCO study investigated asthma symptoms control, pulmonary function, and quality of life in patients treated with extrafine BDP/FF 100/6 μg pMDI as MART or maintenance only. Still, no detailed comparison was made between the two regimens.33 Finally, Hodzhev et al conducted an observational study to evaluate the effects of BDP/FF therapy using two different inhalers in a real-world setting, focusing on patients with severe chronic obstructive pulmonary disease (COPD).34
Overall, few observational studies have investigated the efficacy and safety of BDP/FF in a real-world setting. To our knowledge, no observational study has simultaneously evaluated using BDP/FF DPI as MART versus maintenance only in a real-life population by examining patient preferences. In this article, we present the design of the NEXThaler in a rEal-World study on The probability Of improving the asthma coNtrol status after 6 months of treatment (NEWTON) study. This observational study will investigate the efficacy and safety of BDP/FF DPI used in clinical practice in patients with inadequately controlled asthma, also focusing on the effects of the MART strategy and the speed of change in the patient’s feeling of health after starting BDP/FF Nexthaler®.
The NEWTON Study
The NEWTON study is a multinational, multicenter, observational, prospective cohort study conducted to estimate the effects of BDP/FF fixed combination administered via a DPI (Nexthaler®) in a real-life context [NCT05168995].35 The study is ongoing in approximately 57 respiratory medicine centers in six European countries (France, Germany, Hungary, Italy, Romania, and Spain). Over 12 months, approximately 650 patients will be recruited and followed for 6 months each.
The study aims to evaluate the probability of improving patients’ asthma control status after 6 months of treatment, as well as to describe the safety profile of BDP/FF 100/6 µg per actuation inhalation powder and its effects on asthma control, treatment adherence, and patient satisfaction after 3 and 6 months. The study will also simultaneously evaluate two different approaches, BDP/FF DPI MART versus BDP/FF DPI only as maintenance therapy and a separate reliever. Moreover, the study will use electronic patient-reported outcomes (ePROs) to better capture patients’ perspectives on asthma control, quality of life, and treatment adherence/satisfaction. Finally, a sub-study involving only Italian patients will perform a psychometric validation of a newly developed questionnaire designed to measure the speed of change in patients’ feelings of health after starting a new treatment.
Study Population and Eligibility Criteria
The study population consists of adult patients with inadequately controlled asthma treated with BDP/FF DPI 100/6 µg in clinical practice. Key inclusion and exclusion criteria are detailed in Box 1. Briefly, the study will include adult men and women with not-well-controlled or poorly controlled asthma who have started treatment with BDP/FF DPI within a maximum of 14 days and have not been treated with extrafine formulations in the 6 months preceding the start of BDP/FF DPI and are willing to sign the informed consent to participate in the study and the processing of personal data. Patients with a recent history of life-threatening asthma, COPD, uncontrolled/clinically significant diseases, or under treatment with biological agents and/or extemporary or fixed triple combinations will be excluded from the study, as well as those who are unable to understand and complete the required questionnaires and those participating in other experimental trials. Notably, the study will not exclude specific categories of asthmatic patients, such as asthmatic smokers or pregnant women (usually excluded from randomized controlled trials in asthma), to better capture the real-life situation of patients with asthma.
 |
Box 1 Inclusion and Exclusion Criteria |
Study Plan
Approximately 650 eligible patients will be enrolled in the study to obtain at least 540 evaluable patients, accounting for the potential loss of non-evaluable patients. The study will enroll patients who have been prescribed BDP/FF DPI (Chiesi Farmaceutici S.p.A., Parma, Italy) 100/6 µg as per standard clinical practice and who either start the prescription on the same day of enrollment (Visit 1) or have been using BDP/FF DPI for 14 days maximum. The decision to prescribe and start treatment with BDP/FF DPI must have been taken according to standard clinical practice, irrespective of the patient’s participation in this study.
Patients will be prescribed BDP/FF DPI in two different regimens:
- Maintenance treatment only: BDP/FF DPI will be taken as regular maintenance treatment (one or two inhalations twice daily, with a maximum daily dose of four inhalations), and a separate short-acting bronchodilator will be used as needed as a reliever;
- MART: BDP/FF DPI will be taken as regular maintenance treatment (one inhalation twice daily) and as needed in response to asthma symptoms (one–two additional inhalations as needed in response to symptoms, with a maximum daily dose of eight inhalations).
Enrolled patients will be followed up to 6 months from the first visit, as per routine local clinical practice; data from three visits [enrollment (Visit 1), after 3 months (Visit 2), and after 6 months (Visit 3)] will be collected as part of the study. If treatment starts at the enrollment visit, visit 1 will also correspond to the baseline; otherwise, a retrospective observation window will be defined from the start of BDP/FF DPI to enrollment (Figure 1).
 |
Figure 1 NEWTON study plan and procedures. |
During the first visit, the patient’s demographic data, medical history, smoking status, and asthma history will be collected, as well as the start date of BDP/FF DPI and mode of administration. Both at enrollment and during the follow-up visits, the following parameters will be collected: lung function tests (spirometry, oscillometry, plethysmography), fractional exhaled nitric oxide, asthma control status (Asthma Control Questionnaire-5 questions, ACQ-5), patients’ quality of life (European Quality of Life Five Dimension and Five Levels, EQ-5D-5L), treatment adherence (Test of Adherence to Inhalers), patient satisfaction, any changes in treatment dose, concomitant medications and treatments, need for unscheduled visits, asthma exacerbations, and occurrence of adverse events.
Study Objectives and Endpoints
The study’s primary objective is to evaluate the probability of improving patients’ asthma control status over 6 months of treatment with BDP/FF DPI 100/6, using the ACQ-5. This will be measured as the proportion of patients improving their ACQ-5 score (from poorly controlled to not well-controlled nor poorly controlled, from poorly controlled to well controlled, from not well-controlled nor poorly controlled to well controlled) from baseline to 6 months.
The study’s secondary objectives will evaluate the effects and safety profile of BDP/FF DPI at 3 and 6 months, as detailed in Box 2. Briefly, the effects of BDP/FF DPI will be measured in terms of ACQ-5 improvements, changes in lung function parameters, quality of life, treatment adherence, patient satisfaction with the inhaler (through a three-item questionnaire), and incidence of asthma exacerbation after both 3 and 6 months of treatment. The safety of BDP/FF DPI will be measured in terms of the occurrence of treatment-emergent adverse events and treatment-emergent serious adverse events, adverse drug reactions, discontinuation due to any reason, and occurrence of specific special situations during the study.
 |
Box 2 Study Objectives |
e-PROs and Validation of a New PRO: Speed of Change in the Feeling of Health
To better assess patients’ health improvement in the real-world setting, different ePROs will be used to capture patients’ perspectives on their asthma status, impact on quality of life, and adherence/satisfaction with the prescribed treatment. The ePRO questionnaires will be electronically available through the Bring Your Own Device technology. Each patient will complete the questionnaire/structured questions using their electronic device, such as a smartphone, tablet, or personal computer (laptop and desktop). The investigators will train patients on how to use ePROs to complete the questionnaires autonomously, with minimal support from the investigators, only as needed. The ePROs will be completed at baseline and after 3 and 6 months and will include the ACQ-5 to monitor asthma control, the EQ-5D-5L to assess the quality of life and the 12-TAI to assess treatment adherence; patients will also be asked to complete a structured questionnaire consisting of three simple questions to assess their satisfaction with the device.
Furthermore, a sub-study will be conducted to perform the psychometric validation of a new questionnaire designed to assess the speed of change in the feeling of health after starting a new treatment. This newly developed tool presents an innovative perspective as it does not focus on patients’ reported symptoms but on their perceptions of their global health status, their confidence in the prescribed treatments, and the degree of change from their previous condition at the start of the new treatment. This will also provide direct information on the change in terms of feeling healthy under various perspectives (both physical and psychological), as recognized by the patients themselves.
The sub-study is a multicenter, observational, prospective cohort study conducted only in Italian sites participating in the NEWTON study and will enroll approximately 200 patients from 20 Italian respiratory medicine centers over 12 months. Patients will be observed for 30 days starting from the enrollment visit. They will be asked to complete the “Speed of change in the feeling of health” questionnaire along with other PROs, such as ACQ-5, the Stanford Expectation of Treatment Scale, and the Global Rating Scale, at three different time points: 7, 14, and 30 days. All the questionnaires will be provided as ePROs to allow patients to fill them out remotely through electronic devices (ie, smartphones and tablets) (Figure 2).
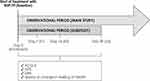 |
Figure 2 NEWTON Italian sub-study plan and procedures. |
Statistical Methods
The sample size calculation for the NEWTON study was based on the proportion of patients expected to improve their level of asthma control at 6 months, which was conservatively assumed to be 60% based on the GOAL study,30 the PRISMA study36 and a Malaysian study.37 A sample size of 540 patients yields a two-sided 95% confidence interval with a width equal to 0.083 when the sample proportion is 0.60, using the large sample normal approximation.38 Assuming a 17% proportion of drop-out or non-evaluable patients, a total number of 650 patients is needed to obtain relevant data from at least 540 evaluable patients.
The study has a descriptive aim: results of statistical analyses from the study data will be presented as descriptive statistics. Continuous variables will be summarized by the number of observations, mean, standard deviation (SD), median, quartiles, minimum, and maximum. Categorical variables will be summarized using counts of patients and percentages. For the primary analysis, the count and proportion of patients improving the level of asthma control from baseline to V3 or early termination will be calculated. For the secondary analysis, we will measure number and proportion of patients improving asthma control, improving ACQ-5 score and achieving well-controlled asthma at different follow-up visits compared to baseline, we will also measure intra-patient changes in lung function parameters, quality of life, treatment adherence, patient satisfaction with the inhaler, and incidence of asthma exacerbation throughout the observational period and compared to baseline.
Ethical Consideration
The study will be conducted in accordance with the ethical principles of the Declaration of Helsinki and in adherence to the study protocol, Good Pharmaco-epidemiology Practices, Good Epidemiological Practices and applicable aspects of Good Clinical Practices, as well as applicable laws and country-specific regulations under which the study is being conducted.
Conclusion
Achieving optimal asthma control is the main focus of asthma treatment but is still difficult to attain for many patients treated in clinical practice, despite the availability of safe and effective medications.9,39 The NEWTON study will investigate the effects of BDP/FF DPI in improving the asthma control status in patients with inadequately controlled asthma treated in real-world clinical practice. Given its observational nature, the study will allow a better characterization of the patient’s treatment in common clinical practice, understanding the effects of treatment in a more heterogeneous population with different features and risk factors. Moreover, the study will compare the results obtained with MART therapy, recommended by current guidelines, versus separate maintenance and reliever therapy with two different inhalers to assess the advantages of the MART regimen with a single inhaler. The study will also use ePRO measures to better assess patients’ perspectives on their asthma status, quality of life, adherence, and satisfaction with treatment. Finally, a nested study will be conducted only among Italian patients to validate a specific questionnaire that measures the speed of change in patients’ feelings of health after starting a new treatment.
Ethics Approval and Informed Consent
The Ethics 225 Committee of each participating center will approve the study protocol before the study starts. The informed consent for participation in the study and use of personal data will be obtained from all patients before any study-related procedures are performed.
Consent for Publication
All authors confirm they consent in the publication of the manuscript.
Acknowledgments
The abstract of this paper was presented at the virtual 2021 European Respiratory Society International Congress (5–8 September 2021) as a poster presentation with interim findings. The poster’s abstract was published in ‘Poster Abstracts’ in European Respiratory Journal 2021 58: PA561. https://erj.ersjournals.com/content/58/suppl_65/PA561. Medical writing and editorial assistance were provided by Ambra Corti, Valentina Attanasio, and Aashni Shah (Polistudium, srl) and were supported by Chiesi Italia S.p.A.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Chiesi Italia S.p.A.
Disclosure
AP, EI, and LC are employees of Chiesi Italia S.p.A. SB is an employee of Chiesi Farmaceutici S.p.A. The authors report no other conflicts of interest in this work.
References
1. Oladunni E, Sumita S. The global impact of asthma in adult populations. Ann Glob Health. 2019;85(1):2. doi:10.5334/aogh.2412
2. Global Initiative for Asthma. Global strategy for asthma management and prevention, 2023. Available from: www.ginasthma.org.
3. Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3(1):1. doi:10.1186/s40733-016-0029-3
4. Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi:10.1034/j.1399-3003.1999.14d29.x
5. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi:10.1016/j.jaci.2003.09.008
6. Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010;2010(4):CD005533.
7. Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma: a double-blind, randomised controlled trial. Lancet Respir Med. 2013;1(1):23–31. doi:10.1016/S2213-2600(13)70012-2
8. Huchon G, Magnussen H, Chuchalin A, Dymek L, Gonod FB, Bousquet J. Lung function and asthma control with beclomethasone and formoterol in a single inhaler. Respir Med. 2009;103(1):41–49. doi:10.1016/j.rmed.2008.09.002
9. Allegra L, Cremonesi G, Girbino G, et al. for PRISMA (PRospectIve Study on asthMA control) Study Group, Real-life prospective study on asthma control in Italy: cross-sectional phase results. Respir Med. 2012;106(2):205–2014. doi:10.1016/j.rmed.2011.10.001
10. Müller V, Gálffy G, Eszes N, et al. Asthma control in patients receiving inhaled corticosteroid and long-acting beta2-agonist fixed combinations. A real-life study comparing dry powder inhalers and a pressurized metered dose inhaler extra-fine formulation. BMC Pulm Med. 2011;11(1):40. doi:10.1186/1471-2466-11-40
11. Papi A. Inhaled BDP/formoterol extra-fine combination. Evidence and future perspectives. Pneumologie. 2009;63(Suppl 2):S102–106. doi:10.1055/s-0029-1214716
12. Virchow JC, Poli G, Herpich C, et al. Lung deposition of the dry powder fixed combination beclometasone dipropionate plus formoterol fumarate using NEXThaler® device in healthy subjects, asthmatic patients, and COPD patients. J Aerosol Med Pulm Drug Deliv. 2018;31(5):269–280. doi:10.1089/jamp.2016.1359
13. Watz H, Barile S, Guastalla D, et al. Targeting the small airways with inhaled corticosteroid/long-acting beta agonist dry powder inhalers: a functional respiratory imaging study. J Aerosol Med Pulm Drug Deliv. 2021;34(5):280–292. doi:10.1089/jamp.2020.1618
14. Chetta A, Facciolongo N, Franco C, Franzini L, Piraino A, Rossi C. Impulse oscillometry, small airways disease, and extra-fine formulations in asthma and chronic obstructive pulmonary disease: windows for new opportunities. Ther Clin Risk Manag. 2022;18:965–979. doi:10.2147/TCRM.S369876
15. Emeryk A, Janeczek K. Feedback systems in multi-dose dry powder inhalers. Postepy Dermatol Alergol. 2023;40(1):16–21. doi:10.5114/ada.2022.117039
16. Corradi M, Chrystyn H, Cosio BG, et al. NEXThaler, an innovative dry powder inhaler delivering an extrafine fixed combination of beclometasone and formoterol to treat large and small airways in asthma. Expert Opin Drug Deliv. 2014;11(9):1497–1506. doi:10.1517/17425247.2014.928282
17. Chetta A, Yorgancioglu A, Scuri M, Barile S, Guastalla D, Dekhuijzen PNR. Inspiratory flow profile and usability of the NEXThaler, a multidose dry powder inhaler, in asthma and COPD. BMC Pulm Med. 2021;21(1):65. doi:10.1186/s12890-021-01430-9
18. Singh D, van den Berg F, Leaker B, et al. Comparison of the effect of beclometasone/formoterol in asthma patients after methacholine-induced bronchoconstriction: a noninferiority study using metered dose vs. dry powder inhaler. Br J Clin Pharmacol. 2019;85(4):729–736. doi:10.1111/bcp.13847
19. Kanniess F, Scuri M, Vezzoli S, Francisco C, Petruzzelli S. Extrafine beclomethasone/formoterol combination via a dry powder inhaler (NEXThaler(®)) or pMDI and beclomethasone monotherapy for maintenance of asthma control in adult patients: a randomised, double-blind trial. Pulm Pharmacol Ther. 2015;30:121–127. doi:10.1016/j.pupt.2014.07.006
20. Ruessel K, Luecke E, Schreiber J. Inhaler devices in a geriatric patient population: a prospective cross-sectional study on patient preferences. Patient Prefer Adherence. 2020;14:1811–1822. doi:10.2147/PPA.S262057
21. Voshaar T, Spinola M, Linnane P, et al. Comparing usability of NEXThaler(®) with other inhaled corticosteroid/long-acting β2-agonist fixed combination dry powder inhalers in asthma patients. J Aerosol Med Pulm Drug Deliv. 2014;27(5):363–370. doi:10.1089/jamp.2013.1086
22. Schreiber J, Sonnenburg T, Luecke E. Inhaler devices in asthma and COPD patients - A prospective cross-sectional study on inhaler preferences and error rates. BMC Pulm Med. 2020;20(1):222. doi:10.1186/s12890-020-01246-z
23. Varacca G, D’Angelo D, Glieca S, et al. The impact of possible improper use on the performance in vitro of NEXThaler in comparison with Ellipta inhaler. Eur J Pharm Sci. 2023;183:106385. doi:10.1016/j.ejps.2023.106385
24. Farkas Á, Tomisa G, Kugler S, et al. The effect of exhalation before the inhalation of dry powder aerosol drugs on the breathing parameters, emitted doses and aerosol size distributions. Int J Pharm X. 2023;5:100167. doi:10.1016/j.ijpx.2023.100167
25. Cloutier MM, Dixon AE, Krishnan JA, Lemanske RF, Pace W, Schatz M. Managing asthma in adolescents and adults: 2020 asthma guideline update from the national asthma education and prevention program. JAMA. 2020;324(22):2301–2317. doi:10.1001/jama.2020.21974
26. National Institute for Health and Care Excellence (NICE)Asthma: Diagnosis, Monitoring and Chronic Asthma Management. London: National Institute for Health and Care Excellence (NICE); 2021.
27. Sobieraj DM, Weeda ER, Nguyen E, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA. 2018;319(14):1485–1496. doi:10.1001/jama.2018.2769
28. Di Marco F. Today’s improvement in asthma treatment: role of MART and Easyhaler. Multidiscip Respir Med. 2020;15(1):649. doi:10.4081/mrm.2020.649
29. Wilkinson A, Woodcock A. The environmental impact of inhalers for asthma: a green challenge and a golden opportunity. Br J Clin Pharmacol. 2022;88(7):3016–3022. doi:10.1111/bcp.15135
30. Bateman ED, Boushey HA, Bousquet J, et al. for GOAL Investigators Group, Can guideline-defined asthma control be achieved? The gaining optimal asthma control study. Am J Respir Crit Care Med. 2004;170(8):836–844. doi:10.1164/rccm.200401-033OC
31. García-Marcos L, Chiang CY, Asher MI, et al. Asthma management and control in children, adolescents, and adults in 25 countries: a global asthma network Phase I cross-sectional study. Lancet Glob Health. 2023;11(2):e218–e228. doi:10.1016/S2214-109X(22)00506-X
32. Bakakos P, Chatziapostolou P, Katerelos P, Efstathopoulos P, Korkontzelou A, Katsaounou P. Extrafine beclometasone dipropionate/formoterol nexthaler on device usability, adherence, asthma control and quality of life. A panhellenic prospective, non-interventional observational study in patients with asthma-the NEXT-step study. J Pers Med. 2022;12(2):146. doi:10.3390/jpm12020146
33. Ulmeanu R, Bloju S, Vittos O. Assessment of symptoms control, pulmonary function and related quality of life in asthmatic patients treated with extrafine beclomethasone dipropionate/formoterol fumarate 100/6 μg pMDI: results of a multicenter observational study in Romania (ALFRESCO Study). J Asthma Allergy. 2022;15:919–933. doi:10.2147/JAA.S358798
34. Hodzhev VA, Kenderov AN, Ivanov YY, Gospodinova-Vulkova DP, Kalinov K. Assessment of extrafine beclomethasone/formoterol for the treatment of chronic obstructive pulmonary disease: a non-interventional study in a Bulgarian population. Pulm Pharmacol Ther. 2022;77:102169. doi:10.1016/j.pupt.2022.102169
35. Chiesi Italia. Effects of beclometasone dipropionate/formoterol fumarate via NEXT(Haler) in a real-world study on asthma control (NEWTON); Available from: https://www.clinicaltrials.gov/ct2/show/NCT05168995NLMidentifier:NCT05168995.
36. Terzano C, Cremonesi G, Girbino G, et al. PRISMA (PRospectIve Study on asthMA control) Study Group, 1-year prospective real-life monitoring of asthma control and quality of life in Italy. Respir Res. 2012;13(1):112. doi:10.1186/1465-9921-13-112
37. Manap RA, Loh LC, Ismail TS, et al. Satisfaction levels and asthma control amongst Malaysian asthmatic patients on budesonide/formoterol maintenance and reliever therapy: experience in a real-life setting. Patient Relat Outcome Meas. 2012;3:71–78. doi:10.2147/PROM.S19211
38. Dixon WJ, Massey J. Introduction to Statistical Analysis.
39. Braido F, Scichilone N, Lavorini F, et al. Manifesto on small airway involvement and management in asthma and chronic obstructive pulmonary disease: an interasma (Global Asthma Association - GAA) and World Allergy Organization (WAO) document endorsed by Allergic Rhinitis and its Impact on Asthma (ARIA) and Global Allergy and Asthma European Network (GA2LEN) [published correction appears in world allergy organ J. 2022;15(2):100596]. World Allergy Organ J. 2016;9(1):37. doi:10.1186/s40413-016-0123-2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
