Back to Journals » OncoTargets and Therapy » Volume 9
BCL3 exerts an oncogenic function by regulating STAT3 in human cervical cancer
Authors Zhao H, Wang WL, Zhao QH, Hu GM, Deng KH, Liu YL
Received 26 July 2016
Accepted for publication 20 September 2016
Published 26 October 2016 Volume 2016:9 Pages 6619—6629
DOI https://doi.org/10.2147/OTT.S118184
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Min Li
Hu Zhao,1 Wuliang Wang,1 Qinghe Zhao,1 Guiming Hu,2 Kehong Deng,1 Yuling Liu1
1Department of Gynecology and Obstetrics, 2Department of Pathology, The Second Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China
Abstract: Aberrant expression of oncogenes and/or tumor suppressors play a fundamental effect on the pathogenesis and tumorigenicity of cervical cancer (CC). B-cell CLL/lymphoma 3 (BCL3) was previously found to be a putative proto-oncogene in human cancers and regulated signal transducer and activator of transcription 3 (STAT3), a critical oncogene, in CC cell line. However, its expression status, clinical significance and biological functions in CC remain largely unclear. The expressions of BCL3 and STAT3 in CC specimens were determined by immunohistochemistry. MTT, colony formation assays and flow cytometry analysis were carried out to test proliferation and cell cycle of CC cells. Here, the levels of BCL3 were overexpressed in CC compared to adjacent cervical tissues. Furthermore, high levels of BCL3 protein were confirmed by immunoblotting in CC cells as compared with normal cervical epithelial cells. The positive expression of BCL3 was correlated with adverse prognostic features and reduced survival rate. In addition, BCL3 regulated STAT3 abundance in CC cells. STAT3 was found to be upregulated and positively correlated with BCL3 expression in CC specimens. BCL3 overexpression resulted in prominent increased proliferation and cell cycle progression in Hela cells. By contrast, inhibition of BCL3 in CaSki cells remarkably suppressed proliferative ability and cell cycle progression. In vivo studies showed that knockdown of BCL3 inhibited tumor growth of CC in mice xenograft model. Notably, we confirmed that STAT3 mediated the oncogenic roles of BCL3 in CC. In conclusion, we suggest that BCL3 serves as an oncogene in CC by modulating proliferation and cell cycle progression, and its oncogenic effect is mediated by its downstream target gene, STAT3.
Keywords: B-cell CLL/lymphoma 3, cervical carcinoma, cell proliferation, cell cycle, signal transducer and activator of transcription 3
Introduction
Cervical cancer (CC), a highly malignant cancer, ranks the third commonest female cancer, causing around 200,000 deaths every year all over the world.1 Currently, surgical resection, chemotherapy and radiotherapy are the therapeutic options for CC patients.2 Furthermore, some chemical compounds were recently used as alternative therapies for CC patients.3–5 Yet, the current prognosis of CC patients is still poor, and the molecular mechanisms underlying CC pathogenesis remain largely unknown.
Numerous studies have confirmed that oncogenes and/or tumor suppressors act as critical regulators in various cellular processes,6,7 including cell proliferation, apoptosis, differentiation and movements. Moreover, emerging studies have demonstrated that dysregulation of oncogenes and/or tumor suppressors modulates several signaling pathways in cancer cells and they were actively implicated in the initiation of human cancers including CC.8–10 Oncogenes and/or tumor suppressors have been regarded as attractive therapeutic targets and promising biomarkers for human malignancies.11,12
B-cell CLL/lymphoma 3 (BCL3) is an atypical member of the IkB family and can bind NF-κB homodimeric complexes of p50 or p52, which switches the transcriptional properties of the homodimers from a repressive to an activating state.13,14 The mRNA and protein levels of BCL3 have been reported to be overexpressed in breast cancer,13,15 nasopharyngeal carcinoma,16 endometrial cancer,17 hepatocellular carcinoma18 and colorectal cancer.19 Functionally, BCL3 was found to regulate the colony formation and cell cycle progression by regulating ubiquitination-mediated degradation of c-Myc in colorectal cancer.20 Recently, Tu et al reported that BCL3 promotes tumor growth of hepatocellular carcinoma by regulating cell proliferation and cell cycle proceeding through cyclin D1.18 Previous study demonstrated that STAT3, an important oncogene in human cancers, was a bona fide target of BCL3 in CC cell line.21 However, the status of BCL3 and its exact roles in CC have not been investigated.
Here, BCL3 was found to be overexpressed in CC. Increased BCL3 level conferred adverse clinical parameters and reduced survival rate of CC patients. Moreover, BCL3 promoted proliferation and cell cycle progression. Notably, STAT3 was recognized as a direct functional mediator of BCL3 in CC.
Materials and methods
CC specimens and cell culture
Sixty pairs of CC and matched normal adjacent cervical tissues were collected from The Second Affiliated Hospital of Zhengzhou University. Diagnoses were confirmed by pathologist. None of the patients received preoperative immunotherapy, chemotherapy, hormone therapy or radiotherapy. These clinical samples were fixed by 10% formalin and embedded with paraffin after surgical resection. Written informed consent was obtained from every patient included in this study. The clinical stage and histological grade were based on 2014 International Federation of Gynecology and Obstetrics (FIGO) staging classification.22 The demographic and clinical information of 60 patients are shown in Table 1. This study and the protocols involving clinical tissues in this study were approved by the Ethics Review Committee of Zhengzhou University.
Four CC-derived cell lines (Hela, SW756, CaSki and ME-180 cells) were obtained from American Type Culture Collection (Manassas, VA, USA), and the human normal cervical epithelial cells were bought from CHI Scientific, Inc (Maynard, MA, USA). All cells were cultured in Dulbecco’s Modified Eagle’s Medium (HyClone, Logan, UT, USA) along with fetal bovine serum (10%) (HyClone), 100 U/mL penicillin and 100 μg/mL streptomycin (Sigma, St Louis, MO, USA). Cell cultures were kept in an incubator containing 5% CO2 and humidified atmosphere at 37°C.
Immunohistochemistry
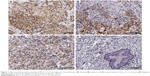
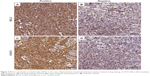
Before immunohistochemistry (IHC) staining, tissue samples were fixed with 10% formalin and embedded with paraffin. Then, the embedded tissues were cut into 4 μm thick sections. IHC staining was performed to evaluate the expression levels of BCL3 (sc-185; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and STAT3 (#9139; Cell Signaling Technology, Boston, MA, USA) in tissue samples following standard protocols. The percentage of positive staining cells was graded as per the following criteria: 0, less than 10%; 1, 10%–30%; 2, 31%–50%; 3, more than 50%.
Cell transfection
BCL3 shRNA (sc-29789-SH) and corresponding non-targeting (NT) shRNA (sc-108060) were obtained from Santa Cruz Biotechnology. shRNAs transfection was performed using plasmid transfection reagent (sc-108061; Santa Cruz Biotechnology). Retroviral vectors pMMP-BCL3 and pMMP-STAT3 were generated by inserting the cDNA into pMMP. Retrovirus packaging and transduction were described previously.23
MTT and colony formation assays
For cell viability, CC cells (4×103) were seeded into 96-well plates and stained with sterile MTT (Sigma) for 4 h at 37°C, following which the culture medium was discarded and an extra 150 μL dimethyl sulfoxide (Sigma) was then added into each well. At 24, 48 and 72 h after transfection, the absorbance at 490 nm was examined. Regarding colony formation assay, CC cells were seeded on 6-well plates. Two weeks after cell seeding, the colonies were stained with 1% crystal violet (MCE China, Shanghai, People’s Republic of China) and the number of colonies was counted.
Cell cycle assay
At 48 hours after transfection, CC cells were collected with trypsin and fixed overnight at 4°C using 80% ethanol. CC cells were then incubated with propidium iodide (Sigma) for 20 min. Then, flow cytometry assays for CC cells were performed with a FACSCalibur (BD Biosciences, Bedford, MA, USA).
Western blot
Cell proteins were collected with RIPA lysis buffer (Santa Cruz Biotechnology), and 40 μg protein was subjected to 4%–20% sodium dodecyl sulfate gel electrophoresis (Sigma) and was then transferred to polyvinylidene fluoride membranes (Roche, Indianapolis, IN, USA). Then, 5% skimmed milk (Shanghai Haoran Bio Technologies Co., Ltd., Shanghai, People’s Republic of China)-blocked membranes were incubated with BCL3 (Santa Cruz Biotechnology) and STAT3 (Cell Signaling Technology) antibodies, respectively, and subsequently incubated with matched secondary antibodies (#7074 and #7076; Cell Signaling Technology). Then, signals for each protein expression were detected with the Bio-Rad Gel imaging system (Bio-Rad, Hercules, CA, USA). GAPDH (#5174; Cell Signaling Technology) was used as a loading control.
In vivo experiments
The subcutaneous tumor formation experiments were performed in nude mice. In all, 5×106 CaSki cells with BCL3 knockdown or control cells were suspended in phosphate-buffered saline and were implanted into the back of mice via subcutaneous injection. Tumor volume was measured with calipers every 3 days. Tumor volume was calculated with volume = length × width × width/2. The protocols of in vivo experiments were approved by the Institutional Animal Care and Use Committee of Zhengzhou University.
Statistical analysis
All data were collected and shown as mean ± standard error. Statistical analyses including Pearson chi-squared test, two-tailed Student’s t-test, analysis of variance, Kaplan–Meier survival analysis, log-rank test and Spearman’s correlation analysis were performed with GraphPad Prism 5 software (GraphPad Software, Inc, San Diego, CA, USA). P<0.05 was considered to be statistically different.
Results
The expression of BCL3 was increased in CC
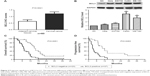
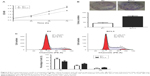
IHC was initially performed to detect BCL3 expression in tumor and matched non-tumor tissues. Compared with that in noncancerous tissues, the level of BCL3 was significantly upregulated in CC tissues (P<0.05, Figures 1 and 2A). Furthermore, our data explored that BCL3 expressions in four CC cell lines (Hela, SW756, CaSki and ME-180 cells) were evidently increased compared to normal cervical epithelial cells (P<0.05, Figure 2B). These data indicate that increased level of BCL3 probably contributes to the initiation and development of CC.
Increased BCL3 level confers poor clinical parameters and prognosis of CC patients
After confirming the overexpression of BCL3 in CC, we disclosed the clinical significance and prognostic value of BCL3 expression. BCL3 staining was considered as positive expression in 37 cases of CC according to IHC scores (≥1). As shown in Table 1, the positive expression of BCL3 in CC patients was correlated with large tumor size (P=0.020) and advanced FIGO stage (P=0.023). Then, we investigated the predicting value of BCL3 for the prognosis of CC patients. Compared with those with negative expression of BCL3, patients who showed positive expression of BCL3 had significant reduced overall survival (OS) (P=0.0051, Figure 2C) and progression-free survival (PFS) (P=0.0283, Figure 2D). These indicate that BCL3 can potentially act as a biomarker and a prognostic indicator for CC.
BCL3 positively regulates STAT3 abundance in CC
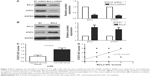
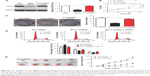
Previous study demonstrated that STAT3, an important oncogene in human cancer, was a bona fide target of BCL3 in CC cell line.21 Hela cells showed the lowest level, while CaSki cells presented the highest level of BCL3 expression. Thus, Hela and CaSki cells were used for gain- and loss-of-function experiments. CaSki cells were transduced with NT shRNA and BCL3 shRNA and were subjected to immunoblotting for BCL3 and STAT3 expression, respectively. As expected, BCL3 knockdown significantly reduced STAT3 expression in vitro (P<0.05, Figure 3A). By contrast, BCL3 overexpression resulted in an obvious increase of STAT3 protein expression in Hela cells (P<0.05, Figure 3B). Furthermore, IHC was performed to detect the expression of STAT3 in CC specimens. The expression of STAT3 was prominently overexpressed in CC compared to adjacent cervical tissues (P<0.05, Figure 3C). Notably, Spearman’s correlation analysis indicated that BCL3 was positively correlated with STAT3 expression in CC specimens (r=0.734, P=0.012, Figures 3D and 4).
BCL3 promotes CC cell proliferation and cell cycle progression
Next, we disclosed the exact roles of BCL3 in CC cells. Since increased proliferation, aberrant cell cycle progression and reduced apoptosis were well-recognized hallmarks of human cancer, we examined whether BCL3 regulated these biological processes of CC cells. Infection of BCL3 retroviruses led to a significant increase of cell viability in Hela cells (P<0.05, Figure 5A). Subsequently, the proliferative ability of Hela cells was enhanced after BCL3 overexpression as suggested by colony formation assays (P<0.05, Figure 5B). Meanwhile, BCL3 overexpression facilitated cell cycle progression in Hela cells (P<0.05, Figure 5C). On the other hand, transfection of BCL3 shRNA led to a significant decrease of STAT3 level (P<0.05, Figure 6A). Functionally, BCL3 knockdown inhibited CaSki cell viability, proliferation and cell cycle progression (P<0.05, Figure 6B–D). Furthermore, a subcutaneous tumor formation model was used to investigate whether BCL3 could affect the growth of CC cells in mice xenograft model. As shown in Figure 6E, BCL3 knockdown significantly reduced tumor growth of CC in mice (P<0.05). Thus, BCL3 facilitates CC growth by promoting proliferation and cell cycle progression.
STAT3 is a functional mediator of BCL3
Since STAT3 was recognized as a downstream target of BCL3 in CC, we further investigated if STAT3 mediated the functions of BCL3. STAT3 retroviruses were employed for STAT3 overexpression in CaSki cells with BCL3 silencing. STAT3 retroviruses significantly increased the expression of STAT3 in CaSki cells with BCL3 knockdown (P<0.05, Figure 6A). Notably, STAT3 restoration abolished the tumor suppressive effects of BCL3 shRNA on CaSki cells with increased cell proliferation (P<0.05, Figure 6B and C) and cell cycle progression (P<0.05, Figure 6D). Our data indicate that STAT3 mediates the functions of BCL3 in CC cells.
Discussion
Increasing evidence confirms that oncogenes play critical roles in the progression of human malignancies including CC.24,25 Functionally, oncogenes were found to modulate the proliferation, apoptosis and metastasis of CC cells.8,26 Moreover, oncogenes were proposed as novel biomarkers and therapeutic targets for CC.27 Therefore, elucidation of the expression level, clinical significance and biological function of specific oncogenes in CC will greatly contribute to the diagnosis and treatment of CC. Overexpression of BCL3 was observed in CC specimens and cells, and the positive expression of BCL3 in CC was directly correlated with adverse clinical features including large tumor size and advanced FIGO stage. Importantly, we suggested that the positive expression of BCL3 conferred a reduced OS and PFS of CC patients. Therefore, these data in this study demonstrate that BCL3 can act as a promising biomarker and a prognostic indicator for CC.
BCL3, a well-known proto-oncogene, is deregulated in solid malignancies and exerts an oncogenic role by regulating proliferation and cell death.21 Study of breast cancer confirmed that BCL3-dependent stabilization of CtBP1 was crucial for the inhibition of apoptosis and tumor progression.28 Moreover, BCL3 promoted the proliferation of ovarian cancer cells.29 In this study, in vitro experiments confirmed that BCL3 promoted the proliferative ability and cell cycle progression of CC cells. Moreover, in vivo experiments showed that BCL3 knockdown could inhibit tumor growth of CC in nude mice. Therefore, this study confirms that BCL3 promotes tumor growth of CC by modulating proliferation and cell cycle progression.
STAT3, an important oncogene in human cancer, was found to be overexpressed in cancers.30–32 Previous study showed that STAT3 was upregulated in CC and promoted tumor growth, invasion and metastasis.33 Furthermore, microRNA-551-3p contributed to resistance to apoptosis and increased survival and proliferation of ovarian cancer cells by activating STAT3 transcription.34 A recent study demonstrated that STAT3, an important oncogene in human cancer, was a bona fide target of BCL3 in CC cell line.21 In this study, BCL3 regulated the protein level of STAT3 in CC cells. Moreover, a positive correlation between BCL3 and STAT3 expression was observed in CC specimens. These data further demonstrate that STAT3 is a downstream target of BCL3 in CC. Notably, STAT3 restoration could abrogate the influence of BCL3 shRNA on proliferation and the process of cell cycle in CC cells. Therefore, these results indicate that STAT3 serves as not only a downstream target but also a functional mediator of BCL3.
In all, we present that BCL3 is overexpressed in CC and its positive expression is correlated with poor clinical parameters and survival. BCL3 promotes tumor growth of CC by regulating proliferative ability and the process of cell cycle. Mechanistically, this study finds that BCL3 functions as an oncogenic factor by activating STAT3. Taken together, BCL3 may serve as a clinical indicator and a drug target for CC patients.
Conclusion
Overall, we suggest that overexpression of BCL3 contributes to the progression of CC, potentially promoting cell proliferation and cell cycle proceeding by activating STAT3, and we infer that BCL3 may function as a clinical biomarker and a therapeutic target for CC, as well.
Acknowledgment
The authors thank all the patients who participated in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Arbyn M, Verdoodt F, Snijders PJ, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15(2):172–183. | ||
Li X, Li J, Wu X. Incidence, risk factors and treatment of cervical stenosis after radical trachelectomy: a systematic review. Eur J Cancer. 2015;51(13):1751–1759. | ||
Fernández-Herrera MA, López-Muñoz H, Hernández-Vázquez JM, et al. Synthesis and selective anticancer activity of steroidal glycoconjugates. Eur J Med Chem. 2012;54:721–727. | ||
Fernandez-Herrera MA, Sandoval-Ramirez J, Sanchez-Sanchez L, Lopez-Munoz H, Escobar-Sanchez ML. Probing the selective antitumor activity of 22-oxo-26-selenocyanocholestane derivatives. Eur J Med Chem. 2014;74:451–460. | ||
Fernández-Herrera MA, López-Muñoz H, Hernández-Vázquez JM, et al. Synthesis of 26-hydroxy-22-oxocholestanic frameworks from diosgenin and hecogenin and their in vitro antiproliferative and apoptotic activity on human cervical cancer CaSki cells. Bioorg Med Chem. 2010;18(7):2474–2484. | ||
Viale A, Pettazzoni P, Lyssiotis CA, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514(7524):628–632. | ||
Antal CE, Hudson AM, Kang E, et al. Cancer-associated protein kinase C mutations reveal kinase’s role as tumor suppressor. Cell. 2015;160(3):489–502. | ||
Li XY, Wang X. The role of human cervical cancer oncogene in cancer progression. Int J Clin Exp Med. 2015;8(6):8363–8368. | ||
Ruttkay-Nedecky B, Jimenez Jimenez AM, Nejdl L, et al. Relevance of infection with human papillomavirus: the role of the p53 tumor suppressor protein and E6/E7 zinc finger proteins (Review). Int J Oncol. 2013;43(6):1754–1762. | ||
He C, Mao D, Hua G, et al. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol Med. 2015;7(11):1426–1449. | ||
Corsini LR, Bronte G, Terrasi M, et al. The role of microRNAs in cancer: diagnostic and prognostic biomarkers and targets of therapies. Expert Opin Ther Targets. 2012;16(suppl 2):S103–S109. | ||
Bentivegna E, Gouy S, Maulard A, Chargari C, Leary A, Morice P. Oncological outcomes after fertility-sparing surgery for cervical cancer: a systematic review. Lancet Oncol. 2016;17(6):e240–e253. | ||
Cogswell PC, Guttridge DC, Funkhouser WK, Baldwin AS Jr. Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene. 2000;19(9):1123–1131. | ||
Bours V, Franzoso G, Azarenko V, et al. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell. 1993;72(5):729–739. | ||
Choi HJ, Lee JM, Kim H, et al. Bcl3-dependent stabilization of CtBP1 is crucial for the inhibition of apoptosis and tumor progression in breast cancer. Biochem Biophys Res Commun. 2010;400(3):396–402. | ||
Thornburg NJ, Pathmanathan R, Raab-Traub N. Activation of nuclear factor-kappaB p50 homodimer/Bcl-3 complexes in nasopharyngeal carcinoma. Cancer Res. 2003;63(23):8293–8301. | ||
Pallares J, Martinez-Guitarte JL, Dolcet X, et al. Abnormalities in the NF-kappaB family and related proteins in endometrial carcinoma. J Pathol. 2004;204(5):569–577. | ||
Tu K, Liu Z, Yao B, et al. BCL-3 promotes the tumor growth of hepatocellular carcinoma by regulating cell proliferation and the cell cycle through cyclin D1. Oncol Rep. 2016;35(4):2382–2390. | ||
Puvvada SD, Funkhouser WK, Greene K, et al. NF-kB and Bcl-3 activation are prognostic in metastatic colorectal cancer. Oncology. 2010;78(3–4):181–188. | ||
Liu Z, Jiang Y, Hou Y, et al. The IkappaB family member Bcl-3 stabilizes c-Myc in colorectal cancer. J Mol Cell Biol. 2013;5(4):280–282. | ||
Maldonado V, Espinosa M, Pruefer F, et al. Gene regulation by BCL3 in a cervical cancer cell line. Folia Biol (Praha). 2010;56(4):183–193. | ||
Mutch DG, Prat J. FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol. 2014;133(3):401–404. | ||
Tu K, Li J, Verma VK, et al. Vasodilator-stimulated phosphoprotein promotes activation of hepatic stellate cells by regulating Rab11-dependent plasma membrane targeting of transforming growth factor beta receptors. Hepatology. 2015;61(1):361–374. | ||
Pellicano F, Mukherjee L, Holyoake TL. Concise review: cancer cells escape from oncogene addiction: understanding the mechanisms behind treatment failure for more effective targeting. Stem Cells. 2014;32(6):1373–1379. | ||
Ekalaksananan T, Sookmai W, Fangkham S, et al. Activity of andrographolide and its derivatives on HPV16 pseudovirus infection and viral oncogene expression in cervical carcinoma cells. Nutr Cancer. 2015;67(4):687–696. | ||
Qureshi R, Arora H, Rizvi MA. EMT in cervical cancer: its role in tumour progression and response to therapy. Cancer Lett. 2015;356(2 Pt B):321–331. | ||
Durzynska J. IGF axis and other factors in HPV-related and HPV-unrelated carcinogenesis (review). Oncol Rep. 2014;32(6):2295–2306. | ||
Choi HJ, Lee JM, Kim H, et al. Bcl3-dependent stabilization of CtBP1 is crucial for the inhibition of apoptosis and tumor progression in breast cancer. Biochem Biophys Res Commun. 2010;400(3):396–402. | ||
Guan Y, Yao H, Zheng Z, Qiu G, Sun K. MiR-125b targets BCL3 and suppresses ovarian cancer proliferation. Int J Cancer. 2011;128(10):2274–2283. | ||
Wu ZL, Song YQ, Shi YF, Zhu J. High nuclear expression of STAT3 is associated with unfavorable prognosis in diffuse large B-cell lymphoma. J Hematol Oncol. 2011;4(1):31. | ||
Chen Y, Wang J, Wang X, et al. STAT3, a poor survival predicator, is associated with lymph node metastasis from breast cancer. J Breast Cancer. 2013;16(1):40–49. | ||
Takemoto S, Ushijima K, Kawano K, et al. Expression of activated signal transducer and activator of transcription-3 predicts poor prognosis in cervical squamous-cell carcinoma. Br J Cancer. 2009;101(6):967–972. | ||
Fan Z, Cui H, Xu X, et al. MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget. 2015;6(28):25266–25280. | ||
Chaluvally-Raghavan P, Jeong KJ, Pradeep S, et al. Direct upregulation of STAT3 by microRNA-551b-3p deregulates growth and metastasis of ovarian cancer. Cell Rep. 2016;15(7):1493–1504. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.