Back to Journals » International Journal of Nanomedicine » Volume 14
BCESA: a nano-scaled intercalator capable of targeting tumor tissue and releasing anti-tumoral β-carboline-3-carboxylic acid
Authors Wu J , Cui Y , Zhang X, Gui L, Wang Y , Peng S , Zhao M
Received 14 September 2018
Accepted for publication 20 March 2019
Published 30 April 2019 Volume 2019:14 Pages 3027—3041
DOI https://doi.org/10.2147/IJN.S187600
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Jianhui Wu,1,2 Yue Cui,1,2 Xiaoyi Zhang,1,2 Lin Gui,1,2 Yaonan Wang,1,2 Shiqi Peng,1,2 Ming Zhao1–3
1Beijing Area Major Laboratory of Peptide and Small Molecular Drugs, School of Pharmaceutical Sciences, Capital Medical University, Beijing 100069, People’s Republic of China; 2Engineering Research Center of Endogenous Prophylactic of Ministry of Education of China, School of Pharmaceutical Sciences, Capital Medical University, Beijing 100069, People’s Republic of China; 3Beijing Laboratory of Biomedical Materials and Key Laboratory of Biomedical Materials of Natural Macromolecules (Beijing University of Chemical Technology), Ministry of Education, Beijing, People’s Republic of China
Background: In the discovery of DNA intercalators, β-carbolines compose one member of the most interesting alkaloid family and are of clinical importance. In the efforts, N-(3-benzyloxycarbonyl-β-carboline-1-yl)ethyl-Ser-Ala-OBzl (BCESA) was designed as a nano-scaled DNA intercalator without Dox-like toxicity.
Methods: Based on the structural analysis and CDOCKER energy comparison, BCESA was rationally designed as such a nano-scaled intercalator. The anti-tumor activity, the toxicity and the tumor targeting action of BCESA were evaluated on mouse models.
Results: The in vitro proliferation of cancer cells, but not non-cancer cells, was effectively inhibited by BCESA. On S180 mouse model BCESA dose-dependently slowed the tumor growth, and 0.01 μmol/kg/day was found as a minimal effective dose. Both BCESA and its moiety were found in the tumor tissue, but not in the organs and the blood, of S180 mice.
Conclusion: BCESA should be a nano-scaled intercalator capable of targeting tumor tissue to release anti-tumoral β-carboline-3-carboxylic acid and its 1-methyl derivative, while Ser-Ala-OBzl is a simple and desirable carrier.
Keywords: nano-medicine, β-carboline, intercalator, d(AGACGTCT)2, anti-tumor
Introduction
As DNA intercalator β-carbolines are one member of the most interesting alkaloid family and of clinical importance.1–5 This leads to the discovery of the diverse β-carbolines, such as tetrahydro-β-carbolines6 and their hydrantoin hybrids,7 β-carboline-benzimidazole conjugates,8 N-heterocyclic carbenes from β-carboline,9 various harmines and related β-carboline,10–14 ruthenium (II) complexes of β-carbolines,15 β-carbolines derived from harmine,16,17 β-carbolines with phenylheterocycles,18 as well as 1-aryl or 1-indolyl substituted β-carbolines.19,20
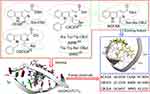
In our previous papers, the diverse intercalators of DNA were reported.21–25 On the other hand, however, their effective dose reached 8.9 μmol/kg/day. Besides, the previous evaluation of N-[(3-benzyloxycarbonyl-β-carboline-1-yl)ethyl]-AA-OBzl showed that N-[(3-benzyloxycarbonyl-β-carboline-1-yl)ethyl]-Ser-OBzl and N-[(3- benzyloxycarbonyl-β-carboline-1-yl)ethyl]-Ala-OBzl had the best efficacy. To decrease the effective dose, Ser-OBzl and Ala-OBzl were combined, used to replace the AA-OBzl of N-[(3-benzyloxycarbonyl-β-carboline-1-yl)ethyl]-AA-OBzl and N-(3-benzyloxycarbonyl-β-carboline-1-yl)ethyl-Ser-Ala-OBzl (BCESA) was designed. The rationality of the design was firstly examined by docking investigation. The CDOCKER energies indicate that among 6 intercalators BCESA has the lowest energy (Figure 1). Besides, the docking feature of BCESA in the active site of d(AGACGTCT)2 shows 8 π-π stacking interactions and 6 hydrogen bond interactions, suggesting BCESA well fits the active site of d(AGACGTCT)2. These encouraged us to hypothesize that BCESA could be able to target tumor tissue and release the anti-tumoral pharmacophores wherein. In this context, the synthesis, structural analysis, nano-scaled assembly, anti-tumoral evaluation, tumor targeting action and release profile of BCESA were performed here.
Materials and methods
Reagents and instruments
L-Amino acids, reagents and solvents for the synthesis were obtained commercially and used without further purification unless otherwise specified. Qingdao silica gel (GF254) was used for TLC. Silica gel (H60, Qingdao Haiyang Chemical Co. Ltd, Qingdao China) was used for chromatography. Melting points were measured on an XT5 hot stage microscope (Beijing key electro-optic factory). The spectra of 1H NMR (300 MHz and 500 MHz) and 13C NMR (75 MHz and 125 MHz) were recorded on Bruker AMX-300 and AMX-500 spectrometers. DMSO-d6 and TMS were used as the solvent and the internal standard, respectively. Perkin-Elmer 983 instrument was used to record IR spectra. ZQ 2000 (Waters, USA) mass spectrometer and 9.4 T SolariX Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometer (Bruker, USA) were used to record the electrospray ionization mass spectra (ESI-MS), both of which had ESI/matrix-assisted laser desorption/ionization (MALDI) dual ion source. The high-performance liquid chromatography (HPLC) purity of BCESA was conducted on an Agilent Technologies 1200 Series HPLC system (Agilent Technologies, Santa Clara, CA, USA), and Eclipse XDB C18 column (5 μm, 4.6×150 mm) was used. The column temperature was 40°C. BCESA was eluted with methanol/H2O. The gradient consisted of 60% methanol (0–5 min), 70% methanol (5–10 min), 80% methanol (10–20 min) and 90% methanol (20–30 min). The flow rate was 0.8 mL/min. Human colorectal carcinoma (HCT-8), murine sarcoma (S180), human neuroblastoma (SH-sy5y), human leukemia (HL60), human non-small-cell lung cancer (A549) and human breast cancer (MCF-7) cell line as well as non-carcinoma cell human keratinocytes cell line (HaCaT) were purchased from KeyGen Biotech Co. Ltd. (Nanjing,China).
Animals and ethics
The in vivo assay was performed under the supervisory control of Ethics Committee of Capital Medical University. The committee approved that this assay can use ICR mice only. Male ICR mice (20–22 g) were commercially obtained from the Laboratory Animal Center of the University. The evaluation protocols were reviewed and approved by the committee. The committee assured that the requirements of Animal Welfare Act and NIH Guide for Care and Use of Laboratory Animals were maintained. With SPSS 19.0 program and one-way ANOVA all data resulted from the in vivo assay were statistically analyzed, and P-value <0.05 was considered statistically significant.
Docking investigation
By following the literature, the conformation of BCESA was generated to perform the docking investigation.26,27 For this purpose, 10 energy optimized conformations of BCESA were docked into the active pocket of d(AGACGTCT)2 (PDB:1N37).28,29
Synthesis of BCESA
Benzyl 1-(2,2-dimethoxyethyl)-β-4H-carboline-3-carboxylate (1)
At room temperature, a suspension of 20.0 mL (78.6 mmol) of 1,1,3,3-tetramethoxypropane, 20 mL of CF3CO2H and 200 mL of CH2Cl2 was stirred for 45 mins, into which 18 g (0.37 mol) of L-Trp-OBzl were added and stirred for additional 48 hrs. The reaction mixture was washed successively with saturated aqueous NaHCO3 (200 mL ×3) and saturated aqueous NaCl (200 mL ×3), dried over anhydrous Na2SO4 for 12 hrs and then filtrated. The filtrate was evaporated under vacuum, the residue was chromatographically purified on silica gel column (petroleum ether/ethyl acetate, 2/1) to obtain 1 as pale yellow oil. Yield: 60%. ESI-MS (m/e): 395 [M + H]+.1H NMR (300 MHz, CDCl3) δ 8.50 (s, 1H), 5.22 (d, J =3.0 Hz, 2H), 4.69 (m, 1H), 4.42 (m, 1H), 4.02 (dd, J =7.6, 5.2 Hz, 1H), 3.44 (s, 3H), 3.38 (s, 3H), 3.20–3.13 (m, 1H), 3.08–2.97 (m, 1H), 2.13–1.99 (m, 2H).
Benzyl 1-(2,2-dimethoxyethyl)-β-carboline-3-carboxylate (2)
At 0ºC, the suspension of 10.8 g (20 mmol) of 1 in 100 mL of THF was stirred to form a clear solution. Into this solution 7.8 g (40 mmol) of KMnO4 were added, stirred at 0ºC for 1 hr, at room temperature for 3 hrs, TLC (CHCl3/MeOH, 15/1) indicated the complete disappearance of 1 and the formed precipitates were removed by filtration. The filtrate was evaporated under vacuum, and the residue was dissolved in 100 mL of ethyl acetate. The ethyl acetate solution was washed with saturated aqueous NaCl, dried over anhydrous Na2SO4 for 12 hrs and evaporated under vacuum. The residue was chromatographically purified on silica gel column (petroleum ether/ethyl acetate, 3/1) to obtain 2 as yellow powders. Yield: 68%. ESI-MS (m/z): 391 [M+H]+1H NMR (300 MHz, DMSO-d6) δ 9.68 (s, 1H), 8.81 (s, 1H), 8.16 (d, J =8.0 Hz, 1H), 7.55 (m, 4H), 7.35 (m, 4H), 5.53 (s, 2H), 4.82 (s, 1H), 3.58 (d, J =4.0 Hz, 2H), 3.41 (s, 6H).
Benzyl 1-carbonylmethyl-β-carboline-3-carboxylate (3)
At room temperature, the mixture of 10 g (2.0 mmol) of 2, 7.2 mL of acetic acid, 0.9 mL of hydrochloric acid and 0.9 mL of water was stirred at room temperature for 4 hrs. Into this mixture, 50 g of ice were added, and the formed precipitates were collected by filtration to provide 3 for the next reaction without purification. Yield: 67%. ESI-MS (m/e) 345 [M+H]+.1H NMR (300 MHz, DMSO-d6) δ 10.97 (s, 2H), 9.79 (s, H), 8.79 (s, 1H), 8.27 (d, J =7.9 Hz, 1H), 7.40 (t, J =8.4 Hz, 4H), 7.10 (t, J =6.9 Hz, 2H), 6.99 (t, J =6.9 Hz, 2H), 6.23 (m, 2H), 5.50 (s, 2H), 4.67 (m, 2H).
Ser-Ala-OBzl
A solution of 2.7 g (13.30 mmol) of Boc-Ser, 2.16 g (16.02 mmol) of HOBt, 3.30 g (16.02 mmol) of DCC and 50 mL of anhydrous THF was stirred for 30 mins. Into this solution, a solution of 4.41 g (12.02 mmol) of Ala-OBzl in 10 mL of anhydrous THF was added. By using N-methylmorpholine (NMM) the reaction mixture was adjusted to pH 9, then at 0ºC stirred for 3 hrs and at room temperature for 12 hrs. By filtration the precipitates of DCU were removed, the filtrate was evaporated under vacuum and the residue was diluted with 30 mL of ethyl acetate. This solution was successively washed with saturated aqueous NaHCO3 (20 mL ×3), 5% aqueous KHSO4 (20 mL ×3) and saturated aqueous NaCl (20 mL ×3) and finally dried over anhydrous Na2SO4 for 12 hrs. After filtration, the filtrate was evaporated under vacuum and the residue was chromatographically purified on silica gel column (CH2Cl2/MeOH, 30/1) to give 3 g of Boc-Ser-Ala-OBzl in 63% yield.
At 0ºC, 1 g (2.73 mmol) of Boc-Ser-Ala-OBzl was stirred in a solution of hydrogen chloride in anhydrous ethyl acetate (10 mL, 4 M) for 2 hrs and evaporated under vacuum. The residue was dissolved in 10 mL of anhydrous ethyl acetate, and the solution was evaporated under vacuum. This procedure was repeated for 3 times to thoroughly remove the excess hydrogen chloride. The residue was triturated with ether to provide 0.6 g of Ser-Ala-OBzl as colorless powders. The yield was 83% and ESI(-)-FT-ICR-MS was 267.1275.
N-[(3-Benzyloxycarbonyl-β-carboline-1-yl)ethyl]-Ser-Ala-OBzl (BCESA)
A mixture of 733 mg (2.42 mmol) of Ser-Ala-OBzl, 40.4 mg (1.01 mmol) of NaOH, 50 mL of MeOH and 695 mg (2.02 mmol) of 3 was stirred at room temperature for 30 mins, and then 127 mg (2.02 mmol) of NaBH3CN were added. The reaction mixture was stirred at room temperature for 20 hrs, adjusted to pH 2 with hydrochloric acid (3 M) and evaporated under vacuum. The residue was dissolved in 20 mL of water. This solution was washed with ether (50 mL ×3), adjusted to pH 8 with aqueous NaOH (1 M) and extracted with ethyl acetate (50 mL ×3). The ethyl acetate phase was washed with saturated aqueous NaCl and dried over anhydrous Na2SO4 for 12 hrs. After filtration, the filtrate was evaporated under vacuum, and the residue was chromatographically purified on silica gel column (petroleum ether/ethyl acetate, 5/1) to give 575 mg of BCESA as pale yellow powers. Yield: 48%. Mp 167–168ºC. [α]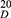 =−2.43 (c=0.37, CH3OH). ESI-FT-ICR-MS (m/z) of [C34H34N4O6+ H]+: calcd 595.2551, found 595.2568. IR (KBr): 3230, 3088, 3062, 3032, 2935, 2868, 1712, 1651, 1568, 1500, 1456, 1379, 1348, 1307, 1247, 1213, 1153, 1139, 1103, 1053, 900, 786, 744, 696 cm−1.1H NMR (300 MHz, CDCl3) δ 10.85 (s, 1H), 8.65 (s, 1H), 8.05 (d, J =7.8 Hz, 1H), 8.00 (d, 7.8 Hz, 1H), 7.48 (d, J =9.0 Hz, 4H), 7.39–7.32 (m, 3H), 7.27 (m, 6H), 5.42 (s, 2H), 5.09 (dd, J =30.4, 12.2 Hz, 2H), 4.61 (m, 1H), 3.97 (mz, 1H), 3.77 (m, 1H), 3.46–3.31 (m, 2H), 3.13 (m, 3H), 1.34 (d, J =7.2 Hz, 3H).13C NMR (75 MHz, CDCl3) δ 173.1, 172.8, 166.1, 144.2, 140.9, 136.4, 136.1, 136.0, 135.1, 128.5, 128.4, 128.3, 128.1, 127.9, 121.8, 121.6, 120.5, 116.7, 112.3, 67.2, 67.1, 66.3, 64.5, 63.0, 58.9, 47.9, 46.7, 34.2, 18.7, 17.8. HPLC Purity: 95.9%.
=−2.43 (c=0.37, CH3OH). ESI-FT-ICR-MS (m/z) of [C34H34N4O6+ H]+: calcd 595.2551, found 595.2568. IR (KBr): 3230, 3088, 3062, 3032, 2935, 2868, 1712, 1651, 1568, 1500, 1456, 1379, 1348, 1307, 1247, 1213, 1153, 1139, 1103, 1053, 900, 786, 744, 696 cm−1.1H NMR (300 MHz, CDCl3) δ 10.85 (s, 1H), 8.65 (s, 1H), 8.05 (d, J =7.8 Hz, 1H), 8.00 (d, 7.8 Hz, 1H), 7.48 (d, J =9.0 Hz, 4H), 7.39–7.32 (m, 3H), 7.27 (m, 6H), 5.42 (s, 2H), 5.09 (dd, J =30.4, 12.2 Hz, 2H), 4.61 (m, 1H), 3.97 (mz, 1H), 3.77 (m, 1H), 3.46–3.31 (m, 2H), 3.13 (m, 3H), 1.34 (d, J =7.2 Hz, 3H).13C NMR (75 MHz, CDCl3) δ 173.1, 172.8, 166.1, 144.2, 140.9, 136.4, 136.1, 136.0, 135.1, 128.5, 128.4, 128.3, 128.1, 127.9, 121.8, 121.6, 120.5, 116.7, 112.3, 67.2, 67.1, 66.3, 64.5, 63.0, 58.9, 47.9, 46.7, 34.2, 18.7, 17.8. HPLC Purity: 95.9%.
Characterization of BCESA
NOESY 2D1H NMR spectrum of BCESA
To reveal the pattern of the molecular assembly of BCESA, the NOESY 2D1H NMR spectrum was on Bruker 800 MHz spectrometer recorded by following the literature.30,31
FT-ICR-MS and qCID spectra of BCESA
To reveal the molecular assembly, the FT-ICR-MS and qCID spectra of BCESA were recorded on Bruker 9.4 T solariX FT-ICR mass spectrometer equipped with an ESI/MALDI dual ion source.26,27
SEM, TEM and AFM feature of BCESA
The nano-features of BCESA in solid state, in water and in mouse plasma were imaged by scanning electron microscopy (SEM), transmission electron microscopy (TEM) and atomic force microscopy (AFM) experiments, respectively.32–34
Mesoscale simulation of BCESA
By following the method of the literature the size of the nano-particles of BCESA a mesoscale simulation was performed.35
Faraday-Tyndall effect, particle size and zeta potential tests of BCESA
By following the method of the literature, the nano-property of BCESA in water was identified by Faraday-Tyndall effect, particle size and zeta potential.36 Aimed at this, the solutions (10 μM, 1 μM, 0.1 μM, 0.01 μM) of BCESA in the ultrapure water of pH 6.8 and 1.2, as well as in PBS of pH 7.4 were prepared. The 650 nm laser was used for inducing Faraday-Tyndall effect.
Bioassays of BCESA
In vitro anti-proliferation assay of BCESA
In T-25 flasks, all the stock cultures were grown. The suspensions of the freshly trypsinized cells were seeded in 96-well microtiter plates, and the density was 5000 cells per well. At 37ºC, the cells were in the high glucose-DMEM or the RPMI-1640 medium cultured for 4 hrs. The medium contained 10% (v/v) fetal calf serum, 60 μg/mL of penicillin and 100 μg/mL of streptomycin. After the addition of 25 μL of the solution of Dox or BCESA, the cells were cultured for 48 hrs, then 25 μL of MTT solution (5 μg/mL) were added and the cells were incubated for additional 4 hrs. The medium was removed, and MTT-formazan was dissolved in 100 μL of DMSO. On a microplate reader, the optical density was measured at 570 nm. The IC50 was calculated based on the survival curve and linear regression analysis. Each test was repeated in quadruplicate, and the results were expressed as mean±SD.
In vivo anti-tumor assay of BCESA on S180 mouse model
In this evaluation ICR mice (male, 22±2 g) were used. For initiation of subcutaneous tumors S180 ascites tumor cells were obtained from the tumor-bearing mice that were serially transplanted once per week. By injecting 0.2 mL saline containing 1×107 viable tumor cells under the skin on the right armpit subcutaneous tumors were implanted, 24 hrs later the implanted mice were randomly divided into experimental groups (12 per group). To clarify the validity of the mouse model and the intercalation mechanism of BCESA, Dox was used as the positive control, while the mice in this group were given a daily i.p injection of 2 μmol/kg/day of Dox for 9 consecutive days. To clarify the validity of the mouse model, 0.5% CMC-Na was used as the negative control, while the mice in this group were orally given 0.5% CMC-Na (10 mL/kg/day) for 9 consecutive days. The mice in BCESA groups were orally given a suspension of BCESA in 0.5% CMC-Na (0.01 μmol/kg/day or 0.1 μmol/kg/day or 1.0 μmol/kg/day) for 9 consecutive days. During the experimental period, the body weights of all mice were recorded every day, while 24 hrs after the last administration all mice were weighed, sacrificed by diethyl ether anesthesia and collected 1 mL of fresh blood. Then, the tumors and organs were dissected and weighed immediately.
In vivo liver and kidney injury of BCESA to S180 mice
The collected blood from the mice receiving in vivo antitumor assay was centrifuged at 1000 rpm for 15 mins to prepare the serum. By following the guidance of the kits (AST/GOT testing kit, ALT/GPT testing kit; JCBIO Co., Nanjing, People’s Republic of China) the serum ALT and AST were measured. By following the standard protocol (creatinine assay kit, JCBIO Co., Nanjing, PR China), the serum Cr was measured.
UV spectrum and the interaction of BCESA towards CT DNA
To visualize the intercalation of BCESA towards calf thymus DNA (CT DNA) the ultraviolet (UV) spectrum assay was performed. In this assay, the UV spectra of CT DNA (10 μM, in PBS of pH 7.4) in the presence of BCESA (0 µM, 5 µM, 10 µM, 15 µM, 20 µM, 25 µM, 30 µM, 35 µM and 40 µM in PBS of pH 7.4) were monitored (Shimadzu 2550 spectrophotometer, 200–320 nm wavelength).
Fluorescent spectrum and the interaction of BCESA towards CT DNA
To visualize the intercalation of BCESA towards CT DNA, the fluorescent spectrum assay was performed. In this assay, the fluorescent spectra of BCESA (10 μM, in pH 7.4 PBS) in the presence of CT DNA (pH 7.4, 0 µM, 9 µM, 18 µM, 27 µM, 36 µM, 45 µM, 54 µM, 63 µM and 72 µM, in PBS of pH 7.4) were monitored (Shimadzu RF-5310PC spectrofluorometer, 258 nm of fluorescence excitation wavelength).
Relative viscosity and the interaction of BCESA towards CT DNA
To visualize the intercalation of BCESA towards CT DNA, the relative viscosity of CT DNA was measured on Ubbeholde viscometer immersed in a thermostated water bath of 25°C. In this assay into the solution of CT DNA in PBS (13 mL, 80 μM) the solutions of BCESA in PBS were added to get a series of ratios of [BCESA]/[CT DNA] that fell in a range of 0.13–0.82. After a thermal equilibrium time of 5 mins, the flow times of the ratios were measured. For this purpose, the equation, η=(t - t0)/t0, was used, wherein t0 and t are the measured flow times in the absence and the presence of BCESA, respectively. The relative viscosity of CT DNA in the presence and the absence of BCESA are calculated and presented as (η/η0)1/3 versus binding ratio, wherein η and η0 are the viscosities of CT DNA in the presence of BCESA and CT DNA alone, respectively.
CD spectrum and the interaction of BCESA towards CT DNA
To visualize the intercalation of BCESA towards CT DNA, the circular dichroism (CD) assay was performed. In this assay, the solution systems consisting of CT DNA in PBS (pH 7.4, 100 µM) and BCESA in PBS (pH 7.4, 50 μM, 100 μM, 150 μM and 200 μM) were incubated at 37ºC for 3 hrs to measure their CD spectra by following a standard procedure.
Results
BCESA was satisfactorily synthesized
To satisfactorily synthesize BCESA, a route of Scheme 1 was used. First L-Trp-OBzl and 1,1,3,3-tetramethoxypropane received Pictet–Spengler condensation to give benzyl 1-(2,2-dimethoxyethyl)-β-tetrahydro-carboline-3-carboxylate (1, 60% yield). Secondary the aromatization of 1 gave benzyl 1-(2,2-dimethoxyethyl)-β-carboline-3-carboxylate (2, 68% yield). Thirdly the hydrolysis of 2 gave benzyl 1-carbonylmethyl-β-carboline-3-carboxylate (3, 67% yield). Besides, the solution-phase method provided Ser-Ala-OBzl in 52% yield. Finally, the coupling of 3 and Ser-Ala-OBzl generated BCESA in 48% yield.
Self-assembly and the tetramer of BCESA
The self-assembly of small molecule has been well documented.29,37 Here the self-assembly of BCESA in ultrapure water (1 nM) was explored with its FT-MS spectrum. Figure 2A shows an ion peak at 2378.00696, the mass of a tetramer plus H. Besides, an ion peak at 1211.50060, the mass of a dimer plus Na and an ion peak at 617.24120, the mass of a monomer plus Na, are also indicated. To clarify the relationship between the tetramer, the dimer and the monomer, a qCID spectrum was recorded. Figure 2B indicates that in FT-MS condition the tetramer can be split to the dimer and the monomer.
 | Figure 2 FT-MS and NOESY 2D1H NMR spectra of BCESA. (A) FT-MS spectrum of BCESA in ultrapure water (1 nM), the spectrum of the tetramer (2378.00696) is locally amplified and inserted; (B) qCID spectrum of BCESA in ultrapure water (1 nM); (C) NOESY 2D1HNMR spectrum of BCESA, in which an interesting cross-peak is marked with red ring; (D) Energy-minimized conformations of the monomer and the tetramer, in the impeller-like conformation of the tetramer the distance is locally amplified and inserted.Abbreviation: BCESA, N-[(3-Benzyloxycarbonyl-β-carboline-1-yl)ethyl]-Ser-Ala-OBzl. |
To know BCESA how to form the tetramer its NOESY 2D1HNMR spectrum was recorded. Figure 2C shows an interesting cross-peak labeled red circle. According to NOESY, this cross-peak is resulted from the interaction of the pyrrole H in the carboline moiety of one molecule and the hydroxyl H of Ser residue in another molecule. This means that the distance between these H is less than 4Ǻ. Based on this, the monomer of BCESA was first energy minimized, and then the assembly of four energy minimized monomers was manually accessed to form a tetramer. This operation leads the tetramer of BCESA to an impeller-like conformation (Figure 2D).
Nano-property of aqueous BCESA
To show the nano-property of aqueous BCESA the Faraday-Tyndall effect was tested. Figure 3A indicates that the radiation of 650 nm laser does not induce the ultrapure water occurring Faraday-Tyndall effect. Figure 3B-3E, Figure 3F-3I and Figure 3J-3M indicate that 650 nm laser-radiation induces the solution of BCESA in pH6.8 ultrapure water, in pH7.4 PBS and in pH 1.2 ultrapure water (10, 1, 0.1 and 0.01 μM) consistently occurring Faraday-Tyndall effect. This suggests that in tissue juice, in biological phase and in gastric juice BCESA has nano-property.
The nano-property of the solution of BCESA in ultrapure water and PBS was also examined on nano-laser particle size analyzer. Figure 3N indicates that during 7 days the sizes of BCESA in ultrapure water (0.01, 0.1 and 1 μM, pH 6.8 and 1.2) and PBS (0.01, 0.1 and 1 μM, pH 7.4) fall in the range of 150 nm to 275 nm, mirroring a typical nano-property.
The nano-property of the solutions of BCESA in ultrapure water and PBS was further examined on a ZetaPlus Potential Analyzer. Figure 3O-3Q indicates that the zeta potentials of the solutions of BCESA in ultrapure water of pH 6.8 (0.01 μM), in PBS of pH 7.4 (0.01 μM) and in ultrapure water of pH 1.2 (0.01 μM) are −32.1 mV, −22.4 mV and −27.5 mV, respectively, again mirroring a typical nano-property.
Nano-feature of BCESA
To visualize the nano-species of BCESA in water and in solids, the TEM and SEM features were imaged. Figure 4A and B indicates that in water (0.01 μM) BCESA forms the particles of 20–120 nm in diameter, and most diameters of the particles are less than 120 nm. Figure 4C and D indicates that the powders lyophilized from aqueous BCESA (0.01 μM) are the particles of 20–63 nm in diameter, and most diameters of the particles are less than 70 nm. Therefore, in solution and solid states, BCESA consistently exists as nanoparticles having similar size.
To visualize the nano-species of BCESA in ultrapure water of pH 6.8 and in mouse plasma, the AFM features were imaged. Figure 4E indicates that in ultrapure water of pH 6.8 (0.01 μM), BCESA forms nanoparticles of 17.06 nm in height. Figure 4F indicates that in mouse plasma (0.01 μM), BCESA forms nanoparticles of 53.58 nm in height. Figure 4G indicates that mouse plasma alone gives no any comparable nanoparticle.
Mesoscale simulation for nanoparticle of BCESA
To simulate the formation and predict the size of the nanoparticle of BCESA a calculation was performed by using mesoscale simulation software. Figure 5 shows that 324 tetramers, ie, 1296 molecules of BCESA can form a nanoparticle of 61.59 nm in diameter. This simulation may help us to estimate how much molecules of BCESA are involved in a nanoparticle of exact size.
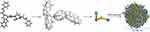 | Figure 5 The course of mesoscale simulation to predict the formation and the size of a nanoparticle of BCESA.Abbreviation: BCESA, N-[(3-Benzyloxycarbonyl-β-carboline-1-yl)ethyl]-Ser-Ala-OBzl. |
Intercalation of BCESA towards CT DNA
To examine the intercalation of BCESA towards CT DNA the UV, fluorescence, CD, viscosity and melting temperature experiments were performed.
The UV spectra of CT DNA in PBS of pH 7.4 (10 μM) and CT DNA plus BCESA in PBS of pH 7.4 (final concentration: 0, 5, 10, 15, 20, 25, 30, 35 and 40 µM) were recorded in the region of 200–320 nm. Figure 6A shows that BCESA induces the bands at 218 nm and 258 nm of CT DNA to have hypochromic effect. The two bands also have small hypsochromic shift. The changes of CT DNA mean that BCESA intercalates the base pairs of CT DNA.
The fluorescence spectra of BCESA in PBS of pH 7.4 (10 μM) and BCESA plus CT DNA in PBS of pH 7.4 (final concentration: 0, 9, 18, 27, 36, 45, 54, 63 and 72 µM) were recorded. Figure 6B shows that with the concentration of CT DNA been increased from 0 µM to 72 μM the fluorescence intensity of BCESA is lowered from 360 to 170 (intensity is decreased by 52%), giving a sharp quenching phenomenon and suggesting the intercalation of BCESA towards the base pairs of CT DNA.
The CD spectra of CT DNA in PBS of pH 7.4 (200 μM) and CT DNA plus BCESA in PBS of pH 7.4 (final concentration: 50, 100, 150 and 200 μM) were recorded. Figure 6C shows that around 245 nm and 276 nm, the CD spectrum of CT DNA alone gives a negative peak and a positive peak, respectively. In the presence of 50, 100, 150 and 200 μM of BCESA, the ellipticities of the positive and negative bands of CT DNA been decreased by 46.5% and 60.6%, respectively. These mean that BCESA intercalates the base pairs of CT DNA.
The relative viscosities of [BCESA]/[CT DNA] in the ratios of 0.13–0.82 were recorded. Figure 6D indicates that with the increase of the concentration of BCESA the relative viscosity of CT DNA is increased steadily. These mean that BCESA intercalates the base pairs of CT DNA.
In thermal denaturation experiments, the curves of CT DNA alone (100 µM) and CT DNA (100 µM) plus BCESA (100 µM) were drawn to show the change of the Tm, and consequently to give the change of melting temperature (ΔTm) of 2.5ºC (Figure 6E).
BCESA inhibiting the proliferation of cancer cells, but not non-carcinoma cells
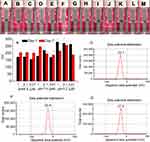
The in vitro anti-proliferation activity of BCESA was evaluated on HCT-8, S180, SH-sy5y, HL-60, A549, MCF-7 and HaCaT by using MTT assay, and Dox was the positive control. Figure 7A shows that the IC50 of BECSA against HCT-8, S180, SH-sy5y, HL60, A549, and HaCaT cells are 7.2 μM, 2.7 μM, 27.7 μM, 5.9 μM, 10.0 μM, 4.9 μM and >100 μM, respectively.
BCESA dose-dependently inhibits tumor growth
The dose-dependent assay of BCESA was performed on S180 mouse model and three doses (1.0 μmol/kg/day, 0.1 μmol/kg/day and 0.01 μmol/kg/day) were used, in which Dox was the positive control. Figure 7B indicates that when the dose of BCESA is from 0.01 μmol/kg/day via 0.1 μmol/kg/day increased to 1.0 μmol/kg/day, the tumor weight is gradually decreased, suggesting a dose-dependent inhibition.
BCESA has no systematic toxicity to S180 mice
The systematically chemotherapeutic toxicity consists of organ and body atrophy. To explain this toxicity the weights of the body, heart, liver, spleen, kidney and brain of the S180 mice treated with BCESA are shown in Figure 8A and B. As seen, the weights of the body, the liver and the spleen of S180 mice treated with 2.0 μmol/kg/day Dox, but not 1.0 μmol/kg/day BCESA, are significantly lower than those of S180 mice treated with CMCNa, suggesting Dox, but not BCESA, may induce body, liver and spleen atrophy. This comparison demonstrates that BCESA has no Dox-like side effects.
BCESA does not change the level of serum ALT, AST and Cr of S180 mice
To ensure the safety of BCESA therapy, the blood of S180 mice treated by CMCNa and BCESA (0.01 μmol/kg/day, 0.1 μmol/kg/day and 1.0 μmol/kg/day) was collected to prepare serum and to measure alanine transaminase (ALT), aspartate transaminase (AST) and creatinine (Cr). In the measurements, the guidance of AST/GOT, ALT/GPT and Cr testing kits was followed. Figure 8C, 8D and 8E indicates that the levels of ALT, AST and Cr in the serum of S180 mice treated with BCESA are equal to those of S180 mice treated with CMCNa.
BCESA targeting tumor tissue
By analyzing the FT-MS spectra of the extracts of the homogenates prepared with the tumor tissue, the brain, the heart, the lung, the liver, the spleen, the kidney and the blood of the treated S180 mice, a tumor-targeting action of BCESA was identified. Figure 9A is the FT(+)-MS spectrum of the extract of blood homogenate and only an ion peak of BCESA plus H occurs at 595.25368 (see the local amplified inset). This means that in blood circulation BCESA is delivered without degradation. Figure 9B is the FT(+)-MS spectrum of the extract of tumor tissue homogenate. The local amplified insets show that in the tumor tissue there are of BCESA and its metabolites, including β-carboline-3-carboxylic acid, 1-methyl-β-carboline-3-carboxylic acid, 1-carbonyl- methyl-β-carboline-3-carboxylic acid, 1-methyl-β-carboline-3-carboxylic benzyl ester, 1-carbonylmethyl-β-carboline-3-carboxylic benzyl ester, N-[(3-carboxyl- β-carboline-1-yl)ethyl]-Ser-Ala, N-[2-hydroxyl-(3-benzyloxycarbonyl-β-carboline-1-yl)ethyl]-Ser-Ala-OBzl, N-[(3-benzyloxycarbonyl-β-carboline-1-yl)ethenyl]-Ser-Ala- OBzl and Ser-Ala. Figure 9C-9H is the FT-MS(+)-MS spectra of the extracts of the homogenates prepared from the heart, the liver, the spleen, the lung, the kidney and brain. The local amplified insets show that these extracts give no any ion peak related to BCESA.
Discussion
The synthetic route could satisfactorily provide BCESA with desired yield and good purity. The experiments of docking, UV, fluorescence, CD, viscosity and melting temperature ensure that BCESA can intercalate the base pairs of CT DNA.
FT-MS spectrum of BCESA reveals that in water BCESA carries out self-assembly, thereby forms the tetramer as the sole and final form. NOESY 2D 1H NMR spectra show that the self-assembly processes by the pyrrole H of the carboline moiety in one molecule to approach the hydroxyl H of Ser-residue in another molecule. Energy minimization gives the tetramer an impeller-like conformation. Mesoscale simulation software-assisted calculation predicts that 324 tetramers (1296 molecules) of BCESA can form a nanoparticle of 61.59 nm in diameter.
Faraday-Tyndall effect, nano-size analysis, SEM image, TEM image and AFM image show that in solution, solids and gastric juice BCESA consistently exists as similar nanoparticles. Besides, in blood circulation BCESA exists as the nanoparticles of small size, so that can effectively avoid macrophage phagocytosis and could be safely delivered.38 This kind of nano-particles should benefit BCESA to exhibit a targeting action.
In vitro MTT assay reveals that the IC50 values of BCESA against the proliferation of HCT-8, S180, SH-sy5y, HL60 and A549 cells range from 2.7 μM to 27.7 μM. Besides, the IC50 value of BCESA against non-carcinoma cell, HaCaT, is more than 100 μM. This means that BCESA inhibits the proliferation of the cancer cells, but not the non-carcinoma cells. Furthermore, in this assay, S180 is the most sensitive cell to BCESA. This specific behavior led to the selection of S180 mouse model for the in vivo assay.
On S180 mouse model, the tumor weights of the mice treated with BCESA (0.01, 0.1 and 1.0 μmol/kg/day) for 9 consecutive days were lower than that of the mice treated with CMCNa, suggesting BCESA was an active antitumor agent and slowed the growth of the tumor in a dose-dependent manner. The activity of 1.0 μmol/kg/day of BCESA was equal to that of 2.0 μmol/kg/day of Dox, suggesting its activity was 2 folds of that of Dox.
The serum levels of ALT, AST and Cr of S180 mice treated with 1.0 μmol/kg/day of BCESA were equal to those of S180 mice treated with CMCNa. Therefore, at the dose of 1.0 μmol/kg/day, BCESA did not injure the liver and kidney of the mice.
FT-MS spectra of the extracts of the homogenates of the organs, the blood and the tumor tissue of the treated S180 mice showed that from the blood circulation BCESA entered the tumor tissue, but not the organs, and released the anti-tumoral β-carbolines wherein.
As a tumor target intercalator, the release profile of BCESA in the tumor tissue was addressed based on the FT-MS peaks of the extract of the homogenate of tumor tissue, and two release approaches were proposed (Figure 10).
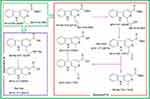 | Figure 10 FT-MS(+)-MS spectra based degradation course of BCESA in tumor tissue.Abbreviation: BCESA, N-[(3-Benzyloxycarbonyl-β-carboline-1-yl)ethyl]-Ser-Ala-OBzl. |
In approach A, BCESA was first converted to N-[(3-benzyloxycarbonyl-β-carboline-1-yl)ethenyl]-Ser-Ala-OBzl and N-[2-hydroxyl-(3-benzyloxycarbonyl-β-carboline-1-yl)ethyl]-Ser-Ala-OBzl that was converted to Ser-Ala and 1-carbonyl-methyl-β-carboline-3-carboxylic benzyl ester. Next, 1-carbonylmethyl-β-carboline-3-carboxylic benzyl ester was converted to 1-methyl-β-carboline-3-carboxylic benzyl ester and 1-carbonylmethyl-β-carboline-3-carboxylic acid. Finally, 1-carbonylmethyl-β-carboline-3-carboxylic acid was converted to 1-methyl-β-carboline-3-carboxylic acid. In approach B, BCESA was successively converted to N-(3-carboxyl-β-carboline-1-yl)-ethyl-Ser-Ala, β-carboline-3-carboxylic acid and Ser-Ala. According to the release profile of BCESA, Ser-Ala-OBzl should be the carrier of 1-methyl-β-carboline-3-carboxylic acid and β-carboline-3-carboxylic acid. In both approaches, Ser-Ala was from Ser-Ala-OBzl, while 1-methyl-β-carboline-3-carboxylic acid and β-carboline-3-carboxylic acid were known as the anti-tumoral agents.39,40
Conclusion
Anti-tumoral pharmacophore analysis and CDOCKER energy comparison resulted in the rational design of BCESA as an intercalator, which had no Dox-like toxicity. BCESA was a nano-scaled small molecule capable of targeting tumor tissue, releasing anti-tumoral 1-methyl-β-carboline-3-carboxylic acid and β-carboline-3-carboxylic acid wherein. In our opinion, Ser-Ala-OBzl was a simple and desirable carrier of β-carbolines.
Acknowledgments
The authors thank the Special Project of China (2018ZX097201003), NSFC (81703332, 81673303), BNSF (7172028), KZ201610025029, KM201810025010, KM201810025011 for financial support.
Disclosure
The authors report no conflicts of interest in this work
References
1. Shankaraiah N, Jadala C, Nekkanti S, et al. Design and synthesis of C3-tethered 1,2,3-triazolo-beta-carboline derivatives: anticancer activity, DNA-binding ability, viscosity and molecular modeling studies. Bioorg Chem. 2016;64:42–50. doi:10.1016/j.bioorg.2015.11.005
2. Ling Y, Guo J, Yang Q, et al. Development of novel beta-carboline-based hydroxamate derivatives as HDAC inhibitors with antiproliferative and antimetastatic activities in human cancer cells. Eur J Med Chem. 2018;144:398–409. doi:10.1016/j.ejmech.2017.12.061
3. Fiore M, Forli S, Manetti F. Targeting Mitogen-Activated Protein Kinase-Activated Protein Kinase 2 (MAPKAPK2, MK2): medicinal chemistry efforts to lead small molecule inhibitors to clinical trials. J Med Chem. 2016;59(8):3609–3634. doi:10.1021/acs.jmedchem.5b01457
4. Suh YG, Lim C, Sim J, Lee JK, Surh YJ, Paek SM. Construction of the azacyclic core of tabernaemontanine-related alkaloids via tandem Reformatsky-Aza-Claisen rearrangement. J Org Chem. 2017;82(3):1464–1470. doi:10.1021/acs.joc.6b02648
5. Kovvuri J, Nagaraju B, Nayak VL, et al. Design, synthesis and biological evaluation of new beta-carboline-bisindole compounds as DNA binding, photocleavage agents and topoisomerase I inhibitors. Eur J Med Chem. 2018;143:1563–1577. doi:10.1016/j.ejmech.2017.10.054
6. Zheng C, Fang Y, Tong W, et al. Synthesis and biological evaluation of novel tetrahydro-beta-carboline derivatives as antitumor growth and metastasis agents through inhibiting the transforming growth factor-beta signaling pathway. J Med Chem. 2014;57(3):600–612. doi:10.1021/jm401117t
7. Shankaraiah N, Nekkanti S, Chudasama KJ, et al. Design, synthesis and anticancer evaluation of tetrahydro-beta-carboline-hydantoin hybrids. Bioorg Med Chem Lett. 2014;24(23):5413–5417. doi:10.1016/j.bmcl.2014.10.038
8. Ling Y, Xu C, Luo L, et al. Novel beta-carboline/hydroxamic acid hybrids targeting both histone deacetylase and DNA display high anticancer activity via regulation of the p53 signaling pathway. J Med Chem. 2015;58(23):9214–9227. doi:10.1021/acs.jmedchem.5b01052
9. Dighe SU, Khan S, Soni I, et al. Synthesis of beta-carboline-based N-heterocyclic carbenes and their antiproliferative and antimetastatic activities against human breast cancer cells. J Med Chem. 2015;58(8):3485–3499. doi:10.1021/acs.jmedchem.5b00016
10. Frederick R, Bruyere C, Vancraeynest C, et al. Novel trisubstituted harmine derivatives with original in vitro anticancer activity. J Med Chem. 2012;55(14):6489–6501. doi:10.1021/jm300542e
11. Dai J, Dan W, Schneider U, Wang J. β-Carboline alkaloid monomers and dimers: occurrence, structural diversity, and biological activities. Eur J Med Chem. 2018;157:622–656. doi:10.1016/j.ejmech.2018.08.027
12. Yang F, Zhang T, Wu H, et al. Design and optimization of novel hydroxamate-based histone deacetylase inhibitors of Bis-substituted aromatic amides bearing potent activities against tumor growth and metastasis. J Med Chem. 2014;57(22):9357–9369. doi:10.1021/jm5012148
13. Behforouz M, Cai W, Stocksdale MG, et al. Novel lavendamycin analogues as potent HIV-reverse transcriptase inhibitors: synthesis and evaluation of anti-reverse transcriptase activity of amide and ester analogues of lavendamycin. J Med Chem. 2003;46(26):5773–5780. doi:10.1021/jm0304414
14. Sathish M, Chetan Dushantrao S, Nekkanti S, et al. Synthesis of DNA interactive C3-trans-cinnamide linked beta-carboline conjugates as potential cytotoxic and DNA topoisomerase I inhibitors. Bioorg Med Chem. 2018;26(17):4916–4929. doi:10.1016/j.bmc.2018.08.031
15. Chen J, Peng F, Zhang Y, et al. Synthesis, characterization, cellular uptake and apoptosis-inducing properties of two highly cytotoxic cyclometalated ruthenium(II) β-carboline complexes. Eur J Med Chem. 2017;140:104–117. doi:10.1016/j.ejmech.2017.09.007
16. Carvalho A, Chu J, Meinguet C, et al. A harmine-derived beta-carboline displays anti-cancer effects in vitro by targeting protein synthesis. Eur J Pharmacol. 2017;805:25–35. doi:10.1016/j.ejphar.2017.03.034
17. Atteya R, Ashour ME, Ibrahim EE, Farag MA, El-Khamisy SF. Chemical screening identifies the beta-Carboline alkaloid harmine to be synergistically lethal with doxorubicin. Mech Ageing Dev. 2017;161(Pt A):141–148. doi:10.1016/j.mad.2016.04.012
18. Samundeeswari S, Chougala B, Holiyachi M, et al. Design and synthesis of novel phenyl −1, 4-beta-carboline-hybrid molecules as potential anticancer agents. Eur J Med Chem. 2017;128:123–139. doi:10.1016/j.ejmech.2017.01.014
19. Bai B, Li XY, Liu L, Li Y, Zhu HJ. Syntheses of novel beta-carboline derivatives and the activities against five tumor-cell lines. Bioorg Med Chem Lett. 2014;24(1):96–98. doi:10.1016/j.bmcl.2013.11.076
20. Liew LPP, Fleming JM, Longeon A, et al. Synthesis of 1-indolyl substituted β-carboline natural products and discovery of antimalarial and cytotoxic activities. Tetrahedron. 2014;70(33):4910–4920. doi:10.1016/j.tet.2014.05.068
21. Wu JH, Li CY, Zhao M, Wang WJ, Wang YJ, Peng SQ. A class of novel carboline intercalators: their synthesis, in vitro anti-proliferation, in vivo anti-tumor action, and 3D QSAR analysis. Bioorg Med Chem. 2010;18(17):6220–6229. doi:10.1016/j.bmc.2010.07.043
22. Wu JH, Zhao M, Qian KD, Lee KH, Morris-Natschke S, Peng SQ. Novel N-(3-carboxyl-9-benzyl-beta-carboline-1-yl)ethylamino acids: synthesis, anti-tumor evaluation, intercalating determination, 3D QSAR analysis and docking investigation. Eur J Med Chem. 2009;44(10):4153–4161. doi:10.1016/j.ejmech.2009.05.006
23. Wu JH, Wei L, Zhao M, Wang YJ, Kang GF, Peng SQ. N-[2(3-Carboxyl-9-benzyl-carboline-1-yl)ethyl-1-yl]-amino acids: correlation of spectral property with in vivo anti-tumor activity. Med Chem Res. 2012;21(1):116–123. doi:10.1007/s00044-010-9504-1
24. Serfilippi LM, Pallman DR, Russell B. Serum clinical chemistry and hematology reference values in outbred stocks of albino mice from three commonly used vendors and two inbred strains of albino mice. Contemp Top Lab Anim Sci. 2003;42(3):46–52.
25. Zhang XY, Yang YF, Zhao M, et al. A class of Trp-Trp-AA-OBzl: synthesis, in vitro anti-proliferation/in vivo anti-tumor evaluation, intercalation-mechanism investigation and 3D QSAR analysis. Eur J Med Chem. 2011;46(8):3410–3419. doi:10.1016/j.ejmech.2011.05.004
26. Feng Q, Zhao M, Gan T, et al. DHDMIQK(KAP): a novel nano-delivery system of dihydroxyl-tetrahydro-isoquinoline-3-carboxylic acid and KPAK towards the thrombus. J Mater Chem B. 2016;4(36):5991–6003. doi:10.1039/C6TB00874G
27. Wu J, Zhu H, Zhao M, et al. IQCA-TASS: a nano-scaled P-selectin inhibitor capable of targeting thrombus and releasing IQCA/TARGD(S)S in vivo. J Mater Chem B. 2017;5(5):877–1120.
28. Wu J, Zhao M, Wang Y, et al. N-(3-hydroxymethyl-β-carboline-1-yl-ethyl-2-yl)-L-Phe: development toward a nanoscaled antitumor drug capable of treating complicated thrombosis and inflammation. Drug Des Devel Ther. 2017;11:225–239. doi:10.2147/DDDT.S123919
29. Zhu H, Song Y, Wang Y, et al. Design, synthesis and evaluation of a novel π–π stacking nano-intercalator as an anti-tumor agent. Med Chem Commun. 2016;7(2):247–257. doi:10.1039/C5MD00507H
30. Wu J, Zhu H, Yang G, et al. Design and synthesis of nanoscaled IQCA-TAVV as a delivery system capable of antiplatelet activation, targeting arterial thrombus and releasing IQCA. Int J Nanomedicine. 2018;13:1139–1158. doi:10.2147/IJN.S150205
31. Xu X, Wang Y, Wu J, et al. ATIQCTPC: a nanomedicine capable of targeting tumor and blocking thrombosis in vivo. Int J Nanomedicine. 2017;12:4415–4431. doi:10.2147/IJN.S129989
32. Jin SM, Wang YN, Zhu HM, et al. Nanosized aspirin-Arg-Gly-Asp-Val: delivery of aspirin to thrombus by the target carrier Arg-Gly-Asp-Val tetrapeptide. ACS Nano. 2013;7(9):7664–7673. doi:10.1021/nn402171v
33. Hu X, Zhao M, Wang Y, et al. Tetrahydro-β-carboline-3-carboxyl-thymopentin: a nano-conjugate for releasing pharmacophores to treat tumor and complications. J Mater Chem B. 2016;4:1384–1397. doi:10.1039/C5TB01930C
34. Xu W, Zhao M, Wang Y, et al. Design, synthesis, and in vivo evaluations of benzyl Nω-nitro-Nα-(9H-pyrido[3,4-b]indole-3-carbonyl)-l-argininate as an apoptosis inducer capable of decreasing the serum concentration of P-selectin. Med Chem Commun. 2016;7(9):1730–1737. doi:10.1039/C6MD00215C
35. Ma H, Zhao M, Wang Y, et al. Cholyl-l-lysine-carboxylbutyryl adriamycin prodrugs targeting chemically induced liver injury. J Mater Chem B. 2017;5(3):470–478. doi:10.1039/C6TB02205G
36. Jiang X, Zhao M, Wang Y, et al. RGD(F/S/V)-Dex: towards the development of novel, effective, and safe glucocorticoids. Drug Des Devel Ther. 2016;10:1059–1076. doi:10.2147/DDDT.S99568
37. Wu JH, Wang YJ, Wang YN, et al. Cu2+-RGDFRGDS: exploring the mechanism and high efficacy of the nanoparticle in antithrombotic therapy. Int J Nanomedicine. 2015;10:2925–2938. doi:10.2147/IJN.S76691
38. Fujita Y, Mie M, Kobatake E. Construction of nanoscale protein particle using temperature-sensitive elastin-like peptide and polyaspartic acid chain. Biomaterials. 2009;30(20):3450–3457. doi:10.1016/j.biomaterials.2009.03.012
39. Chen YF, Lin YC, Chen JP, et al. Synthesis and biological evaluation of novel 3,9-substituted beta-carboline derivatives as anticancer agents. Bioorg Med Chem Lett. 2015;25(18):3873–3877. doi:10.1016/j.bmcl.2015.07.058
40. Zhao M, Bi L, Wang W, et al. Synthesis and cytotoxic activities of beta-carboline amino acid ester conjugates. Bioorg Med Chem. 2006;14(20):6998–7010. doi:10.1016/j.bmc.2006.06.021
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.