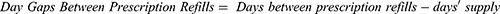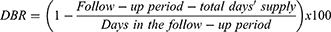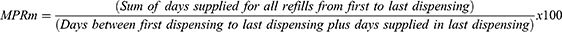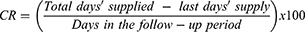Back to Journals » Patient Preference and Adherence » Volume 16
Baseline Characteristics and Secondary Medication Adherence Patterns Among Patients Receiving Tafamidis Prescriptions: A Retrospective Analysis Using a National Specialty Pharmacy Dispensing Database
Authors Roy A, Peterson A, Marchant N, Alvir J, Bhambri R, Lynn J, Benjumea D, Prasad S, O'Brien A, Chen Y, Kemner J, Parasuraman B
Received 15 December 2021
Accepted for publication 14 April 2022
Published 29 April 2022 Volume 2022:16 Pages 1115—1129
DOI https://doi.org/10.2147/PPA.S352332
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Johnny Chen
Anuja Roy,1 Andrew Peterson,2 Nick Marchant,1 Jose Alvir,3 Rahul Bhambri,4 Jason Lynn,4 Darrin Benjumea,5 Sapna Prasad,6 Alex O’Brien,6 Yong Chen,7 Jason Kemner,8 Bhash Parasuraman8
1Global HEOR, Patient & Health Impact, Rare Diseases BU, Pfizer Inc, New York, NY, USA; 2Department of Pharmacy Practice/Pharmacy Administration, University of the Sciences, Philadelphia, PA, USA; 3Statistical Research and Data Science Center Global Product Development, Pfizer Inc, New York, NY, USA; 4Medical Affairs, Pfizer Inc, New York, NY, USA; 5Evidence Strategy, Genesis Research, Hoboken, NJ, USA; 6Clarify Insights Services, Clarify Health Solutions, New York, NY, USA; 7Rare Disease, Pfizer Inc, Collegeville, PA, USA; 8Patient & Health Impact, Pfizer Inc, Collegeville, PA, USA
Correspondence: Anuja Roy, Global HEOR, Patient & Health Impact, Rare Diseases BU, Pfizer Inc, New York, NY, USA, Email [email protected]
Introduction: Transthyretin amyloid cardiomyopathy (ATTR-CM) is a serious, underrecognized condition, which leads to heart failure and early mortality if left untreated. Until recently, heart transplantation was the only treatment for ATTR-CM. Regulatory approval of tafamidis transformed treatment for patients. In the phase 3 Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT), which established the safety and efficacy of tafamidis, medication adherence was high with 97.2% of patients taking ≥ 80% of scheduled doses. Evidence of real-world adherence to cardiology drugs demonstrates low adherence and suboptimal outcomes; however, real-world adherence to tafamidis has not been investigated. The main objective of this study was to describe adherence patterns of patients filling tafamidis in the Symphony Health database.
Methods: This retrospective analysis of the Symphony Health Solutions claims database used secondary adherence measures, including modified medication possession ratio (MPRm), days between fills adherence rate, and compliance rate, to assess adherence patterns of 2020 patients filling tafamidis free acid 61-mg capsules or tafamidis meglumine 4x20-mg capsules from June 1, 2019 to August 31, 2020.
Results: Patients receiving a tafamidis formulation had characteristics consistent with the expected patient population; 71.6% were aged 75– 84 years, 83.2% were male, and the highest proportion resided in the Northeast region (30.5%) of the United States. Adherence for tafamidis was high, as 75% to 100% of the patients across subgroups met or exceeded the commonly defined adherence threshold of 80%. Median number of refills ordered and received was six refills per patient. Most patients received refills with no gap (n=1633) or a gap < 30 days (n=1267/1317 patients). Adherence was high across follow-up time, sex, and age subgroups. Adherence varied by geographic region, with the Northeast being significantly higher than the Midwest (mean MPRm 94.41% vs 88.21%, p=0.0007).
Conclusion: These results provide evidence that real-world adherence to tafamidis in patients with ATTR-CM is high.
Keywords: claims analysis, amyloidosis, cardiomyopathy, transthyretin amyloid, adherence
Introduction
Transthyretin amyloid cardiomyopathy (ATTR-CM) is a life-threatening, yet manageable cause of heart failure whose prevalence is difficult to estimate as it is underdiagnosed and undertreated.1–3 The underlying etiology of ATTR-CM is the deposition of misfolded aggregates of transthyretin in tissues including the myocardial interstitial space.4 Formation of transthyretin amyloid fibrils may occur as a result of a destabilizing gene mutation (hereditary) or spontaneous age-linked process (wild type).5 Left untreated, patients progress to severe disease and experience rapid reductions in quality of life and increased healthcare resource utilization, including hospitalization.4 Ultimately, the disease proves fatal (median survival 2 to 6 years).6 Prior to 2019, no proven pharmacotherapies were available for patients with ATTR-CM, and the only treatment was heart transplant.4,7–10 The discovery and subsequent regulatory approval of tafamidis was transformative to the treatment paradigm.5,11
Vyndaqel® (tafamidis meglumine 4x20-mg capsules once daily) and Vyndamax™ (tafamidis free acid 61-mg capsule once daily), which are collectively referred to as tafamidis from here on in, are first-in-class therapies approved in the United States (US) by the Food and Drug Administration (FDA) as treatment for wild-type or hereditary ATTR-CM in adults.12 The mechanism of action for tafamidis is to prevent the amyloidogenic cascade by stabilizing transthyretin at the thyroxine binding sites.13,14 Its safety and efficacy were established in the pivotal phase 3 Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT), in which tafamidis treatment was associated with significantly reduced mortality and cardiovascular-related healthcare utilization, as well as reductions in functional decline and better maintained quality of life versus placebo.15
The World Health Organization (WHO) has reported that for long-term treatment of chronic illnesses, average adherence rates in developed countries are approximately 50%.16 Across chronic diseases, and particularly in cardiovascular conditions, medication adherence is essential to successful treatment and are associated with better disease and economic outcomes.17–19 Nonadherence to medications used for cardiac conditions increases cardiac event risk, healthcare resource utilization, and mortality.20 In ATTR-ACT, adherence was high with 97.2% of patients taking at least 80% of their scheduled doses.15,21 Studies evaluating real-world data often utilize secondary adherence, which measures whether patients receive refills as prescribed during a defined observation period.19 To ensure consistency throughout, the term adherence was used in place of secondary adherence from here on in. Evidence suggests that real-world adherence rates are generally lower than in clinical trials, and this discrepancy may lead to disparities between clinical efficacy and effectiveness.19 Thus, investigation into the adherence of tafamidis is needed to account for the real-world behaviors of patients and provide a comparative perspective to the findings of ATTR-ACT.
Given the relative recency of the FDA approval for tafamidis, to our knowledge there is no published real-world evidence describing both treatment patterns and adherence rates in the US. Moreover, the patient population receiving tafamidis has yet to be characterized.
Study Objectives
The primary objective of the study was to describe adherence patterns of patients filling tafamidis in the Symphony Health dataset population. The secondary objective was to evaluate the use of concomitant medications in the same patient population.
Materials and Methods
Overview
This non-interventional, observational, retrospective cohort study of patients who have been prescribed tafamidis in the US utilized de-identified individual patient data (point-of-sale prescription data, non-retail invoice data, and demographic data) from Symphony Health’s specialty pharmacy dispensing database. The Symphony database includes commercial as well as Managed Medicaid and Medicare Advantage pharmacy claims data of over 280 million patients across the US and approximately 65% of the US specialty market.22–24
Study Population
Adult patients (≥18 years) with at least one prescription claim and a days’ supply greater than zero across all claims for tafamidis in the Symphony Health Solutions (SHS) administrative claims database between June 1, 2019 and August 31, 2020 were included. The earliest accepted tafamidis prescription date was used as the index date and patients were subsequently followed until their last (most recent) tafamidis prescription. These data were used to inform the patient demographic and clinical characteristics of patients with evidence of tafamidis prescription fills.
Further inclusion and exclusion criteria were used to determine the patient cohort for the tafamidis adherence analysis. For inclusion in the tafamidis adherence analysis, patients must have had at least two fully adjudicated prescription claims for tafamidis and a 3-month minimum follow-up period; thus, the last month of treatment initiation/enrollment was May 2020 (with 3-month follow-up going through August 2020) (Figure 1). Patients who had a single claim, claims on a single day, <3 months follow-up, and whose first and last claims were the same were excluded.
The study was considered to be exempt from the requirements for “human subjects research” in the US as it only used commercially available de-identified secondary data sources, under DHHS regulations 45 CFR 46.104. Thus, review and informed consent from the institutional review board/independent ethics committee was not necessary. Analyses performed for this study were not required to undergo an approval process by Pfizer board members. The study was conducted in accordance with legal and regulatory requirements and followed the generally accepted research practices described in Good Practices for Outcomes Research issued by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).25–27
Measures of Medication Day Gaps and Adherence
Follow-up intervals were calculated based on the first prescription claim of tafamidis treatment until August 2020 (end of data availability) or once prescription activity was no longer seen within the study period. Follow-up was defined as the time (in months) between the first accepted claim and the last claim of any status (accepted, reversed, or rejected). Patient follow-up periods differed based on their individual prescription fill dates. Uptake over time was defined as the number of first-time fills in each month of the study period. Additionally, days’ supply was provided by the Symphony database across both accepted and reversed claims. Prescribed daily doses were not provided by the Symphony database.
Refill gaps were measured using the following metrics: Day Gaps Between Prescription Refills and Days Between Fills Adherence Rate (DBR).28 Day Gaps Between Prescription Refills were calculated as the days between prescription refills minus the days’ supply of the prescription. When the time between fills exceeded the days’ supply of the previous fill, there was considered to be a gap in fills. Gaps were reported as specific categories (0 days, 1 to 30 days, 31 to 60 days, 61 to 90 days, and >90 days).
DBR was calculated as the days in the follow-up period minus total days’ supply divided by the days in the follow-up period and was reported as a percentage. There is a potential for adherence values to exceed 100%, as the calculation does not take into account the possibility of patients picking up their medication refill before the previous supply is exhausted, creating a total days’ supply that is greater than the follow-up period.
As this was an exploratory study, multiple measures including modified Medication Possession Ratio (MPRm) and Compliance Rate (CR) were used to evaluate adherence to tafamidis. MPRm was calculated as the days’ supply of tafamidis dispensed throughout the observation period from first to last dispensing, divided by the number of days between first and last dispensing plus the days’ supply of the last dispensing, multiplied by 100 and reported as a percentage.29 MPRm was chosen as the MPR variation used in this study because it included the days’ supply dispensed with last dispensing in the denominator.29 CR was utilized as it provides an adherence rate that includes all days’ supplied with treatment up until the last day of refill.29 CR was calculated as total days’ supply minus last accepted days’ supply for each patient, divided by the number of days in the follow-up period. CR was multiplied by 100 and reported as a percentage.
Both adherence measures were calculated for the overall cohort and in sub-cohorts based on the length of follow-up between first and last prescription claim: >0 months to 3 months, >3 months to ≤6 months, >6 months to ≤12 months, and >12 months. Additionally, adherence was reported in subgroups defined by baseline characteristics, including age, gender, region, and payment plan type. Patients were categorized as adherent (Yes/No) based on a calculated MPRm ≥70%, 75%, and 80% threshold. Eighty percent is a commonly employed adherence threshold30 and was the defined adherence cut-off in the ATTR-ACT trial. However, given the recency of therapy approval, various thresholds were assessed.
Statistical Methods and Data Analyses
Data were handled and analyzed using Athena Engine software, v2 or later Copyright © -2021, Amazon Web Services, Inc. Patient characteristics, prescription characteristics, and adherence were reported using descriptive statistics. Categorical variables were summarized by the number of available observations, frequency, percentage, and 95% confidence limits. Continuous variables were summarized by the number of available observations, mean, standard deviation, 95% confidence limits, median, quartiles, minimum, and maximum, where appropriate. Missing categorical data were included as a separate “missing” category. Missing continuous data were not included in the summaries and analyses and no imputations were performed. This study did not have any hypotheses specified a priori. All statistical analyses were descriptive and exploratory. An α=0.05 was utilized to determine statistical significance; statistical tests included chi-square test, one-way ANOVA, post-hoc Tukey’s test, and Mann–Whitney U-test.
Results
Study cohort attrition is presented in Table 1. Among the 2020 patients who had >0 days’ supply across all accepted/reversed claims, 1365 patients were included in the MPRm and CR adherence analyses and 1366 patients were included in the DBR adherence analyses. These patients met the inclusion criteria for adherence analyses as they had at least two fully adjudicated prescription claims for tafamidis and a 3-month minimum follow-up period. A total of 1317 patients were found to have at least one gap in fill and were therefore included in the gaps in fills analyses.
 |
Table 1 Study Cohort Attrition |
Patient Characteristics
Baseline patient characteristics included sex, age groups, payment plan types, and US geographic region (Table 2). Overall, there was a significantly higher proportion of males (83.2% vs 16.8%, p<0.0001) and mean age was 75 years. Most patients had coverage that included Medicare (70.4%) or pharmacy benefit management organizations (PBMs) (14%). The proportion of patients receiving tafamidis varied significantly by geography; the Northeast had the highest proportion of patients receiving tafamidis, whereas the Midwest and West had fewer patients. Prescription characteristics included duration of follow-up, tafamidis uptake over time, refills ordered and received, days’ supply, prescribing physician specialties, and tafamidis meglumine and tafamidis free acid patient count. Across cohorts, the patient count was evenly distributed. The mean duration of follow-up was 6 months. Most patients with 0 months of follow-up were observed initiating treatment with tafamidis in the last month of the study period, accounting for the lack of follow-up for these patients (Figure 2).
 |
Table 2 Baseline Patient Characteristics |
 |
Figure 2 Patient count across specified follow-up periods. |
Preliminary Prescription Information
The highest rates of tafamidis uptake were observed in July and October of 2019, with even distribution throughout the remaining months (Figure 3). Among all claims and accepted claims, the most frequent prescriber specialties reported were cardiology and interventional cardiology; however, specialty information was not reported for the majority of providers. For patients with fully adjudicated claims, more received tafamidis meglumine than tafamidis free acid (n=995 vs 529, respectively) and approximately 10% switched between products during the study period.
 |
Figure 3 Patient uptake of tafamidis over time. |
There was a mean of seven refills ordered per patient and six refills received per patient. The median refills received and ordered were six refills per patient. Most tafamidis prescriptions were dispensed as a 30-day supply. The mean total days’ supply per patient was approximately 198 days (~6.6 months; range: 1 month to 22 months). Total days’ supply across the study period aligned with the corresponding duration of follow-up.
Prescription Characteristics Over Follow-Up
Distribution of Gaps in Fills
The vast majority of patients had either no gaps or one gap in fill (equating to a 1- to 30-day supply gap). The proportion of patients with more than one gap in fill was low. Of the 1317 patients who had at least one gap in fill, 96% had a <30-day gap. The average number of gaps in fill for these patients was two gaps, with a mean gap of 10 days (Table 3).
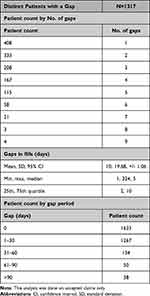 |
Table 3 Gap Periods and Number of Gaps |
Adherence to Tafamidis
DBR was calculated for a sub-cohort of 1366 patients; one patient was excluded from MPRm and CR analyses because the patient’s first and last accepted claim were the same.
Adherence to tafamidis was high, as evidenced by mean MPRm of 92%. Among those with at least 9 months and 12 months of follow up, adherence rates were similar, and the proportion of patients with MPRm ≥80% was 79% and 81%, respectively. Adherence rates were similar in males and females and across age groups; no statistically significant differences were observed. Among patients aged 55 to 84 years, the highest proportion of adherence (87%) was seen in the 55–64 age group. Adherence varied across geographic regions, though all regions demonstrated high rates of adherence, generally. Statistically significant differences were observed between the Northeast and Midwest with MPRm (94.41% vs 88.21%, p=0.0007). Median MPRm was similar among patients who took tafamidis meglumine or tafamidis free acid for the duration of the study period (98.9% and 94.0%, respectively); approximately 10% of the patients switched therapies. DBR, MPRm, and CR results by subgroup are presented in Table 4.
 |
Table 4 MPRm, DBR, and CR by Subgroup |
Most concomitant medications in this patient population were indicated for use in heart failure, thrombosis, hypertension, hypothyroidism, neuralgia, seizures, restless leg syndrome (RLS) and gastroesophageal reflux disease. Polypharmacy among patients was common, with the most frequently prescribed medications including furosemide, apixaban, torsemide, spironolactone, atorvastatin calcium, metoprolol succinate, tamsulosin, allopurinol, levothyroxine sodium, and gabapentin (Table 5).
 |
Table 5 Concomitant Therapy Use |
Discussion
According to a WHO report, average adherence rates for long-term therapy of chronic illnesses in developed countries are roughly 50%.16 Poor adherence over the course of a long-term treatment regimen severely diminishes its effectiveness and leads to poor outcomes, decreased quality of life, increased healthcare costs, and is a significant concern, particularly with respect to cardiovascular therapies. Without treatment, patients with ATTR-CM experience severe and progressive disease that correlates to frequent hospitalizations and poor survival.6 Patients with an accumulation of variant (ATTRv) transthyretin amyloid fibrils also experience a more rapid disease progression and diminished quality of life.6 Based on its mechanism of stabilization of transthyretin to prevent the formation and the accumulation of amyloid plaques, timely diagnosis and treatment, including with tafamidis, is paramount to limiting disease progression, cardiovascular-related hospitalizations, and mortality.13–15,17–20 The current analysis demonstrates high rates of adherence among patients taking tafamidis in the real world (75% to 100% of the patients having adherence ≥80%), especially by real-world standards and within the cardiology setting.31,32 Although the proportion of patients with ≥80% adherence across subgroups were generally lower than adherence levels reported in the ATTR-ACT trial (97.2% of the patients having adherence ≥80%), this was to be expected.15 The level of scrutiny that controlled trials undergo to ensure patients administer their medications consistently and appropriately is not usually present in real-world non-interventional studies.
This study included a sample that is representative of the ATTR-CM population expected to be users of tafamidis. The majority of patients were over 65 years of age and had prescription coverage provided by Medicare. In this analysis 83% of the patients were male, a proportion that is less skewed than published reports with male to female ratio estimates ranging from 10:1 to 20:1,33–38 but in-line with the level of male predominance reported in two recent systematic literature (83% and 86%).39,40 Among healthcare practitioners whose specialty was specified, cardiologists were the primary prescribers of tafamidis.
The ratio of refills ordered to refills received was 1. Moreover, most patients receiving tafamidis during the study period did not have gaps between refills and those patients with a gap in therapy were likely to have one for <30 days, suggesting the majority of patients are adherent. The observed MPRm (92%; 95% CI 90.89 to 93.19) is well over the commonly defined adherence threshold reported in most clinical trials of 80%.30 Polypharmacy was common among patients treated with tafamidis; however, medications were generally consistent with both the age and diagnosis of the patient population and included medications used for hypertension, thrombosis, heart failure, hypothyroidism, neuralgia, seizures, restless legs syndrome, and gastroesophageal reflux disease. Medication lists were used as a proxy for patient comorbidity data as they were not captured in the Symphony specialty pharmacy claims database. Comorbidity data is instead captured through medical claims data. Overall, these results provide evidence that not only is tafamidis being used by the expected population, but that among patients who initiate tafamidis, adherence is high. The proportion of patients taking only tafamidis meglumine (n=995) was higher than those only taking tafamidis free acid (n=529). Though the single-pill dosage of tafamidis free acid offers convenience to patients that might contribute to adherence, mean MPRm for both formulations were over 90% and only 10% of the patients switched. The similar adherence rates may be attributed to both being administered once daily (tafamidis free acid 61-mg capsule once daily or tafamidis meglumine 4×20-mg capsules once daily). Further, results were generally consistent across the various measures used to assess adherence as well as when stratified by patient characteristics.
Overall, adherence measures were similar for differing follow-up durations, age groups, and sexes. In general, high adherence was noted across all measures regardless of the region, with at least 75% of the population being designated “adherent” at the commonly employed 80% threshold. However, some geographic variations were noted. Adherence across all measures was significantly higher in the Northeast than the Midwest; of note, the Midwest had lower number of patients receiving tafamidis, whereas the Northeast region had the highest. This finding is in line with an analysis by Gilstrap et al, in which the highest incidence and prevalence of ATTR-CM was seen in the Northeast; the authors attributed this observation to better detection of ATTR-CM and presence of amyloidosis centers of excellence in the region.41 A center of excellence may offer specialists with a higher level of expertise and enhanced patient support for the specific disease state; it is plausible that these characteristics may also contribute, at least in part, to the observed higher adherence in the Northeast. Conversely, lower uptake in the Midwest and lower adherence in the region might be linked. Future studies may provide better understanding of contributing factors and identify opportunities for educational interventions to further improve adherence in patients in the Midwest. Due to the rarity of ATTR-CM in claims data, other patient characteristics such as race/ethnicity were not able to be assessed. These variables are likely confounders in any associations between treatment and adherence.42 They also constitute key privacy concerns for datasets of patients with rare diseases. Due to the low prevalence of patients with ATTR-CM receiving tafamidis in the specialty pharmacy database, race did not pass the expert determination Privacy Review process.
The concepts of implementation and adherence presented in this study align with other standardized taxonomies including the taxonomy provided by the Ascertaining Barriers to Compliance (ABC) project team, as well as adherence reporting guidelines that utilize ABC taxonomy such as the ESPACOMP Medication Adherence Reporting Guideline (EMERGE).43,44 We operationalized the concepts using the terminology defined by ISPOR, which places an enhanced focus on refill data.44 This was deemed to be appropriate, as metrics included for adherence analysis in this study required multiple fills of tafamidis prescriptions.
Limitations
There are limitations to pharmacy claims studies for assessing adherence; the limitations of this analysis are consistent with other studies. Fully adjudicated claims data were employed to mitigate errors in data entry and potential biases that may arise due to the inability to provide quality control or consistency for data collection. While the Symphony database has been used successfully in adherence research to demonstrate both relatively high and low levels of adherence,22,23 its use is subject to limitations common to all retrospective claims-based analyses, including incorrect data entry, the inability to recognize partial adherence, failure to record prescriptions filled outside of the database, and lack of documented reasoning for refill adherence behavior of patients.45
An increased amount of screening and awareness of ATTR-CM is needed to provide a complete and accurate perspective of the population with ATTR-CM diagnoses.1,3 Until this occurs, tafamidis as a treatment of ATTR-CM may be underutilized, resulting in patient selection biases, and thus may affect available real-world data.
Our results can only be considered as a proxy to real-world adherence of tafamidis, as adherence measures provide an overview of prescription fills. It is not known whether filled tafamidis is taken as prescribed. Claims databases are not equipped to provide further insights on these types of barriers to adherence. In addition, because patients may fill their medication before they have exhausted their previous supply, there is a potential to have adherence values over 100% as calculated by MPRm, DBR, and CR. While some studies cap the MPR at one, in this study, such capping was not done. Instead, median values were calculated and relied upon to observe central tendencies.
As the majority of tafamidis prescriptions were dispensed as a 30-day supply, we are unable to report whether there was a difference between patients that received a 30-day supply or 90-day supply. Recently, in a study utilizing the Symphony database, patients who were prescribed a 90-day supply of medications had significantly higher adherence rates in comparison to those who were prescribed a 30-day supply within 1 year post-discharge.23 Subsequent studies may explore the influence that days’ supply has on patient adherence to tafamidis. Additionally, the manner in which refills were ordered was not available in the Symphony database. Considering the average age of patients receiving tafamidis, the logistics of refill ordering and pickup may influence adherence.
It should be noted that in this analysis, discontinuation was not assessed. Medication discontinuation in electronic database studies can only be assessed within the context of a pre-specified operational definition for the required number of days without medication available (maximum permissible gap). However, in this study, we did not have a known or agreed-upon maximum permissible gap. Given the very recent approval of tafamidis and resulting lack of any baseline understanding of prescription-taking behavior, studying or inferring discontinuation from this data was not deemed appropriate. These data may allow for future research on gaps and medication taking behavior.
Patterns of nonadherence vary and may also include non-initiation, incorrect implementation, and early discontinuation.46 Non-initiation was not possible to examine based on the study design. There are many factors that influence adherence. Cost and adverse events are often assumed reasons for discontinuation and/or nonadherence; however, because fully adjudicated claims were used for this study, cost of treatment and its impact on adherence was not considered. Currently in the US, the cost of tafamidis treatment is covered through various methods, including financial assistance programs that patients may qualify for.47 Future studies may investigate the impact that varying modes of coverage for tafamidis costs and adverse events associated with tafamidis have on adherence to the medication.
The large numbers of patients receiving tafamidis in the Symphony Health dataset, including both Medicare and non-Medicare prescriptions, allowed assessment of adherence across age groups, including those in the <65 years of age population. These patients represented a small but not insignificant proportion of patients (~7%). The consistency of multiple adherence measures across age groups, sex, and regions provides strong evidence that adherence is high for patients on tafamidis, regardless of their individual follow-up periods. Other studies have utilized the Symphony health database to demonstrate high adherence rates in their respective pharmacotherapies of interest.23,48,49 These studies were also able to utilize similar subgroups such as follow-up time, age groups, and gaps in fills to characterize the patient population they evaluated.23,48,49
As the first study examining adherence among tafamidis users in a real-world setting, this study provides key insights and will lay the foundation for future research to further elucidate patient and physician behavior. The limitations of this study will provide an opportunity for future studies to collect or incorporate additional data elements to help move beyond descriptive analyses to causal relationships. Potential topics for exploration will aim to provide insight into adherence at all stages, including initiation and persistence. Deeper understanding of adherence in vulnerable minority populations and factors contributing to nonadherence in different segments of patient populations, will help to minimize any potential health disparities and further optimize treatment outcomes through various education programs. Additional research could extend to determining the impact this level of adherence has on patient outcomes and its value to payers.
Conclusion
This is the first study reporting real-world adherence of tafamidis in patients with ATTR-CM in the US. Based on our analysis, the characteristics of patients receiving tafamidis are consistent with the expected patient population being older, predominantly male, and with the highest proportion of patients in the Northeast US. Patients were generally prescribed tafamidis by their cardiologist and received concomitant medications primarily indicated for heart failure, thrombosis, and hypertension. Adherence to tafamidis was high across patients of various age groups as well as in males and females. While adherence was generally high across all regions, there is room for improvement in the Midwest. In addition, patients filled as many prescriptions as they were prescribed by their physician. Gaps in refills were minimal; moreover, on average, patients with gaps had one to two gaps of 1 to 30 days. These findings suggest that overall, this patient population exhibits strong real-world adherence behaviors, which may contribute to the effectiveness of tafamidis in ATTR-CM.
Acknowledgments
The authors wish to thank Elizabeth Hubscher, PhD, and Holden Young, PharmD, MBA both of Cytel Inc, for manuscript writing support, which was funded by Pfizer.
Funding
This study was supported by Pfizer, Inc.
Disclosure
Darrin Benjumea is an employee of Genesis Research who has been contracted by Pfizer, Inc. for involvement in this study. Andrew Peterson is an employee of University of the Sciences who has been contracted by Pfizer, Inc. for involvement in this study. Sapna Prasad and Alex O’Brien are employees of Clarify Health Solutions and were contracted by Pfizer, Inc. for involvement in this study. Anuja Roy, Nick Marchant, Jose Alvir, Rahul Bhambri, Jason Lynn, Yong Chen, Jason Kemner, and Bhash Parasuraman are employees of Pfizer and own stock and/or stock options. The authors report no other conflicts of interest in this work.
References
1. Kittleson MM, Maurer MS, Ambardekar AV, et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2020;142(1):e7–e22. doi:10.1161/CIR.0000000000000792
2. Feng KY, Loungani RS, Rao VN, et al. Best practices for prognostic evaluation of a patient with transthyretin amyloid cardiomyopathy. journal article review. JACC CardioOncol. 2019;1(2):273–279. doi:10.1016/j.jaccao.2019.11.006
3. Garcia-Pavia P, Bengel F, Brito D, et al. Expert consensus on the monitoring of transthyretin amyloid cardiomyopathy. Eur J Heart Fail. 2021;23(6):895–905. doi:10.1002/ejhf.2198
4. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709–716. doi:10.1016/j.jchf.2019.04.010
5. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(22):2872–2891. doi:10.1016/j.jacc.2019.04.003
6. Nativi-Nicolau J, Judge DP, Hoffman JE, et al. Natural history and progression of transthyretin amyloid cardiomyopathy: insights from ATTR-ACT. ESC Heart Fail. 2021;8:3875–3884. doi:10.1002/ehf2.13541
7. Castaño A, Drachman BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20(2):163–178. doi:10.1007/s10741-014-9462-7
8. Maurer MS, Bokhari S, Damy T, et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circulation. 2019;12(9):e006075. doi:10.1161/CIRCHEARTFAILURE.119.006075
9. Rubin J, Maurer MS. Cardiac amyloidosis: overlooked, underappreciated, and treatable. Annu Rev Med. 2020;71:203–219. doi:10.1146/annurev-med-052918-020140
10. Teng C, Li P, Bae JY, Pan S, Dixon RAF, Liu Q. Diagnosis and treatment of transthyretin-related amyloidosis cardiomyopathy. Clin Cardiol. 2020;43(11):1223–1231. doi:10.1002/clc.23434
11. Hanna M, Damy T, Grogan M, et al. Impact of tafamidis on health-related quality of life in patients with transthyretin amyloid cardiomyopathy (from the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial). Am J Cardiol. 2021;141:98–105. doi:10.1016/j.amjcard.2020.10.066
12. FDA. FDA approves new treatments for heart disease caused by a serious rare disease, transthyretin mediated amyloidosis; 2021. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatments-heart-disease-caused-serious-rare-disease-transthyretin-mediated.
13. Bulawa CE, Connelly S, Devit M, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012;109(24):9629–9634. doi:10.1073/pnas.1121005109
14. Park J, Egolum U, Parker S, Andrews E, Ombengi D, Ling H. Tafamidis: a first-in-class transthyretin stabilizer for transthyretin amyloid cardiomyopathy. Ann Pharmacother. 2020;54(5):470–477. doi:10.1177/1060028019888489
15. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–1016. doi:10.1056/NEJMoa1805689
16. Sabaté E. Adherence to Long-Term Therapies: Evidence for Action. World Health Organization; 2003:198.
17. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi:10.1161/CIRCULATIONAHA.108.768986
18. Leslie KH, McCowan C, Pell JP. Adherence to cardiovascular medication: a review of systematic reviews. J Public Health. 2019;41(1):e84–e94. doi:10.1093/pubmed/fdy088
19. van Onzenoort HAW, Menger FE, Neef C, et al. Participation in a clinical trial enhances adherence and persistence to treatment: a retrospective cohort study. Hypertension. 2011;58(4):573–578. doi:10.1161/HYPERTENSIONAHA.111.171074
20. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–314. doi:10.4065/mcp.2010.0575
21. Vong C, Boucher M, Riley S, Harnisch LO. Modeling of survival and frequency of cardiovascular-related hospitalization in patients with transthyretin amyloid cardiomyopathy treated with tafamidis. Am J Cardiovasc Drugs. 2021;21(5):535–543. doi:10.1007/s40256-021-00464-y
22. Brixner D, Rubin DT, Mease P, et al. Patient support program increased medication adherence with lower total health care costs despite increased drug spending. J Manag Care Spec Pharm. 2019;25(7):770–779. doi:10.18553/jmcp.2019.18443
23. Rymer JA, Fonseca E, Bhandary DD, Kumar D, Khan ND, Wang TY. Difference in medication adherence between patients prescribed a 30-day versus 90-day supply after acute myocardial infarction. J Am Heart Assoc. 2021;10(1):e016215. doi:10.1161/JAHA.119.016215
24. Symphony Health. What we do. Available from: https://symphonyhealth.com/what-we-do.
25. Motheral B, Brooks J, Clark MA, et al. A checklist for retrospective database studies–report of the ISPOR task force on retrospective databases. Value Health. 2003;6(2):90–97. doi:10.1046/j.1524-4733.2003.00242.x
26. Orsini LS, Berger M, Crown W, et al. Improving transparency to build trust in real-world secondary data studies for hypothesis testing-why, what, and how: recommendations and a road map from the real-world evidence transparency initiative. Value Health. 2020;23(9):1128–1136. doi:10.1016/j.jval.2020.04.002
27. Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10(1):3–12. doi:10.1111/j.1524-4733.2006.00139.x
28. Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7–8):1280–1288. doi:10.1345/aph.1H018
29. Raebel MA, Schmittdiel J, Karter AJ, Konieczny JL, Steiner JF. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care. 2013;51(8 Suppl 3):S11–21. doi:10.1097/MLR.0b013e31829b1d2a
30. Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25(9):2303–2310. doi:10.1185/03007990903126833
31. Baroletti S, Dell’Orfano H. Medication adherence in cardiovascular disease. Circulation. 2010;121(12):1455–1458. doi:10.1161/CIRCULATIONAHA.109.904003
32. Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012;125(9):882–7e1. doi:10.1016/j.amjmed.2011.12.013
33. Helder MR, Schaff HV, Nishimura RA, et al. Impact of incidental amyloidosis on the prognosis of patients with hypertrophic cardiomyopathy undergoing septal myectomy for left ventricular outflow tract obstruction. Am J Cardiol. 2014;114(9):1396–1399. doi:10.1016/j.amjcard.2014.07.058
34. Koike H, Hashimoto R, Tomita M, et al. Diagnosis of sporadic transthyretin Val30Met familial amyloid polyneuropathy: a practical analysis. Amyloid. 2011;18(2):53–62. doi:10.3109/13506129.2011.565524
35. Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140(1):16–26. doi:10.1161/circulationaha.118.038169
36. Rapezzi C, Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120(13):1203–1212. doi:10.1161/circulationaha.108.843334
37. Russo M, Mazzeo A, Stancanelli C, et al. Transthyretin-related familial amyloidotic polyneuropathy: description of a cohort of patients with Leu64 mutation and late onset. J Peripher Nerv Syst. 2012;17(4):385–390. doi:10.1111/j.1529-8027.2012.00436.x
38. Siepen FAD, Bauer R, Voss A, et al. Predictors of survival stratification in patients with wild-type cardiac amyloidosis. Clin Res Cardiol. 2018;107(2):158–169. doi:10.1007/s00392-017-1167-1
39. Bruno M, Castaño A, Burton A, Grodin JL. Transthyretin amyloid cardiomyopathy in women: frequency, characteristics, and diagnostic challenges. Heart Fail Rev. 2021;26(1):35–45. doi:10.1007/s10741-020-10010-8
40. Kroi F, Fischer N, Gezin A, Hashim M, Rozenbaum MH. Estimating the gender distribution of patients with wild-type transthyretin amyloid cardiomyopathy: a systematic review and meta-analysis. Cardiol Ther. 2021;10(1):41–55. doi:10.1007/s40119-020-00205-3
41. Gilstrap LG, Dominici F, Wang Y, et al. Epidemiology of cardiac amyloidosis-associated heart failure hospitalizations among fee-for-service medicare beneficiaries in the United States. Circ Heart Fail. 2019;12(6):e005407. doi:10.1161/CIRCHEARTFAILURE.118.005407
42. Gerber BS, Cho YI, Arozullah AM, Lee SY. Racial differences in medication adherence: a cross-sectional study of Medicare enrollees. Am J Geriatr Pharmacother. 2010;8(2):136–145. doi:10.1016/j.amjopharm.2010.03.002
43. De Geest S, Zullig LL, Dunbar-Jacob J, Hughes D, Wilson IB, Vrijens B. Improving medication adherence research reporting: ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Eur J Cardiovasc Nurs. 2019;18(4):258–259. doi:10.1177/1474515119830298
44. Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. doi:10.1111/j.1365-2125.2012.04167.x
45. Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. 2015;2015:217047. doi:10.1155/2015/217047
46. Kronish IM, Thorpe CT, Voils CI. Measuring the multiple domains of medication nonadherence: findings from a Delphi survey of adherence experts. Transl Behav Med. 2021;11(1):104–113. doi:10.1093/tbm/ibz133
47. Masri A, Chen H, Wong C, et al. Initial experience prescribing commercial tafamidis, the most expensive cardiac medication in history. JAMA Cardiol. 2020;5(9):1066–1067. doi:10.1001/jamacardio.2020.1738
48. Lafeuille MH, Grittner AM, Lefebvre P, et al. Adherence patterns for Abiraterone acetate and concomitant prednisone use in patients with prostate cancer. J Manag Care Spec Pharm. 2014;20(5):477–484. doi:10.18553/jmcp.2014.20.5.477
49. Patel AK, Duh MS, Barghout V, et al. Real-world treatment patterns among patients with colorectal cancer treated with trifluridine/tipiracil and regorafenib. Clin Colorectal Cancer. 2018;17(3):e531–e539. doi:10.1016/j.clcc.2018.04.002
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.