Back to Journals » OncoTargets and Therapy » Volume 13
Baicalin Attenuates YAP Activity to Suppress Ovarian Cancer Stemness
Authors Li Y , Wang D, Liu J, Li Y, Chen D, Zhou L, Lang T , Zhou Q
Received 25 March 2020
Accepted for publication 29 June 2020
Published 24 July 2020 Volume 2020:13 Pages 7151—7163
DOI https://doi.org/10.2147/OTT.S254607
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Yucong Li,1– 3 Dong Wang,1 Jingshu Liu,1 Yunzhe Li,1 Duke Chen,1 Lei Zhou,4 Tingyuan Lang,1– 3 Qi Zhou1– 3
1Department of Gynecologic Oncology, Chongqing University Cancer Hospital, Chongqing 400030, People’s Republic of China; 2Chongqing Key Laboratory of Translational Research for Cancer Metastasis and Individualized Treatment, Chongqing University Cancer Hospital, Chongqing 400030, People’s Republic of China; 3Key Laboratory for Biorheological Science and Technology of Ministry of Education (Chongqing University), Chongqing University Cancer Hospital, Chongqing 400044, People’s Republic of China; 4Singapore Eye Research Institute, Singapore, 169856, Singapore
Correspondence: Qi Zhou; Tingyuan Lang
Department of Gynecologic Oncology, Chongqing University Cancer Hospital, Chongqing 400030, People’s Republic of China
Tel +86 18908384529
; +86 18717859346
Fax +86 23 65311341
Email [email protected]; [email protected]
Purpose: This study aims to reveal the mechanism underlying baicalin-suppressing ovarian cancer stemness.
Methods: OVCAR-3 and the primary ovarian cancer cells were used for cell model. The ovarian cancer stem cells were isolated by suspension culture. Cell viability and clonogenicity were examined by CCK-8 assay and colony formation assay. The self-renewal of the cells was evaluated by the determination of sphere-forming capacity and the frequency of in vitro sphere-forming and in vivo tumor-initiating cells. The mRNA and protein levels were relatively quantified by qRT-PCR and Western blot. The transcription regulation of target genes was tested by luciferase reporter assay and a modified nuclear rn-on qRT-PCR assay.
Results: Treatment with a non-toxic dose of baicalin significantly inhibited the spherogenicity of ovarian cancer cells. Moreover, a non-toxic dose of baicalin treatment suppressed the frequency of sphere-forming and tumor-initiating ovarian cancer cells. Furthermore, the expression of ovarian cancer stem cell markers (CD133 and ALDH1A1) was inhibited by a non-toxic dose of baicalin treatment. Baicalin inhibits YAP activity and suppresses RASSF6, a positive regulator of YAP, at the transcriptional level. Overexpression of both YAP and RASSF6 abolished the inhibitory effect of baicalin on the proliferation and stemness of ovarian cancer cells.
Conclusion: The results in this study demonstrated that baicalin suppresses the stemness of ovarian cancer cells by attenuating YAP activity via inhibiting RASSF6 at the transcriptional level. This finding revealed baicalin as a novel YAP inhibitor that could serve as an anti-cancer drug for eradicating ovarian cancer stem cells.
Keywords: baicalin, ovarian cancer, stemness, YAP, RASSF6
Introduction
Ovarian cancer (OC) is the leading cause of death among gynecological malignancies; it is characterized by early metastatic spread, extensively disseminated tumors as well as accumulation of malignant ascites.1,2 Moreover, for most patients, the OC cells develop chemoresistance, which leads to relapse and metastasis after initial therapy.3,4 Despite efforts to develop the therapeutic strategy in past decades, the long-term outcomes in OC patients have not significantly improved.5
Cancer stem cells (CSCs), also named tumor-initiating cells (TICs), are a small number of cancer cells within tumors with stem-like properties; it is characterized by self-renewal and differentiation and thus possesses the ability to sustain hierarchical organization of tumors.6 There is accumulating evidence that tumorigenesis, recurrence, and metastasis are originated from CSCs, and CSCs are the only subpopulation within tumors capable of tumor initiation.7,8 Moreover, CSCs are also responsible for therapeutic resistance, and thus the development of strategies eradicating CSCs would be promising for cancer therapy.9
Ovarian cancer stem cells (OCSCs) are first isolated by Bapat and colleagues; they cultured a single clone from ascites capable of differentiation and tumorigenesis.10 Subsequently, researchers have identified various OCSCs related markers, including CD44, CD24, CD177, CD133, and ALDH1, etc.11,12 Similarly, evidences have shown that OCSCs are responsible for OC initiation, recurrence, metastasis as well as chemoresistance.13 Thus, OCSCs have been recognized as the prime drug target for OC therapy. However, targeting OCSCs with traditional chemotherapeutic (cytotoxic) drugs is challenging as the chemoresistance and relatively fewer number of OCSCs; non-selective cytotoxic drugs may promote progression by OCSCs selection and lead to a serious side effect.
Baicalin, a flavonoid, is a metabolite of baicalein which is isolated from the roots of Scutellaria baicalensis Georgi, also named Huang Qin in Traditional Chinese Medicine (TCM).14 Baicalin is widely used as an alternative remedy in TCM.15 Recently, baicalin has been received attention for novel anticancer drug development as its anticancer activity in a variety of cancer types, including OC, and relatively fewer side effects.16,17 However, the effect of baicalin on OCSCs has remained elusive.
Hippo/YAP signaling pathway was first discovered in Drosophila that is a conserved kinase cascade responsible for the regulation of organ size.18 In mammals, the core components of Hippo signaling consist of nuclear Dbf2-related family protein kinases Lats1 and Lats2, STE20 family protein kinases Mst1 and Mst2 and their downstream target transcription coactivator YAP (Yes-associated protein).19 Mst1/2 phosphorylates and activates Lats1/2, which in turn phosphorylates and inhibits YAP.20 YAP regulates a set of target genes associated with survival and proliferation.21 Recent evidence also showed that YAP activity is important for cell fate decision and maintenance of stemness of both normal and cancer stem cells.22 Thereby, Hippo/YAP signaling is an important target for eradicating cancer stem cells.
In this study, we found that baicalin inhibits the transcription of RASSF6, a negative regulator of Mst1/2,19,20 attenuates YAP activity, and thereby suppressing the stemness of ovarian cancer cells.
Materials and Methods
Reagents, Primers, Antibodies
Baicalin was purchased from Sigma-Aldrich (572667; 95% purity, St Louis, MO, United States). The concentration of DMSO (Dimethyl sulfoxide) stock solution of baicalin is 50 mM. Culture medium containing baicalin was prepared by diluting baicalin with culture medium followed by the addition of supplements such as FBS and antibiotics. The sequences of primers are listed in Supplementary Table 1. The information on antibodies used in this study, including manufacture, catalog number, and dilution, etc., were provided in Supplementary Table 2. All other reagents were purchased from Sigma-Aldrich (St Louis, MO, United States) except where indicated.
Cell Culture
Human epithelial ovarian adenocarcinoma cell line NIH: OVCAR-3 (OVCAR3) was acquired from American Type Culture Collection (ATCC, Manassas, VA, USA) and was grown in RPMI 1640 cell culture medium (Thermo Fisher, Waltham, MA, USA) containing 10% fetal bovine serum (FBS, Thermo Fisher, Waltham, MA, USA), 100 U mL−1 penicillin (Thermo Fisher, Waltham, MA, USA) and 100 μg mL−1 streptomycin (Thermo Fisher, Waltham, MA, USA). The cells were maintained at 37°C in 5% CO2.
Primary Culture
The ovarian cancer specimens were collected during surgery and placed in a sterile container filled ice-cold PBS. The samples were then cut into small pieces and transferred into dispase II (2 U mL−1) (Thermo Fisher, Waltham, MA, USA) in DMEM (Thermo Fisher, Waltham, MA, USA) followed by 30 min incubation at 37°C. The cell slurry was then passed through a cell strainer (70 μm) (Thermo Fisher, Waltham, MA, USA) and centrifuged for 5 min. The cell pellet was resuspended in DMEM containing 10% FBS and incubated at 37°C in 5% CO2. The medium was changed every three days. All tissues were obtained from Chongqing University Cancer Hospital, which was approved by the ethics committee of Chongqing University Cancer Hospital (Chongqing China). All patients provided written informed consent, and that this was conducted in accordance with the Declaration of Helsinki.
Ovarian Cancer Stem Cell Isolation
The cells (cell lines or primary cells) were digested from normal cell culture flask and centrifuged. The cell pellets were then resuspended in 1:1 DMEM: F12 containing 1% FBS, 20 ng mL−1 recombinant epidermal growth factor (EGF) (Thermo Fisher, Waltham, MA, USA), 10 ng mL−1 recombinant basic fibroblast growth factor (FGF) (Thermo Fisher, Waltham, MA, USA), 100 U mL−1 penicillin and 100 μg mL−1 streptomycin and seeded into ultra-low attachment culture plate (Corning, NY, USA). The cells were split into 2 new ultra-low attachment culture plates after 60–80% confluence.
CCK-8 Assay
The CCK-8 assay kit was purchased from Thermo Scientific (Waltham, MA, USA) and the assays were performed according to the manual. The cells were digested and seeded into 96-well plate. After 12 hours of culture for attachment, the cells were treated according to experiment design. Then, after another 24 h incubation, the cell medium was discarded and rinsed with PBS, followed by the addition of 10% Cell Counting Kit-8 containing culture medium at 100 μL per well. The results were read by a spectrometer at 450 nm.
Drug Sensitivity Assay
OVCAR-3 cells or primary human ovarian cancer cells were seeded into 96-well plates at a density of 3,000 cells per well. After 24 h culture for attachment, the cells were exposed to serially diluted baicalin (a three-fold dilution with a starting concentration of 1,000 μM) and incubated for another 24 h. The cell number was examined by CCK-8 assay as mentioned above. Inhibition rate (%) = 1 - (Atreatment-Ablank)/(Avehicle-Ablank) %. IC50 values were calculated with non-linear regression analysis. Experiments were performed in triplicate.
Cell Viability Assay
Both OVCAR-3 cells and primary human ovarian cancer cells were seeded in 96 well plates at a density of 3,000 cells per well. After 12–24 h culture for attachment, the cells were exposed to 40 μM baicalin or vehicle for 24 h. The cell viability was examined by CCK-8 assay. Proliferation rate (%) = (AT-24h-AT-0)/At-0%. Three replicates were performed.
Evaluation of Clonogenicity
The cells were seeded into a 1.5 cm culture dish containing complete culture medium at a density of 1,000 cells per dish. After 12–24 h attachment culture, the cells were exposed to 40 μM of baicalin or vehicle for 24 h. The medium was then changed to complete culture medium without baicalin or vehicle and cultured for 10–14 days followed by staining and counting. The experiments were repeated three times.
Evaluation of Spherogenicity
The cells (cell lines or primary cells) were first cultured in a 6 cm culture dish for 12–24 h. After attachment, the cells were exposed to 15 μM of baicalin or vehicle for 24 h. Then, the cells were digested with trypsin (Thermo Fisher, Waltham, MA, USA) and collected by centrifugation. The sphere-formation was performed according to the protocol from Lombardo et al’s publication.23 Briefly, the cell pellets (1.5 × 104 cells per well) were resuspended in 1:1 DMEM: F12 containing 1% FBS, 20 ng mL−1 recombinant epidermal growth factor (EGF), 10 ng mL−1 recombinant basic fibroblast growth factor (FGF), 100 U mL−1 penicillin and 100 μg mL−1 streptomycin and seeded into 6-well ultra-low attachment culture plate (Corning, NY, USA). After 10–14 days of culture, the spheres were counted under the microscope.
Lentivirus Packaging and Infection
YAP- or RASSF6-overexpressing construct was generated by subcloning PCR-amplified full-length human YAP or RASSF6 cDNA into the pCDH plasmid. Lentiviral particles were produced by transfecting HEK 293 T cells with reconstructed plasmids along with packaging and backbone vectors. The particles were concentrated by virus precipitation reagent (Thermo Fisher, Waltham, MA, USA) followed by centrifugation according to the manufacture’s suggestion. The particles were then added into a cell culture plate for infection. The infected cells were selected with puromycin. The efficiency of gene manipulation was examined by Western blot.
qRT-PCR
Total mRNA was isolated by the Trizol reagent, followed by reverse transcription. qRT-PCR was performed using qRT-PCR Master Mix (Thermo Fisher, Waltham, MA, USA). The data were normalized against GAPDH. The primer sequences are listed in Supplementary Table 1.
Western Blot
The cells were lysed with RIPA lysis buffer. The protein samples were separated by SDS-PAGE and then transferred onto PVDF membrane according to standard protocol. The proteins were then blocked with 5% BSA overnight at 4°C, and incubated with primary antibodies overnight at 4°C. After another 2 h incubation with secondary antibody, the signaling was detected by ECL reagents (Thermo Fisher, Waltham, MA, USA). The antibodies used in this study are listed in Supplementary Table 2. The uncropped gel is provided in Supplementary Figure 4.
Luciferase Reporter Gene Assay
The promoter of RASSF6 was cloned into PGL4 plasmid (Promega, Madison, WI, USA). The cells were seeded into a 24-well plate and treated according to experiment design. The cells were then transfected with reconstructed plasmid or control plasmid by lipofectamine 3000 (Thermo Fisher, Waltham, MA, USA). After another 24 h culture, the luciferase activities were determined according to the standard protocol.
Xenografts
The cells were dissociated and resuspended in cultured medium containing 25% Matrigel (Thermo Fisher, Waltham, MA, USA). The cells were then inoculated into the flank of 4-week old female NOD/SCID mice (Pusheng Technology, Nanjing, China). The mice were then maintained under the standard condition for 25–30 days for observation. All animal experiments were reviewed and approved by the institutional ethics and recombination safety committees of Chongqing University Cancer Hospital (Chongqing, China).
Limiting Dilution Assay (LDA)
One million OVCAR-3 cells were plated into 6 cm culture dish and culture for 12–24 h. After attachment, the cells were treated with 15 μM of baicalin or vehicle for 24 h. the cells were then washed with PBS to remove the baicalin and vehicle and collected. The cells were then resuspended in 1:1 DMEM: F12 containing 1% FBS, 20 ng mL-1 recombinant epidermal growth factor (EGF), 10 ng mL-1 recombinant basic fibroblast growth factor (FGF), 100 U mL-1 penicillin and 100 μg mL-1 streptomycin. Ten, 5, or 1 cells were seeded into a 96-well plate for further culture. Twelve wells for each group were performed. After 10–14 days of culture, the number of wells exhibited spheres was counted. ELDA software was used for calculation of the frequency of sphere-forming cells. All groups were performed in triplicate.
To evaluate tumorigenicity, OVCAR-3 cells were treated with 15 μM of baicalin or vehicle for 24 h. After washing and dissociation, 21, 7, 3 cells were inoculated into the flank of 4-week old female NOD/SCID mice. The mice were then maintained under the standard condition for 25–30 days for observation. The number of mice with tumors was counted. ELDA software was used for calculation of the frequency of tumor-initiating cells. All groups were performed in triplicate.
Evaluation of RASSF6 Transcription
The regulation of RASSF6 transcription was determined by Click-iT™ Nascent RNA Capture Kit (Thermo Fisher, Waltham, MA, USA) via metabolic labeling of nascent RNA according to the manual. This method is similar to nuclear run-on qRT-PCR reported by Roberts et al24 and the advantage of this method is that no radioactive nucleoside analogs are required. Briefly, The OVCAR-3 and primary cells were cultured in a 6-well plate at 25–30% confluence. After 12 h culture for attachment, the cells were treated with 15 μM baicalin or vehicle for 24 h. After removing baicalin or vehicle, the cells were immediately maintained in culture medium (3 mL per well) containing 0.5 mM 5-ethynyl uridine (EU) for 1 h. After incubation, the cells were harvested by centrifugation for RNA isolation. The total RNA was isolated by TRIzol reagent (Thermo Fisher, Waltham, MA, USA) according to standard protocol. Next, mix total RNA with biotin azide (10 ug EU-labeled RNA: 1 mM Biotin Azide) for biotinylation of EU-labeled RNA (EU-RNA) according to the manual provided by the manufacture. After precipitation, the biotinylated EU-RNA was then bound to streptavidin-coupled magnetic beads for EU-RNA purification with a DynaMagTM-2 magnet (Thermo Fisher, Waltham, MA, USA). After washing, the beads with EU-RNA were resuspended and immediately used for cDNA synthesis. cDNA synthesis was performed by SuperScript® VILO™ cDNA synthesis kit. qRT-PCR was performed as previously mentioned. GAPDH was used as control. The experiments were performed in triplicate.
Evaluation of RASSF6 Stabilization
To evaluate whether baicalin affects the stabilization or half-life of RASSF6, a translation inhibitor, cycloheximide (CHX), was employed. CHX inhibits protein synthesis in eukaryotic cells by interfering with the translocation step. Briefly, the cells were cultured in a 6 cm dish at 60% confluence. After attachment, the cells were incubated in complete culture medium containing 10μM of CHX for 4h to inhibit protein synthesis of the cells. Then, the medium was changed to complete culture medium containing 10 μM of CHX and 15 μM of baicalin or an equal volume of vehicle for 24 h. The total proteins in cells before and after baicalin or vehicle treatment were extracted and the protein levels of RASSF6 in the cells before and after baicalin or vehicle treatment were examined by Western blot and anti-RASSF6 antibody as previously mentioned. As the translation of the cells was inhibited by CHX treatment, the degradation of RASSF6 during baicalin or vehicle treatment can be evaluated.
Cellular Fractionation
The cytoplasmic and nuclear protein fractions of OVCAR-3 cells were prepared with a Subcellular Protein Fractionation kit (Thermo Fisher, Waltham, MA, USA) according to the manufacture’s protocol. Briefly, the cells were collected by centrifugation in a microcentrifuge tube. The cell pellet was incubated with cytoplasmic extraction buffer (CEB) at 4°C for 10 min. The lysate was then centrifuged at 500 g for 5 min and the cytoplasmic proteins in the supernatant were collected in a fresh microcentrifuge tube. The pellet was then incubated with membrane extraction buffer at 4°C for 10 min following by centrifugation at 3,000 g for 5 min. The supernatant was discarded and the nuclear proteins were extracted by incubating pellet with IB sample buffer and boiling for 5 min.
Statistics
The data are presented as mean ± SEM. P values were determined by two-tailed Student’s t-test with P < 0.05 considered statistically significant.
Results
Baicalin Inhibits the Stemness of OC Cells
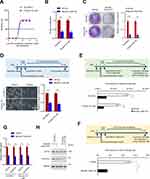
To investigate the effect of baicalin on OC cells, the IC50 values of baicalin on OVCAR-3 and primary OC cells were first determined. As shown in Figure 1A, the IC50 values of baicalin on OVCAR-3 and primary ovarian cancer cells were 31.71 μM and 38.29 μM, respectively, and 15 μM for 24 h treatment is the non-toxic dose for both cells. To confirm the proliferation inhibitory effect of baicalin on OC cells, we performed proliferation and colony formation assay. As shown in Figure 1B and C, treatment with a toxic dose of baicalin (40 μM, 24 h) significantly inhibits the proliferation and clonogenicity of OC cells.
Next, we investigated the effect of baicalin on the stemness of OC cells. To exclude the probability that the observed inhibitory effect is the result of proliferation inhibition, we treated the cells with a non-toxic dose of baicalin (15 μM, 24 h). As shown in Figure 1D, the cells were pretreated with 15 μM of baicalin or an equal volume of vehicle for 24 h followed by suspension culture with medium without compounds for 12–16 days and the result showed that non-toxic dose of baicalin significantly inhibits the spherogenicity of both OVCAR-3 and primary OC cells. Similarly, the cells were pretreated with 15 μM of baicalin or an equal volume of vehicle for 24 h and subjected to LDA assay as mentioned in methods session. As shown in Figure 1E, F and Supplementary Figure 1A and 1B, the frequency of in vitro sphere-forming cells and in vivo tumor-initiating cells was also inhibited by a non-toxic dose of baicalin treatment. Furthermore, the results from qRT-PCR and Western blot assay indicated that the non-toxic dose of baicalin treatment significantly inhibited both mRNA and protein levels of OCSCs markers (CD133 and ALDH1A1) (Figure 1G and H). Taken together, these results demonstrated that baicalin inhibits the stemness of ovarian cancer cells.
Baicalin Attenuates YAP Activity in OC Cells
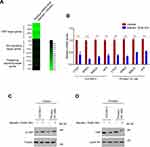
Given the important role of Wnt, Hippo/YAP, and Hedgehog signaling pathway in maintenance of CSCs stemness,25,26 to further investigate the underlying mechanisms of baicalin suppressing the stemness of OC cells, we next tested the inhibitory effect of baicalin on these signaling pathways. As shown in Figure 2A, most of YAP target genes were downregulated by the non-toxic dose of baicalin treatment in OC cells, while, the downstream target genes of Wnt and Hedgehog signaling pathways were not affected, which indicated that Hippo/YAP signaling pathway is the potential target of baicalin in OC cells. This result was further verified by qRT-PCR (Figure 2B). In Hippo/YAP signaling pathway, the kinase cascade, consists of MST1/2 and LATS1/2, phosphorylates YAP and blocks its nuclear translocation.18–22 We thus tested the phosphorylation level of YAP in OC cells after baicalin treatment. As shown in Figure 2C, baicalin treatment significantly increased the protein level of phosphorylated YAP in the cytoplasm, which indicated that Hippo signaling is activated by baicalin. In line with this result, the protein level of nuclear YAP is significantly reduced by baicalin (Figure 2D). These results demonstrated that baicalin activates Hippo signaling and attenuates YAP activity.
Overexpression of YAP Abolished the Inhibitory Effect of Baicalin on the Stemness of OC Cells
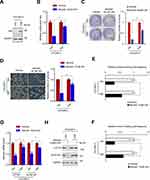
To confirm the role of YAP inhibition in the inhibitory effect of baicalin on the stemness of OC cells, the YAP was overexpressed in OVCAR-3 cells by lentivirus-mediated gene delivery system (Figure 3A). We first examined whether YAP overexpression can abolish the inhibitory effect of baicalin on the proliferation and spherogenicity of OC cells. YAP-overexpressing OVCAR-3 and control cells were treated with a toxic dose of baicalin and the proliferation and spherogenicity of the cells were examined by CCK-8 and colony formation assay. As shown in Figure 3B and C, the ectopic expression of YAP partially abolished the inhibitory effect of baicalin on the proliferation and spherogenicity of OVCAR-3 cells.
Next, we investigated whether YAP overexpression can abolish the inhibitory effect of baicalin on the stemness of OC cells. OVCAR-3 cells transfected with YAP-overexpressing or empty control plasmids were pretreated with a non-toxic dose of baicalin. Then, the cells were subjected to sphere formation and LDA assay to evaluate the self-renew capacities. The results showed that YAP overexpression significantly abolished the inhibitory effect of baicalin on the sphere-forming capacity of OVCAR-3 cells (Figure 3D). And the frequency of sphere-forming and tumor-initiating cells was also restored by YAP overexpression (Figure 3E, F and Supplementary Figure 2A and 2B). In addition, the inhibitory effect of baicalin on the expression of OCSCs markers was also abolished by YAP expression as indicated by qRT-PCR and Western blot (Figure 3G and H). Collectively, these results demonstrated that YAP inhibition is necessary for baicalin inhibiting the stemness of OC cells.
Baicalin Inhibits RASSF6 at the Transcriptional Level
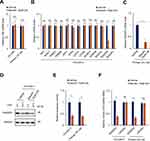
We next investigated the mechanisms underlying baicalin regulating YAP activity. As mentioned above, we found that baicalin increases the phosphorylation level of YAP, which indicated that activating Hippo signaling is a prime mechanism underlying baicalin inhibiting YAP. However, this result could not exclude the probability that baicalin may inhibit the transcription of YAP. We thus tested the mRNA level of YAP in baicalin-treated OC cells. As shown in Figure 4A, there is no significant difference in mRNA level between baicalin-treated and non-treated OC cells, which excluded the probability that baicalin regulates YAP at the mRNA level.
To investigate the mechanism underlying baicalin regulating Hippo signaling, we then performed qRT-PCR array to reveal the exact target of baicalin in Hippo signaling pathway. As shown in Figure 4B, we found that baicalin significantly decreased the mRNA level of RASSF6 in OVCAR-3 cells, which indicated that RASSF6 is the potential target of baicalin in Hippo signaling pathway. This result was further confirmed with primary OC cells (Figure 4C).
Next, we investigated the mechanisms underlying baicalin regulating RASSF6. We first investigated whether or not baicalin regulates the stability of RASSF6. To achieve this, we employed a translation inhibitor, cycloheximide (CHX), which inhibits protein synthesis in eukaryotic cells by interfering with the translocation step. The cells were incubated in complete culture medium containing 10 μM of CHX for 4h to inhibit protein synthesis of the cells followed by the addition of 15 μM of baicalin or an equal volume of vehicle and culture for 24 h. The RASSF6 protein levels in cells before and after treatment were examined by Western blot. As shown in Figure 4D, there are no obvious differences between the half-life of RASSF6 protein in baicalin- and vehicle-treated cells, which indicated that baicalin does not affect the stability of RASSF6 protein.
Next, we investigated whether or not baicalin inhibits RASSF6 at the transcriptional level. We employed luciferase reporter assay to determine the transcriptional activity of RASSF6 promoter and a modified nuclear run-on assay to examine the transcription of RASSF6 gene. As shown in Figure 4E, luciferase reporter assay showed that the activity of RASSF6 promoter was significantly inhibited by baicalin treatment. In addition, results from the modified nuclear run-on assay showed that the transcription of RASSF6 was significantly suppressed by baicalin (Figure 4F). Taken together, these results demonstrated that baicalin inhibits RASSF6 at the transcriptional level in OC cells.
Overexpression of RASSF6 Abolished the Inhibitory Effect of Baicalin on the Stemness of OC Cells
To further confirm that RASSF6 is the target of baicalin in Hippo signaling pathway, the RASSF6-overexpressing cells were established (Figure 5A). Similar with YAP overexpression, RASSF6 overexpression abolished the inhibitory effect of baicalin on the proliferation (Figure 5B), sphere-forming capacity (Figure 5C), the frequency of sphere-forming (Figure 5D and Supplementary Figure 3A) and tumor-initiating (Figure 5E and Supplementary Figure 3B) cells as well as the expression of OCSCs markers (Figure 5F), which demonstrated that RASSF6 is the target of baicalin inhibiting the stemness of OC cells.
In summary, the above results demonstrated that baicalin suppresses the stemness of OCSCs by attenuating YAP activity via suppressing RASSF6 at the transcriptional level.
Discussion
OC is still the leading cause of women’s death worldwide.27 OCSCs were recognized as the root of initiation and progression of ovarian cancer,12,13 however, the strategies eradicating OCSCs are still limited. In this study, we investigated the effect of baicalin, a single compound developed from traditional Chinese herbal, on the stemness of OC cells and revealed that YAP inhibition by transcriptionally inhibiting RASSF6 is the prime mechanism underlying baicalin attenuating OC stemness. This funding thus provides a novel therapeutic strategy for human OC.
The development of methods for eradicating OCSCs is important for OC therapy. CSCs have been recognized as the only subpopulation of tumor cells with the capability of initiation of tumors.6–11 Similar with CSCs and normal stem cells, OCSCs possess the ability to self-renew and differentiate into multiple lineages of differentiated cells.12,13 Thus, methods of eradicating OCSCs would hold the promise to lead to OC cure.
Several mechanisms involved in CSCs maintenance have been revealed in the past decade; the key pathways associated with CSCs include canonical Wnt signaling, Hedgehog signaling, canonical Notch signaling as well as Hippo/YAP singling pathway.25,26 A set of molecules against these pathways have been developed, including PRI-72427 (Dishevelled inhibitor), LGK97428 (Porcupine inhibitor), Vantictumab29 (Anti-Frizzled 7 receptor) and Ipafricept30 (Fc-Frizzled 8 receptor) for Wnt signaling, SMO inhibitor (GDC-0449,31 Sonidegib,32 and Glasdegib33), 5E134 and Genistein35 (Gli1 inhibitor) for Hedgehog signaling and Gamma secretase inhibitor (RO4929097,36 LY900009,37 and PF-0308401438), anti-DLL4 antibody (Enoticumab39 and Demcizumab40) and Tarextumab41 (anti-Notch 2/3) for Notch signaling. Most of them are undergoing Phase I clinical trials for safety evaluation. However, to eradicate CSCs, these compounds are facing challenges. First, CSCs are more resistant to traditional cytotoxic drugs; traditional chemotherapy may promote disease progression through CSCs selection. Second, as the resistance and relatively fewer number of CSCs, CSCs eradication with non-selective cytotoxic drugs may lead to serious side-effects. Thus, the clinically used safety dose of traditional cytotoxic drugs may not be enough for eradicating CSCs.
To achieve the goal of eradicating CSCs, natural products have recently received attention because of their long-term effect and relatively fewer side effects.17 Baicalin is a flavonoid that is isolated from the roots of Scutellaria baicalensis Georgi and is widely used as an alternative remedy in TCM for several diseases, indicating its relatively fewer side effects.14–16 More importantly, baicalin has recently been found both in vitro and in vivo anti-cancer activities in various types of cancer, including OC, which showed its enormous potential as an anti-cancer drug,14–16 however, its effect on OCSCs has remained elusive. In this study, we found that baicalin inhibits the spherogenicity (Figure 1D), the sphere-forming (Figure 1E and Supplementary Figure 1A) and tumor-initiating (Figure 1F and Supplementary Figure 1B) capacities as well as the expression of OCSCs markers (Figure 1G and H), which demonstrated the inhibitory effect of baicalin on the stemness of OC cells. This finding suggests the potential application of baicalin for eradicating OCSCs.
YAP has been shown to be an important player for CSCs maintenance. YAP activation induces CSC properties in several types of human cancer cells.19,20 Moreover, YAP expression is necessary for sustaining the self-renewal capacity of cancer cells.21 Furthermore, YAP upregulates key transcription factors, such as SOX9, to promotes the stemness of cancer cells. Thus, YAP is an important target of anti-cancer drug development.22 The prime upstream regulator of YAP is Hippo signaling pathway, which serves as a negative regulator that phosphorylates YAP and promotes its degradation in cytoplasm.19–22 Several YAP inhibitors have developed, such as verteporfin,21 blocking the binding between YAP and TEAD, and Statins,22 preventing nuclear translocation of YAP. In this study, we demonstrated that baicalin serves a novel YAP inhibitor that attenuates the stemness of OC cells. Baicalin inhibits the expression of YAP target genes (Figure 2) and YAP overexpression abolished the inhibitory effect of baicalin on the stemness of OC cells (Figure 3 and Supplementary Figure 2). Furthermore, YAP inhibits RASSF6 at the transcriptional level (Figure 4) and overexpression of RASSF6 also abolished the effect of baicalin on the stemness of OC cells (Figure 5). These results demonstrated that baicalin inhibits RASSF6, promotes YAP degradation, and thereby suppressing the stemness of OC cells and suggested that baicalin possesses the potential to develop an anti-cancer drug as a YAP inhibitor.
Conclusion
In summary, the results in this study demonstrated that baicalin suppresses the stemness of ovarian cancer cells by attenuating YAP activity via inhibiting RASSF6 at the transcriptional level. This finding revealed baicalin as a novel YAP inhibitor that could serve as an anti-cancer drug for eradicating ovarian cancer stem cells.
Disclosure
The authors have no conflicts of interest in this work.
References
1. Matulonis U, Sood A, Fallowfield L, et al. Ovarian cancer. Nat Rev Dis Primers. 2016;2:16061. doi:10.1038/nrdp.2016.61
2. Karnezis AN, Cho KR, Gilks CB, et al. The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nat Rev Cancer. 2017;17(1):65–74. doi:10.1038/nrc.2016.113
3. Oza AM. Advances in prediction for ovarian cancer treatment stratification. Nat Rev Clin Oncol. 2019;16(2):75–76. doi:10.1038/s41571-018-0147-3
4. Liu J, Matulonis UA. New strategies in ovarian cancer: translating the molecular complexity of ovarian cancer into treatment advances. Clin Cancer Res. 2014;20(20):5150–5156. doi:10.1158/1078-0432.CCR-14-1312
5. Dao F, Schlappe BA, Tseng J, et al. Characteristics of 10-year survivors of high-grade serous ovarian carcinoma. Gynecol Oncol. 2016;141(2):260–263. doi:10.1016/j.ygyno.2016.03.010
6. Nassar D, Blanpain C. Cancer stem cells: basic concepts and therapeutic implications. Annu Rev Pathol. 2016;11:47–76. doi:10.1146/annurev-pathol-012615-044438
7. Henkin RI. Clinical and therapeutic implications of cancer stem cells. N Engl J Med. 2019;381(10):e19. doi:10.1056/NEJMc1908886
8. Nguyen L, Vanner R, Dirks P, et al. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–143. doi:10.1038/nrc3184
9. Lytle NK, Barber A, Reya T. Stem cell fate in cancer growth, progression and therapy resistance. Nat Rev Cancer. 2018;18:669–680. doi:10.1038/s41568-018-0056-x
10. Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65(8):3025–3029. doi:10.1158/0008-5472.CAN-04-3931
11. Motohara T, Masuda K, Morotti M, et al. An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene. 2019;38(16):2885–2898. doi:10.1038/s41388-018-0637-x
12. Zhang S, Cui B, Lai H, et al. Ovarian cancer stem cells express ROR1, which can be targeted for anti-cancer-stem-cell therapy. Proc Natl Acad Sci U S A. 2014;111(48):17266–17271. doi:10.1073/pnas.1419599111
13. Wang L, Xu T, Cui M. Are ovarian cancer stem cells the target for innovative immunotherapy? Onco Targets Ther. 2018;11:2615–2626. doi:10.2147/OTT.S155458
14. Liu H, Dong Y, Gao Y, et al. The fascinating effects of baicalein on cancer: a review. Int J Mol Sci. 2016;17(10):
15. Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35(1):57–68. doi:10.1016/j.ctrv.2008.09.005
16. Gong WY, Zhao ZX, Liu BJ, et al. Exploring the chemopreventive properties and perspectives of baicalin and its aglycone baicalein in solid tumors. Eur J Med Chem. 2017;126:844–852. doi:10.1016/j.ejmech.2016.11.058
17. Avila-Carrasco L, Majano P, Sánchez-Toméro JA, et al. Natural plants compounds as modulators of epithelial-to-mesenchymal transition. Front Pharmacol. 2019;10:715. doi:10.3389/fphar.2019.00715
18. Cordenonsi M, Zanconato F, Azzolin L, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147(4):759–772. doi:10.1016/j.cell.2011.09.048
19. Moya IM, Halder G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol. 2019;20(4):211–226. doi:10.1038/s41580-018-0086-y
20. Zanconato F, Cordenonsi M, Piccolo S. YAP and TAZ: a signalling hub of the tumour microenvironment. Nat Rev Cancer. 2019;19(8):454–464. doi:10.1038/s41568-019-0168-y
21. Ramos A, Camargo FD. The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 2012;22(7):339–346. doi:10.1016/j.tcb.2012.04.006
22. Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18(12):758–770. doi:10.1038/nrm.2017.87
23. Lombardo Y, Giorgio AD, Coombes CR, Stebbing J, Castellano L. Mammosphere formation assay from human breast cancer tissues and cell lines. J Vis Exp. 2015;22(97). doi:10.3791/52671
24. Roberts TC, Hart JR, Kaikkonen MU, Weinberg MS, Vogt PK, Morris KV. Quantification of nascent transcription by bromouridine immunocapture nuclear run-on RT-qPCR. Nat Protoc. 2015;10(8):1198–1211. doi:10.1038/nprot.2015.076
25. Takebe N, Miele L, Harris PJ, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12(8):445–464. doi:10.1038/nrclinonc.2015.61
26. Dandawate PR, Subramaniam D, Jensen RA, Anant S. Targeting cancer stem cells and signaling pathways by phytochemicals: novel approach for breast cancer therapy. Semin Cancer Biol. 2016;40–41:192–208. doi:10.1016/j.semcancer.2016.09.001
27. Okazaki H, Sato S, Koyama K, et al. The novel inhibitor PRI-724 for Wnt/β-catenin/CBP signaling ameliorates bleomycin-induced pulmonary fibrosis in mice. Exp Lung Res. 2019;45(7):188–199. doi:10.1080/01902148.2019.1638466
28. Yang J-M, Huang H-M, Cheng J-J, et al. LGK974, a PORCUPINE Inhibitor, Mitigates Cytotoxicity in an in Vitro Model of Parkinson’s Disease by Interfering With the WNT/β-CATENIN Pathway. Toxicology. 2018;410:65–72. doi:10.1016/j.tox.2018.09.003
29. Lindsey Davis S, Cardin DB, Shahda S, et al. A phase 1b dose escalation study of Wnt pathway inhibitor vantictumab in combination with nab-paclitaxel and gemcitabine in patients with previously untreated metastatic pancreatic cancer. Invest New Drugs. 2020;38(3):821–830. doi:10.1007/s10637-019-00824-1
30. Moore KN, Gunderson CC, Sabbatini P, et al. A phase 1b dose escalation study of ipafricept (OMP54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecol Oncol. 2019;154(2):294–301. doi:10.1016/j.ygyno.2019.04.001
31. Robarge KD, Brunton SA, Castanedo GM, et al. GDC-0449-a potent inhibitor of the hedgehog pathway. Bioorg Med Chem Lett. 2009;19(19):5576–5581. doi:10.1016/j.bmcl.2009.08.049
32. Jain S, Song R, Xie J. Sonidegib: mechanism of action, pharmacology, and clinical utility for advanced basal cell carcinomas. Onco Targets Ther. 2017;10:1645–1653. doi:10.2147/OTT.S130910
33. Goldsmith SR, Lovell AR, Schroeder MA. Glasdegib for the treatment of adult patients with newly diagnosed acute myeloid leukemia or high-grade myelodysplastic syndrome who are elderly or otherwise unfit for standard induction chemotherapy. Drugs Today. 2019;55(9):545–562. doi:10.1358/dot.2019.55.9.3020160
34. Maun HR, Wen X, Lingel A, et al. Hedgehog pathway antagonist 5E1 binds hedgehog at the pseudo-active site. J Biol Chem. 2010;285(34):26570–26580. doi:10.1074/jbc.M110.112284
35. Spagnuolo C, Russo GL, Orhan IE, et al. Genistein and cancer: current status, challenges, and future directions. Adv Nutr. 2015;6(4):408–419. doi:10.3945/an.114.008052
36. Lee SM, Moon J, Redman BG, et al. Phase 2 study of RO4929097, a gamma-secretase inhibitor, in metastatic melanoma: SWOG 0933. Cancer. 2015;121(3):432–440. doi:10.1002/cncr.29055
37. Pant S, Jones SF, Kurkjian CD, et al. A first-in-human Phase I study of the oral notch inhibitor, LY900009, in patients with advanced cancer. Eur J Cancer. 2016;56:1–9. doi:10.1016/j.ejca.2015.11.021
38. Wei P, Walls M, Qiu M, et al. Evaluation of selective gamma-secretase inhibitor PF-03084014 for Its antitumor efficacy and gastrointestinal safety to guide optimal clinical trial design. Mol Cancer Ther. 2010;9(6):1618–1628. doi:10.1158/1535-7163.MCT-10-0034
39. Chiorean EG, LoRusso P, Strother RM, et al. A Phase I first-in-human study of enoticumab (REGN421), a fully human delta-like ligand 4 (Dll4) monoclonal antibody in patients with advanced solid tumors. Clin Cancer Res. 2015;21(12):2695–2703. doi:10.1158/1078-0432.CCR-14-2797
40. McKeage MJ, Kotasek D, Markman B, et al. Phase IB trial of the anti-cancer stem cell DLL4-binding agent demcizumab with pemetrexed and carboplatin as first-line treatment of metastatic non-squamous NSCLC. Target Oncol. 2018;13(1):89–98. doi:10.1007/s11523-017-0543-0
41. Hu ZI, Bendell JC, Bullock A, et al. A randomized Phase II trial of nab-paclitaxel and gemcitabine with tarextumab or placebo in patients with untreated metastatic pancreatic cancer. Cancer Med. 2019;8(11):5148–5157. doi:10.1002/cam4.2425
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.