Back to Journals » Infection and Drug Resistance » Volume 12
Baicalin Attenuates Mycoplasma gallisepticum-Induced Inflammation via Inhibition of the TLR2-NF-κB Pathway in Chicken and DF-1 Cells
Authors Wu Z, Chen C, Miao Y, Liu Y, Zhang Q, Li R, Ding L, Ishfaq M, Li J
Received 20 September 2019
Accepted for publication 10 December 2019
Published 20 December 2019 Volume 2019:12 Pages 3911—3923
DOI https://doi.org/10.2147/IDR.S231908
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Zhiyong Wu,1,* Chunli Chen,1,* Yusong Miao,1 Yuhao Liu,1 Qiaomei Zhang,1 Rui Li,1 Liangjun Ding,1 Muhammad Ishfaq,1 Jichang Li1,2
1College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, People’s Republic of China; 2Heilongjiang Key Laboratory for Animal Disease Control and Pharmaceutical Development, Harbin 150030, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Muhammad Ishfaq; Jichang Li
College of Veterinary Medicine, Northeast Agricultural University, 600 Changjiang Road, Xiangfang District, Harbin 150030, People’s Republic of China
Tel +86 451 5519 0674
Fax +86 451 5519 1200
Email [email protected]; [email protected]
Background: Previous reports demonstrated that baicalin possesses potential anti-inflammatory properties. The present study was conducted to determine the effects of baicalin against inflammatory responses in chicken and DF-1 cells infected with Mycoplasma gallisepticum (MG).
Methods: An MG infection model was developed in chickens to study the anti-inflammatory mechanism of baicalin. Baicalin was mixed in water at a dose of 450 mg/kg per day, and the treatment is continued for 7 consecutive days. Samples were taken at 1, 4, and 7 days post treatment.
Results: By using transmission electron microscopy, ultrastructure of lung and tracheal cells has been examined. It can be seen that the cilia cells in the MG-infected group have pyknosis, degeneration, and necrosis. In the lung tissues, alveolar type-I epithelial cells were severely damaged. In the baicalin-treated group, cilia were swollen, mushroom-shaped edema bubbles formed on the apex, and fused together. Alveolar type I epithelial cells injury was significantly reduced. Compared to MG-infection group, the levels of proinflammatory cytokines IL-1β and TNF-α were significantly decreased (P < 0.01). The corresponding proteins TLR2 and P-p65 decreased in the baicalin-treated group after 1 (p > 0.05), 4 (p < 0.05), and 7 days (p < 0.05), respectively.
Conclusion: The results showed that baicalin can interfere with inflammatory injury by suppressing the release of inflammatory cytokines IL-1β and TNF-α during MG infection both in vivo and in vitro. Meanwhile, baicalin suppressed TLR2-NFκB signaling pathway by inhibiting the phosphorylation of p65 and IκB, thereby affecting the expression of inflammatory factors. The results suggested that baicalin acts as a potential anti-inflammatory agent against MG infection in chicken and DF-1 cells.
Keywords: Mycoplasma gallisepticum, baicalin, inflammation, TLR2, p65
Introduction
Mycoplasma gallisepticum (MG) is widely distributed throughout the world and causes huge economic losses to the poultry industry.1 MG is considered as the most economically important pathogen of the four pathogenic Mycoplasma species due to its economic losses.2 Current methods of controlling MG infections among avian species, include bio-surveillance practices, vaccines, and medication.3
Mycoplasma treatment has also been restricted due to antibiotic resistance, drug residues, and side effects.4 While, the active ingredients of traditional Chinese medicine have been proven to have a variety of biological effects, which not only significantly inhibit the pathogenic microorganisms but also improve the body’s immunity.5 The effect of traditional Chinese medicine on MG has made extensive progress in pharmacodynamics, but the relevant mechanism is still unclear.6 Baicalin is one of the most common monomers of these traditional Chinese medicine.7 Previous reports demonstrated that baicalin possess potential anti-inflammatory properties.8,9 Therefore, baicalin is the major active constituent isolated from the root of Scutellaria baicalensis10 and was used as an anti-inflammatory agent against MG infection in this study.
Ying et al provide evidence11 that MG lipid-associated membrane proteins (LAMPs) activate IL-1β production through the NF-κB pathway via TLR2 and MyD88. Tian Wei found both TLR2-2 and TLR6 were upregulated upon MG infection, followed by upregulation of the downstream NF-κB-mediated inflammatory responses.12 Guo et al demonstrated the anti-inflammatory effects of baicalin in a mouse model against Staphylococcus aureus-induced mastitis.13 The results showed that baicalin reduced the expression of the proinflammatory cytokines IL-1β, TNF-α, and IL-6 by inhibiting NF-κB and p38 phosphorylation. However, it is unknown whether baicalin can play a similar role in MG infection. Thus, this study was conducted to determine the effects of baicalin against inflammatory responses in chicken and in DF-1 cells infected with MG and to better understand the underlying mechanism of action of baicalin.
Materials and Methods
Ethics Approval Statement
The present study was conducted under the approval of Institutional Animal Care and Use Committee of Northeast Agricultural University (Heilongjiang province, China) (SYXK (Hei) 2012–2067) in accordance with Laboratory animal-Guideline for ethical review of animal welfare (GB/T 35892–2018, National Standards of the People’s Republic of China).
Mycoplasma Strains, Drug, and Chickens
The MG strain Rlow was obtained from Harbin Institute of Veterinary Medicine, Chinese Academy of Agricultural Science. The mycoplasma were cultured at 37°C in modified Hayflicks medium supplemented with 20% FBS (Gibco BRL), 10% freshly prepared yeast extract, 0.05% Penicillin, 0.05% thallium acetate, and 0.1% Nicotinamide adenine dinucleotide (NAD).14 MG, in its mid-exponential phase indicated by the color change of phenol red dye from red to orange, which was used to challenge chickens at a density of 1×109 CCU/mL (color change unit per milliliter) in the culture medium.15 Baicalin powder (purity 98.0%) was kindly supplied by the Huifeng Animal Health Co., Ltd. (LOT: RH128699, Heilongjiang, China). Eighty one-day-old commercial Leghorn chickens were obtained from Chia Chau Chicken Farm (Harbin, Heilongjiang, China). The chickens were in healthy conditions, MG and Mycoplasma synoviae-free, and did not undergo vaccination. They were fed with antibacterial-free balanced ad libitum feed and provided free access to ultra-pure water. They were raised to 1 week under controlled conditions at 25°C.
Cell Culture
Chicken fibroblast cell lines (DF-1 cells) were obtained from Harbin Institute of Veterinary Medicine, Chinese Academy of Agricultural Science. The cells were cultured in Dulbecco’s Modified Eagles Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 100 IU/mL penicillin–100 μg/mL streptomycin at 37°C under a humidified air with 5% CO2. DF-1 cells were seeded in 6-well plates at a concentration of 1×107 cells/well and cultured in an incubator at 37 °C with 5% CO2 for at least 12 hrs prior to exposure to MG. The cell vitality was assessed by MTT assay as described previously.16 Significant cell death was observed at a dose greater than 500 MOI (MOI =0:107–5 × 109:107, mycoplasma: cell).17 The cells were divided into six experimental groups and treated with baicalin at the following concentrations: 0, 5, 10, 20, 40, and 60 μg/mL. The cell viability were assessed by MTT assay. To choose the dose of baicalin, DF-1 cells in 12-well plates were infected with MG (MOI = 500). The cells were treated with baicalin (15 μg/mL) for 0, 6, 12, 24, and 48 hrs, respectively. The cell vitality was assessed by MTT assay.
Experimental Grouping
DF-1 cells were divided into 5 groups including control group (control), MG infection group (MG), MG + baicalin low-dose group (low), MG + baicalin medium-dose group (medium), and MG + baicalin high-dose group (high). The final concentrations of baicalin used for subsequent experiments are 5, 10, and 15 μg/mL. Cells were cultured at 37°C for 24 hrs, and the cell supernatant was collected. The levels of TNF-α and IL-1β were detected by enzyme-linked immunosorbent assay (ELISA) kits.
MG Infection Model and Experimental Protocols
After pre-experiments, the model was determined according to the development of MG infection course. Briefly, we injected 7-day-old chickens with 0.2 mL of rejuvenated MG Rlow at a concentration of approximately 1×109 color change units per milliliter (CCU/mL) in logarithmic phase into the bilateral air sacs of the thoracic region. The infection model established in this way have clinical symptoms on the fifth day and reach the peak of infection on the seventh day post-infection. Eighty chickens were divided randomly into 4 groups as follows:
(1) Control group (CG): Chickens in the CG were fed only with basal diet and were direct air sac inoculation with the culture medium 0.2 mL on day 7.
(2) MG-infected control group (MGG): The MG infection model was constructed one time by direct air sac inoculation with 1×109 CCU/mL MG R strain 0.2 mL on day 7.
(3) MG infection + baicalin administration group (MG + BG): The same infection model as MGG, then treated with baicalin mixed water, given orally by gavage. The treatment started on day 10 and continued for a week, once in a day at a dose of 450 mg/kg.18
(4) Baicalin control group (BG): The same dose of baicalin (450 mg/kg) was given orally to chickens by gavage started at day 10 and continued for a week.
Each group provided randomly with 20 chickens and housed in 4 positive pressure fiberglass isolators. The measurement was determined according to the preliminary pre-test results of the laboratory. In our preliminary experiments (Supplementary material 1), the results obtained from the single dose of 450 mg/kg is similar to the recommended dose of tylosin tartrate (Jilin Yigefeng Animal Pharmaceutical Co., Ltd.).
On days 11, 14, and 17 (1, 4, and 7 days post treatment), 6 chickens from each group were sacrificed by cardiac puncture method following euthanasia with sodium pentobarbital as described previously.19
Gross and Histopathological Examination of Lesions
The severity of the gross air sac lesions was scored on a scale of 0–3, using a modification of a system as described previously.20 One centimeter of trachea proximal to the middle part was stored in buffered 10% formalin for histology. The tracheal mucosal thickness was measured in the middle trachea at four different points transected by a vertical and a horizontal line to determine the mean mucosal thickness at each level. The tracheal lesion score was determined from the same three discontinuities of the trachea. The tracheal infection score was also calculated on the basis of 0 to 3 scoring system published by Kirkpatrick, Mahmoudian.21 All scoring systems and the tracheal mucosal thickness were determined by a pathologist unaware of the experimental conditions.
Transmission Electron Microscopy
Transmission electron microscopy was performed as described in a previous study (Zhang et al, 2015). Briefly, lung tissue and the middle part of the trachea were trimmed into small pieces of 1 mm3 and fixed overnight in 2.5% glutaraldehyde. They were washed with PBS twice and post-fixed in 1% osmium tetroxide at 4°C for 1 hr. Next, the tissues were dehydrated by ethanol series and 100% acetone, embedded in epoxy resins. The ultrathin sections were stained with uranyl acetate and lead citrate and then observed under a GEM-1200ES transmission electron microscope (JEOL Ltd., Tokyo, Japan).
Serology
Blood samples were collected from all chickens on day 11, 14, and 17 (1, 4, and 7 days post treatment) of the trial. Serum antibody against MG in each bird was assessed by serum plate agglutination (SPA) assay using freshly collected sera and stained MG agglutination antigen purchased from the China Institute of Veterinary Drug Control (Beijing, China). Results were scored on a scale of 0–4 according to the size of clumps and the clarity of the background. Meanwhile, the serum was assayed for TNF-α and IL-1β levels by using ELISA kits in accordance with the manufacturer’s instructions (Shanghai Enzyme-linked Biotechnology Co., Ltd.).
Real-Time PCR
Real-Time PCR (RT-PCR) was performed to analyze gene expression using a Biosystem 7500 Real-Time PCR System thermocycler (Roche) and Taq SYBR Green qPCR Premix (Beijing Tianxiruizhi Science and Technology Co., Ltd.). Total RNA was extracted using TRI reagent, and 1 μg of RNA was reverse transcribed with All-in-one First-Stand Synthesis MasterMix. Oligo nucleotide primers (Table 1) for GAPDH, IL-1β and TNF-α were designed by Sangon Biotech (Shanghai, China), based on the sequences available in NCBI database. Each sample was analyzed in triplicate. The fold change in gene expression was calculated using the △△cycle time (Ct) method after the expression level was normalized with GAPDH gene taken as internal standard.
 |
Table 1 Sequences of the Oligo Nucleotide Primers Used for RT-PCR |
Western Blot
The cells were washed twice with cold PBS and 100 mg lung tissues, then total proteins were prepared in radio-immunoprecipitation assay (RIPA) lysis buffer containing 0.5 mM PMSF, respectively. The mixture was homogenized for 2 mins at a low frequency of 65 Hz using an automatic sample rapid grinding machine (Shanghai Jingxin Industrial Development Co., Ltd.), followed by high speed centrifugation (12,000 × g for 30 mins) at 4°C. Then, the supernatant protein content was determined by using BCA protein assay kit. Equivalent amounts of protein were separated in a 10–15% gel by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) run on 120 v for 1 hr, and then transferred onto nitrocellulose (NC) membranes run on 120 v for 90 mins.22 After blocking in 5% non-fat dried milk-TBST (25 mM Tris-HCl, 150 mM NaCl, 0.1% Tween-20; pH 7.4) at room temperature for 1 hr, the membranes were incubated overnight on a shaker at 4°C with primary antibodies against: GAPDH (1:200 dilution, Boster Biological Technology Co. Ltd); TLR2 (1:300 dilution, Absin Bioscience Inc. Shanghai, China); p65 (1:1000 dilution); P-p65 (1:1000 dilution); P-IκB (1:1000 dilution); IκB (1:1000 dilution); and MyD88 (1:1000 dilution, Bioss Bioscience Inc. Beijing, China). Secondary anti-mouse or anti-rabbit IgG peroxidase was used for 1 hr, and then bound immune-complexes were detected using enhanced chemiluminescence (ECL) detection.
Statistical Analysis
Data are presented as mean results ± standard deviation (SD). The significance was determined using one-way ANOVA followed by LSD and Dunnett’s T3 test. The data were analyzed using the GraphPad Prism (version 5.01). Values (p ≤ 0.05) were considered as statistically significant.
Results
Histopathological Changes and SPA Score
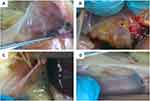
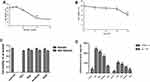
The histopathological changes in the air sacs of each group were examined. No pathological changes were observed in CG and BG (Figure 1A and D). In MGG, the air sacs showed cloudy thickening and more than several yellowish foci, and there were only cloudy appearance and several yellowish foci in MG + BG (Figure 1B and C). Tracheal tissues were harvested at 1, 4, and at 7 days after baicalin treatment. Tracheal sections were subjected to hematoxylin and eosin staining. The MGG showed the tracheal mucosal thickening, cilia stick together, or even fall off residual loss, epithelial cell proliferation, and a large number of lymphocytes infiltrated in the submucosa (Figure 2A and B). While, the lesion in MG + BG reduced significantly, there were slight thickening of the mucosa due to diffuse lymphocytic infiltration (Figure 2A and C). No changes were observed in CG and BG (Figure 2A and D). From the results of SPA score, it can be seen that MGG and MG + BG showed statistical differences after 7 days post-treatment. The SPA score of MG + BG reduced significantly, indicating that baicalin has certain therapeutic effects. Gross air sac lesions were similar to SPA scores and showed significant differences 7 days after treatment. The score of the MG + BG decreased after 7 days of administration, indicating that the therapeutic effect was achieved (Figure 3A).
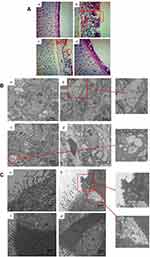
Ultrastructural Observation of Lungs and Trachea
In order to observe the effect of baicalin on MG infection from the ultrastructural level of cells, the lung and tracheal tissues were selected from each group for transmission electron microscopy 7 days after the treatment of baicalin mixed water. In the trachea, we can observe that the ciliated cells of the MGG have ruptured cilia, inverted, cytoplasmic swelling, decreased matrix electron density, and formation of umbrella-like protrusions outside the cell; partial ciliated cells are necrotic; mitochondria, rough endoplasmic reticulum expansion, even complete vacuolization; goblet cell reactive hypertrophy. Baicalin partially ameliorated the abnormal pathological changes in the chickens trachea infected with MG compared to MGG (Figure 2C). In the transmission electron microscopic observation of lung tissue, we found that the cytoplasm of the alveolar type I epithelial cells in MGG was broken and the basement membrane was exposed. In addition, the alveolar type II epithelial cells were damaged and the cytoplasmic vacuoles were formed. While, the degree of lesions in MG + BG was significantly reduced (Figure 2B).
Effect of Baicalin on Inflammatory Cytokines in Lung Tissues and Cells After Treatment
In order to determine the effects of baicalin and/or MG infection on inflammatory markers such as TNF-α and IL-1β, we measured the level of TNF-α and IL-1β in serum and cells supernatant. The results showed that compared with CG, an increase in the expression of explosive inflammatory factors (P < 0.01) has been noted in MGG, and the expression of inflammatory factors decreased significantly 7 days post-treatment. Compared with the MGG, the expression levels of inflammatory factors IL-1β and TNF-α in the MG + BG and the BG were significantly lower (P < 0.01) (Figure 3B and C). After 4 days of treatment (7 days after MG infection), the mRNA expression levels of IL-1β and TNF-α were significantly increased in the MGG compared with other groups, and the MG + BG was significantly different (P < 0.01). It has been noted that the expression of inflammatory factors in the infected control group was significantly decreased (P < 0.05) 7 days post-treatment. After DF-1 cells infected with MG, the secretion levels of TNF-α and IL-1β were significantly increased (p < 0.05) compared to control group. While, the levels of TNF-α and IL-1β were significantly (p < 0.05) decreased with baicalin-treatment in a dose-dependent manner (Figure 5D).
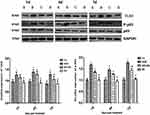
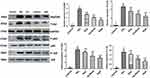
Baicalin Suppressed TLR2 and P65 Expression in the Lung Tissues
Baicalin significantly inhibited the rapid increase of TLR2 expression in MGG (P < 0.05). The results showed that baicalin can inhibit the expression of TLR2 and prevent the activation of its downstream signaling pathway in MG infection as shown in Figure 4. In this study, the expression of p65 protein was detected by Western blotting to analyze the activation of NF-κB signaling pathway. The results showed that the phosphorylation level of p65 was significantly increased in the MGG but was significantly reduced in the MG + BG (P < 0.05) (Figure 4).
Effect of Baicalin on Cell Viability
After incubation with various concentrations of baicalin (0–60 mg/mL), the viability of cells was determined by MTT method. Baicalin reduced cell viability in a dose- and time-dependent manner (Figure 5A and B). Cells exposed to 20 μg/mL of baicalin for 24 hrs exhibited 88.78% cells viability. In order to investigate the effect of baicalin and MG on DF-1 cells viability, the relative cell viability was above 80% after treatment with baicalin at the concentrations of 5, 10, and 20 μg/mL, and there was no significant difference among the three concentrations (Figure 5C). Thus, baicalin at concentrations of 5, 10, and 20 μg/mL were used for subsequent experiments.
Effect of Baicalin on the TLR2-NF-κB Pathway in DF-1 Cells
Compared to the control group, MG infection significantly increased the expression of TLR2, MyD88, P-p65, and P-IκB in DF-1 cells (p < 0.05). The expression of P-p65 and P-IκB in the treatment group was significantly reduced than that in the MG-infected group (p < 0.05) in a concentration-dependent manner. From these results, it has been suggested that baicalin exerts anti-inflammatory effects through the inhibition of NF-κB pathway during MG infection (Figure 6).
Discussion
Previous studies established MG infection model by various methods, such as nasal drops, intratracheal inoculation, air sac inoculation, mixed infection, immunodeficiency models, and so on.21,23 In order to select a suitable model for drug therapy, our laboratory selected four infection methods for comparative experiments. The method was to construct a model of 7-day-old chicks by air sac inoculation with a concentration of 1×109 CCU/mL (0.2 mL) of MG R strain. Therefore, the necropsy samples were taken at 4, 7, and 10 day post infection. In the study of baicalin, researchers found that baicalin can reduce inflammatory cell infiltration24 and inhibit the expression of TLR2 receptor in Staphylococcus aureus mastitis.13,25,26 A preliminary study in our lab found that the Chinese medicinal compound “Man Hu Kang” could also alleviate the inflammatory damage caused by MG infection, and the active ingredients of the compound were confirmed to be baicalin and chlorogenic acid.27
The cytokines produce more inflammatory factors through the intercellular transfer and amplification of the alveolar type I epithelial cells, resulting in a waterfall-like burst of inflammation.28 Among them, IL-1β and TNF-α are the main inflammatory factors, and both of them could activate more inflammatory factors by binding to cell membrane surface receptors.29 Compared with the MG + BG, it can be clearly observed that the release of inflammatory factors decreased significantly after treatment with baicalin. Our findings indicate that baicalin can reduce the damage caused by excessive secretion of inflammatory factors in chicken and DF-1 cells infected with MG. According to the results of pathological clinical manifestations (gross air sac lesion score, tracheal lesion score) and SPA score, it can be found that the tissue damage of the target organ can be reduced and the degree of lesion is significantly reduced after 7 days of treatment with baicalin and found statistical significance.21 MG infection caused mitochondrial damage, rough endoplasmic reticulum disruption, and even vacuolization of the trachea. A large myelin-like structure is common in the cytoplasm, and the ciliated cells are condensed, degenerated, shedded, and necrotic. In the lung tissues, the alveolar type I epithelial cells are severely damaged. The alveolar type II epithelial cells proliferate and form vacuoles between the cells. The MG + BG mainly showed cilia swelling, mushroom-like edema at the top, cilia fusion, and the damage of alveolar type I epithelial cells was significantly reduced. This finding indicated that baicalin had a protective effect on lung tissues.
In recent years, some breakthroughs have been made in the research on the mechanism of MG infection. These advances are important for elucidating the activation of signaling pathways during MG infection. MG-derived lipid-associated membrane proteins (LAMPs) activate NF-κB through TLR2 in a MyD88-dependent pathway in DF-1 cells.9 Recently, Wu et al (2018) reported the preventive effects of baicalin against atherosclerosis through the suppression of oxidative stress and inflammatory responses via NF-κB and MAPK signaling pathway.30 Daniele found that higher-ordered oligomeric α-synuclein induced a proinflammatory microglial phenotype by directly engaging the heterodimer TLR2 at the cell membrane, leading to the nuclear translocation of NF-κB and the increased production of the proinflammatory cytokines TNF-α (tumor necrosis factor-α) and IL-1β (interleukin-1β) in a MyD88-dependent manner.31 Tian reported that TLR2-2 and TLR6 play important roles in MG-induced inflammatory response in chicken embryos.12 Wang et al demonstrated that M. bovis-derived LAMPs activate IL-1β production through NF-kB pathway via TLR2 and MyD88.32 In the present study, MG induced inflammatory responses through activating TLR2-NF-kB signaling pathway. Our in vitro data showed that MG induced inflammatory responses by activating TLR2, MyD88, P-p65 and P-IκB, and baicalin could interfere with the activation of TLR2-NF-kB signaling pathway. Besides NF-κB signaling pathway, further molecular studies are needed to investigate the effect of baicalin on MAPKs, such as JNK and p38 signaling pathway.
NF-κB is a group of important transcription factors involved in many immune processes.33 NF-κB initiates the expression of inflammatory factors in innate immunity.34 In the classical activation pathway of NF-κB, the upstream signal causes activation of the IκK protein kinase complex, promotes phosphorylation of IκB, and then IκB to be degraded by the proteasome. NF-κB is responsible for the phosphorylation of p65 into the nucleus involved in the transcriptional regulation of related genes (inflammatory factors) due to the loss of IκB inhibition.35 In this study, the expression of P-p65 and P-IκB protein was significantly increased after MG infection, while the expression level in baicalin treated group was relatively decreased. This indicated that baicalin could effectively interfere with the activation of NF-κB signaling pathway, reducing the phosphorylation of p65 into the nucleus, and reduce the release of inflammatory factors. In addition, Hong et al (2017) studied molecular docking approach and found that baicalin could bind to IκK and p65-RelA.36 Nevertheless, the exact mechanism of baicalin behind the reduction of p65 phosphorylation is still elusive.
As a receptor for cell recognition of LAMPs, the TLRs act as a response switch during the development of MG infection and can regulate subsequent acquired immune responses. The activation of most TLRs induces cellular immune responses, and then produces IL-1β, IL-6, TNF-α, and chemotactic cytokines.37 Our previous results showed that silencing of TLR2 substantially reduced MG-triggered autophagy in RAW264.7 cells and markedly reduced MG-induced extracellular regulated protein kinase (ERK) in macrophages.17 In the present study, we focused on the role of NF-κB signaling pathway induced by TLR2 involved in MG-induced inflammatory responses. Comparing the expression levels of TLR2-NFκB signaling pathway protein in the MGG, it can be seen that the TLR2 receptor receives negative regulation of certain cytokines throughout the course of MG infection, but the detailed molecular mechanism needs further investigation.
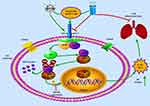
In conclusion, we demonstrated that the anti-inflammatory effects of baicalin (as shown in the schematic diagram (Figure 7) in a chicken model of MG. Baicalin reduced the expression of IL-1β and TNF-α, and alleviated the inflammatory injury caused by MG infection. Furthermore, the anti-inflammatory mechanism might be through interfering with the activation of TLR2-NFκB signaling pathway and by inhibiting the phosphorylation of p65 protein both in chicken lungs and DF-1 cells. Our findings may aid in better understanding of the complex mechanisms during host immune responses upon MG infection, and baicalin may be an effective drug to treat MG infection.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31973005 and 31772801), Project of Youth Innovative Talent Training Program in Heilongjiang Province (UNPYSCT-2018146), and Academic Backbone Project of Northeast Agricultural University (18XG23).
Disclosure
All the authors have no potential conflicts of interests regarding the publication of this manuscript.
References
1. Xie Z, Luo S, Deng X, et al. Development of a duplex real-time PCR for the rapid detection of virulent strain and avirulent vaccine strain of Mycoplasma gallisepticum.
2. Pflaum K, Tulman ER, Beaudet J, Liao X, Geary SJ. Global changes in Mycoplasma gallisepticum phase-variable lipoprotein gene vlhA expression during in vivo infection of the natural chicken host. Inf Immun. 2016;84(1):351. doi:10.1128/IAI.01092-15
3. Feberwee A, Banniseht-Wysmuller T, Vernooij JCM, Gielkens ALJ, Stegeman JA. The effect of vaccination with a bacterin on the horizontal transmission of Mycoplasma gallisepticum. Avian Pathol J Wvpa. 2006;35(1):35–37. doi:10.1080/03079450500465700
4. Mohammadi AR, Afshar M, Sohrabi S. The study of the effects of Tylosin Tartarat on control and treatment of the experimental infection with Mycoplasmagallisepticum in broiler chicks. Resuscitation. 2010;81(2):S96–S96.
5. Liu Y, Feng N. Nanocarriers for the delivery of active ingredients and fractions extracted from natural products used in traditional Chinese medicine (TCM). Adv Colloid Interface Sci. 2015;221:60. doi:10.1016/j.cis.2015.04.006
6. Kahya S, Önat K, Erköse E, Temelli̇ S, Eyi̇Gor A, Carlı KT. Effect of mentofin application on the clearance of Mycoplasma gallisepticum (MG) from naturally infected layer chickens’ trachea. Veteriner Faku¨ltesi Dergisi. 2015;62(1):17–21.
7. Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35(1):57–68. doi:10.1016/j.ctrv.2008.09.005
8. Zheng H, Tianming FU. A randomized parallel controlled study of acupuncture, moxibustion combined with traditional Chinese medicine internal and external use in the treatment of chronic eczema divided by syndrome differentiation. J Pract Traditional Chin Internal Med. 2016.
9. Yu Y, Pei M, Li L. Baicalin induces apoptosis in hepatic cancer cells in vitro and suppresses tumor growth in vivo. Int J Clin Exp Med. 2015;8(6):8958.
10. Gao Z, Huang K, Xu H. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol Res. 2001;43(2):173–178. doi:10.1006/phrs.2000.0761
11. Ying Y, Ying C, Yang W, Yuan L, Lin Z, Xin J. TLR2/MyD88/NF-κB signaling pathway regulates IL-1β production in DF-1 cells exposed to Mycoplasma gallisepticum LAMPs. Microb Pathog. 2018;117:225–231.
12. Tian W, Zhao C, Hu Q, Sun J, Peng X. Roles of Toll-like receptors 2 and 6 in the inflammatory response to Mycoplasma gallisepticum infection in DF-1 cells and in chicken embryos. Dev Compar Immunol. 2016;59:39. doi:10.1016/j.dci.2016.01.008
13. Guo M, Zhang N, Li D, et al. Baicalin plays an anti-inflammatory role through reducing nuclear factor-κB and p38 phosphorylation in S. aureus-induced mastitis. Int Immunopharmacol. 2013;16(2):125–130. doi:10.1016/j.intimp.2013.03.006
14. Cai HY, Bell-Rogers P, Parker L, Ferencz A, Pozder P. Development and field validation of a Mycoplasma iowae real-time polymerase chain reaction assay. J Vet Diagn Investigation Off Publ Am Assoc Vet Lab Diagnosticians Inc. 2008;20(2):230. doi:10.1177/104063870802000216
15. Lin MY, Kleven SH. Transferred humoral immunity in chickens to Mycoplasma gallisepticum. Avian Dis. 1984;28(1):79–87. doi:10.2307/1590130
16. Zhang L, Zhao Y, Ding W, et al. Autophagy regulates colistin-induced apoptosis in PC-12 cells. Antimicrob Agents Chemother. 2015;59(4):2189–2197. doi:10.1128/AAC.04092-14
17. Lu Z, Xie D, Chen Y, et al. TLR2 mediates autophagy through ERK signaling pathway in Mycoplasma gallisepticum-infected RAW264.7 cells. Mol Immunol. 2017;87:161–170. doi:10.1016/j.molimm.2017.04.013
18. Ishfaq M, Chen C, Bao J, et al. Baicalin ameliorates oxidative stress and apoptosis by restoring mitochondrial dynamics in the spleen of chickens via the opposite modulation of NF-kappaB and Nrf2/HO-1 signaling pathway during Mycoplasma gallisepticum infection. Poult Sci. 2019;98:6296–6310. doi:10.3382/ps/pez406
19. Muhammad I, Sun X, Wang H, et al. Curcumin successfully inhibited the computationally identified CYP2A6 enzyme-mediated bioactivation of aflatoxin B1 in arbor acres broiler. Front Pharmacol. 2017;8:143. doi:10.3389/fphar.2017.00143
20. Leigh SA, Branton SL, Evans JD, Collier SD. Effect of infection route and concurrent infectious bronchitis virus vaccination on Mycoplasma gallisepticum disease pathology in an experimental model. Avian Pathol. 2012;41(5):497–503. doi:10.1080/03079457.2012.721925
21. Wijesurendra DS, Kanci A, Tivendale KA, et al. Development of a Mycoplasma gallisepticum infection model in turkeys. Avian Pathol. 2015;44(1):35–42. doi:10.1080/03079457.2014.992390
22. Lu Z, Chen C, Wu Z, et al. A dual role of P53 in regulating colistin-induced autophagy in PC-12 cells. Front Pharmacol. 2017;8:768. doi:10.3389/fphar.2017.00768
23. Xiao X, Zhao DH, Yang X, et al. Mycoplasma gallisepticum and Escherichia coli mixed infection model in broiler chickens for studying valnemulin pharmacokinetics. J Vet Pharmacol Ther. 2014;37(1):99–102. doi:10.1111/jvp.12065
24. Chen YC, Shen SC, Chen LG, Lee JF, Yang LL. Wogonin, baicalin, and baicalein inhibition of inducible nitric oxide synthase and cyclooxygenase-2 gene expressions induced by nitric oxide synthase inhibitors and lipopolysaccharide. Biochem Pharmacol. 2001;61(11):1417–1427. doi:10.1016/S0006-2952(01)00594-9
25. Shi H, Ren K, Lv B, et al. Baicalin fromScutellaria baicalensisblocks respiratory syncytial virus (RSV) infection and reduces inflammatory cell infiltration and lung injury in mice. Sci Rep. 2016;6:35851. doi:10.1038/srep35851
26. Kim DH, Kim HK, Park S, et al. Short-term feeding of baicalin inhibits age-associated NF-κB activation. Mech Ageing Dev. 2006;127(9):719. doi:10.1016/j.mad.2006.05.002
27. Jichang LI, Shi Q, Pan X, Meng Q. Therapeutic test of manhukang oral solution against the artificially infected Mycoplasma gallisepticum. China Poultry. 2008;21.
28. Aye R, Mwirigi MK, Frey J, Pilo P, Jores J, Naessens J. Cyto-adherence of Mycoplasma mycoides subsp. mycoides to bovine lung epithelial cells. BMC Vet Res. 2015;11(1):1–9. doi:10.1186/s12917-015-0347-3
29. Li Z, Mao Z, Lin Y, et al. Dynamic changes of tissue factor pathway inhibitor type 2 associated with IL-1beta and TNF-alpha in the development of murine acute lung injury. Thromb Res. 2008;123(2):361–366. doi:10.1016/j.thromres.2008.03.019
30. Wu Y, Wang F, Fan L, et al. Baicalin alleviates atherosclerosis by relieving oxidative stress and inflammatory responses via inactivating the NF-kappaB and p38 MAPK signaling pathways. Biomed Pharmacother. 2018;97:1673–1679. doi:10.1016/j.biopha.2017.12.024
31. Daniele SG, Beraud D, Davenport C, Cheng K, Yin H, Maguire-Zeiss KA. Activation of MyD88-dependent TLR1/2 signaling by misfolded alpha-synuclein, a protein linked to neurodegenerative disorders. Sci Signal. 2015;8(376):ra45. doi:10.1126/scisignal.2005965
32. Wang Y, Liu S, Li Y, et al. Mycoplasma bovis-derived lipid-associated membrane proteins activate IL-1β production through the NF-κB pathway via Toll-like receptor 2 and MyD88. Dev Compar Immunol. 2016;55:111–118. doi:10.1016/j.dci.2015.10.017
33. Baldwin JA. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14(1):649. doi:10.1146/annurev.immunol.14.1.649
34. Poupko R, Müller K, Krieger C, Zimmermann H, Luz Z. The two NF-kappa B activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25(6):280. doi:10.1016/j.it.2004.03.008
35. Hu J, Haseebuddin M, Young M, Colburn NH. Suppression of p65 phosphorylation coincides with inhibition of IκBα polyubiquitination and degradation. Mol Carcinog. 2005;44(4):274. doi:10.1002/(ISSN)1098-2744
36. Hong C, Schuffler A, Kauhl U, et al. Identification of NF-kappaB as determinant of posttraumatic stress disorder and its inhibition by the chinese herbal remedy free and easy wanderer. Front Pharmacol. 2017;8:181. doi:10.3389/fphar.2017.00181
37. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. 2010;11(5):373. doi:10.1038/ni.1863
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.