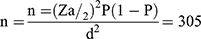Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Bacterial Profiles and Their Associated Factors of Urinary Tract Infection and Detection of Extended Spectrum Beta-Lactamase Producing Gram-Negative Uropathogens Among Patients with Diabetes Mellitus at Dessie Referral Hospital, Northeastern Ethiopia
Authors Alemu M, Belete MA , Gebreselassie S, Belay A, Gebretsadik D
Received 28 May 2020
Accepted for publication 29 July 2020
Published 19 August 2020 Volume 2020:13 Pages 2935—2948
DOI https://doi.org/10.2147/DMSO.S262760
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Mekuanent Alemu,1 Melaku Ashagrie Belete,2 Solomon Gebreselassie,3 Assefa Belay,1 Daniel Gebretsadik2
1Department of Medical Laboratory Science, Dessie Health Science College, Dessie 1212, Amhara, Ethiopia; 2Department of Medical Laboratory Science, College of Medicine and Health Sciences, Wollo University, Dessie 1145, Ethiopia; 3Department of Microbiology, Immunology and Parasitology, College of Health Sciences, Addis Ababa University, Addis Ababa 1176, Ethiopia
Correspondence: Melaku Ashagrie Belete
Department of Medical Laboratory Science, College of Medicine and Health Sciences, Wollo University, Dessie 1145, Ethiopia
Tel +251- 913867849
Fax +251 333115250
Email [email protected]
Purpose: To determine the bacterial profile with its associated risk factors and to identify extended spectrum beta-lactamase producing Gram-negative bacterial uropathogens among diabetic patients at Dessie Referral Hospital, Northeastern Ethiopia.
Materials and Methods: A hospital-based cross-sectional study was conducted from May to September 2018. A total of 336 diabetic patients were included using a simple random sampling technique. A structured questionnaire was used to collect socio-demographic and risk factor-related data. A 10-mL mid-stream urine specimen was collected and transported to the microbiology laboratory for culture, antimicrobial susceptibility testing, and detection of ESBL-producing bacteria. The data were entered into SPSS version 22, and descriptive statistics, bivariate and multivariate logistic regression analyses were performed. A p-value ≤ 0.05 with a 95% confidence interval was considered for statistical significance.
Results: Among 336 diabetic patients, the overall prevalence of UTI was 11.6%. The predominant bacterial isolate was Escherichia coli 12/39 (30.8%), followed by Klebsiella pneumoniae 11/39 (28.2%) and coagulase-negative staphylococci 7/39 (17.9%). Gram-negative isolates showed 100% resistance to ampicillin, whereas Gram-positive isolates showed a high level of resistance to penicillin and tetracycline. Moreover, MDR was observed among 18 (46.2%) of the isolates and 2 of the isolated Gram-negative bacteria were ESBL producers. Being illiterate (AOR=7.226, 95% CI: (1.478, 35.340), p< 0.015), having current symptoms of UTI (AOR = 2.702, 95% CI: (1.102, 6.624), p=0.030), and blood glucose level ≥ 126 mg/dl (AOR = 2.940, 95% CI: (1.080, 8.005), p=0.035) were significantly associated with the occurrence of bacterial UTI.
Conclusion: The overall prevalence of significant bacteriuria (11.6%) in this study was comparable with some studies in Ethiopia and relatively lower than others. A moderately higher rate of resistance to the commonly used antimicrobial agents was noticed for both Gram-negative and Gram-positive isolates. Health information dissemination should be given about UTI, glycemic control, and habit of drug use for diabetes mellitus patients.
Keywords: urinary tract infection, diabetes mellitus, bacterial profile, antimicrobial susceptibility pattern, risk factors, ESBL
Introduction
Diabetes mellitus (DM) is a metabolic disorder characterized by hyperglycemia mainly as a result of defects in insulin action, secretion, or both.1 Globally, an estimated 422 million adults were living with diabetes in 2014. Diabetes caused 1.5 million deaths in 2012. Higher-than-optimal blood glucose caused an additional 2.2 million deaths, by increasing the risks of cardiovascular and other diseases. The majority of people with diabetes are affected by type 2 diabetes. This used to occur nearly entirely among adults, but now occurs in children too.2 By 2030 about 550 million people are projected to be inflicted with the disease globally.1 A systematic review indicates the prevalence of diabetes in Ethiopia ranged from 2.0% to 6.5% with a low of 2% in smaller rural areas.3
Diabetes mellitus is associated with bladder dysfunction, glycosuria, and low immunity, all of which predispose an individual to urinary tract infection (UTI). The risk of developing UTI in diabetic patients is higher compared to non-diabetics.4,5 Diabetes patients are more susceptible to contracting UTI if glucose is not well controlled, diabetes has already attacked the nervous system (neuropathy), it occurs among women, the patient already had kidney or blood vessel complications from diabetes, or the patient has had a urinary tract infection within the last year.6–9
UTIs in diabetic patients may be symptomatic or asymptomatic bacteriuria, and are most commonly considered as intricate and complicated.10 Asymptomatic UTI is the most common form in diabetic patients and can lead to severe kidney damage and renal failure.5 The prevalence of UTI among DM patients has a varied proportion with symptomatic and asymptomatic conditions. Globally, it ranges from 12% to 40.2%911-13 and in Ethiopia its prevalence is between 10.9% and 22.6%.14–18 Several uropathogens have been identified in diabetic patients and the most common uropathogens isolated are Escherichia coli, Klebsiella spp., and Staphylococcus aureus.7,9,12,18 Due to different reasons, these bacterial uropathogens showed wide-ranging resistances to different classes of antibiotics across the world.6,7,1317–19
In diabetic patients, early diagnosis of UTI is essential for its proper management and to avoid the incidence of possible complications. However, in developing countries including Ethiopia, urine culture screening is not routinely done for diabetic follow-ups; and treatment is empirical, which may lead to the emergence and spread of antimicrobial-resistant strains which is a leading cause of treatment failure in UTI.20
One of the leading antimicrobial resistance mechanisms for many UTI-causing Gram-negative bacteria is extended spectrum β-lactamase (ESBL) enzyme production, which hydrolyzes the β-lactam ring of antimicrobials that confer bacterial resistance to commonly prescribed antibiotics, including penicillins, first, second and third-generation cephalosporins, and aztreonam.21 Delay in the detection and reporting of ESBL production by bacterial uropathogens is most commonly associated with a prolonged hospital stay, increased morbidity, mortality, and overwhelming health-care costs.22
There is a paucity of research addressing the etiologies, risk factors, and management of UTI in diabetic patients in most developing countries, and there is little information about the etiologies of UTI in Ethiopian diabetic patients, particularly in the study area where there are no published data. In addition, a recent global report has revealed that the overall epidemiology of ESBL-producing bacteria has not been well understood in resource-limited countries like Ethiopia. Therefore, this study aimed to fill this information gap.
Materials and Methods
Study Design, Area and Period
A hospital-based cross-sectional study was conducted from May to September 2018 at Dessie Referral Hospital, Northeastern, Ethiopia. Dessie is located at the latitude of 11°8′N and longitude of 39°38′E with an elevation between 2400 and 3200 meters above sea level and 401 km northeast of Addis Ababa. The city has one referral hospital, one general hospital (Boru-Meda), six health centers (Dessie, BanbuaWuha, Segno Gebeya, Tita, Kurkur, Meytero), four private hospitals, and several private clinics. The hospital catchment population is about 7 million.
Study Populations
The source population comprises all diabetic patients (inpatients and outpatients) at DRH. The study participants were adult diabetic patients at DRH Diabetic Clinic during the study period.
Inclusion and Exclusion Criteria
Diabetic patients with or without symptoms of UTI who were present at DRH Diabetic Clinic during the study period were included in this study, whereas diabetic patients who were severely ill and who did not give consent to participate in the study were excluded.
Sample Size Determination
A single population proportion formula was used to estimate sample size and the following assumption was considered: 95% confidence interval (Zα/2 = 1.96), 10.9% proportion from previous work,15 and 3.5% margin of error.
The calculated sample size was 305, but in order to compensate for inadequate sample specimens and minimize errors due to sample collection, it was decided to consider an additional 10% of the minimum sample size which made the final sample size 336.
Data and Specimen Collection
A structured questionnaire was used to obtain information about the study participants related to demographic characteristics clinical and risk factor data. About 10 mL freshly voided midstream urine sample was collected using a pre-labeled (date, time, and identification code), leak-proof, wide mouth, sterile, screw-capped plastic container (FL Medical, Italy) by the study participants after appropriate instructions were given.
Specimen Transportation
The collected specimens were immediately transported to Amhara Public Health Institute (APHI), Dessie branch using a cold box, and processed within 30 minutes. When a delay of more than 30 minutes was anticipated, urine specimens were kept refrigerated at 4°C until being processed.
Cultivation and Identification of Isolates
Using a calibrated wire loop (0.001 mL) mid-stream urine samples were inoculated into Cystine Lactose Electrolyte Deficient medium (CLED) (Oxoid Ltd, UK). After cultures were incubated overnight in an aerobic atmosphere at 37°C for 24 hours, colonies were counted to check for the presence of significant growth. Colony counts yielding bacterial growth of ≥105CFU/mL of urine were regarded as significant bacteriuria.23
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing (AST) of all identified bacterial isolates from significant bacteriuria specimens was performed according to the criteria of Clinical and Laboratory Standards Institute (CLSI) using the Kirby–Bauer disk diffusion method on Mueller–Hinton Agar. Pure culture colonies of 24-hour growth were suspended in a tube with 4 mL of physiological saline to get bacterial inoculums equivalent to 0.5 McFarland turbidity standards. A sterile cotton swab was dipped, rotated across the wall of the tube to avoid excess fluid, and was evenly inoculated on Mueller-Hinton agar (Conda Ltd, USA) and then the antibiotic disks were placed on MHA plates.23
The following antimicrobials were used based on the CLSI recommendations and local frequent prescriptions of these drugs for the treatment of UTIs. Penicillin (PEN, 10 µg), ciprofloxacin (CIP, 5 µg), tetracycline (TTC, 30 µg), nitrofurantoin (NIF, 300 μg), norfloxacin (NOR, 10 μg), trimethoprim-sulfamethoxazole (SXT, 25 μg) were used for Gram-positive isolates. Furthermore, ampicillin (AMP, 10 μg), amoxicillin-clavulanic acid (AMC, 20/10 μg), nitrofurantoin (NIF, 300 μg), norfloxacin (NOR, 10 μg), trimethoprim-sulfamethoxazole (SXT, 25 μg), ciprofloxacin (CIP, 5 µg), tetracycline (TTC, 30 µg), gentamicin (GN, 10 μg), ceftriaxone (CRO, 30 µg, amikacin (AM, 30 μg), cefotaxime (CTX, 30 µg), and ceftazidime (CAZ, 30 µg) were used for Gram-negative isolates. All antibiotic disks were from Oxoid, Ltd, UK. The plates were then incubated at 37°C for 24 hours. Diameters of the zone of inhibition around the disks were measured using a digital caliper. The interpretation of the results of the AST was based on the standardized table supplied by the national committee for CLSI criteria23 as sensitive, intermediate, and resistant.
Extended Spectrum Beta-Lactamase Detection
Initial screening for ESBL was done by the diameters of zones of inhibition produced by ceftriaxone (30 µg), ceftazidime (30 µg), or cefotaxime (30 µg) from the antimicrobial susceptibility test on Mueller–Hinton media (Conda Ltd) according to the CLSI screening criteria. These breakpoints indicative of suspicion for ESBL production were for ceftriaxone, ≤ 25 mm; for ceftazidime (30 µg), ≤ 22 mm; and for cefotaxime, ≤ 27 mm. After this initial screening, phenotypic detection of ESBL production was confirmed by double disk synergy (combined disk potentiate) test according to CLSI guidelines.23 The isolate to be tested was spread onto a Mueller–Hinton agar plate using similar procedures as for AST. Ceftazidime (30 µg), cefotaxime (30 µg), and amoxicillin-clavulanic acid (AMC) were used for phenotypic confirmation of the presence of ESBLs. Disks containing cephalosporin (cefotaxime, ceftriaxone, or ceftazidime) were applied next to a disk with clavulanic acid (amoxicillin + clavulanic acid), and after incubation at 37ºC for 24 hours, a positive result was indicated when the inhibition zones around any of the cephalosporin disks are augmented in the direction of the disk containing clavulanic acid. The distance between the disks is critical and 20 mm center-to-center has been found to be optimal for cephalosporin 30 μg disks; however, it may be reduced (15 mm) or expanded (30 mm) for strains with very high or low resistance level, respectively.23
Quality Assurance
All the questions in the structured questionnaire were prepared in a clear and precise way and translated into the local language (Amharic). Data collectors were trained; the entire questionnaires were checked for completeness, during and after data collection by the data collectors. Moreover, all laboratory assays were done by maintaining quality control procedures. Standard operating procedures (SOPs) were strictly followed, verifying that media meet expiration date and quality control parameters including sterility testing per CLSI guidelines. Reference strains of S. aureus (ATCC 25,923); E. coli (ATCC 25,922), and P. aeruginosa (ATCC 27,853) were used as a quality control for culture and susceptibility testing throughout the study. Moreover, E. coli (ATCC 25,922) and K. pneumoniae (ATCC 700,603) reference strains were used as quality control for ESBL detection. All reference strains were obtained from APHI, Dessie branch.
Statistical Analysis
The data were imported and analyzed using Statistical Package for Social Sciences (SPSS) version 22.0 (IBM, USA). Descriptive statistics, binary and multivariate logistic regressions were employed. Binary logistic regression was used to show the association of each variable with the dependent variable. Moreover, a multivariate analysis was computed to identify factors that independently influence the occurrence of dependent variables. A p-value <0.05 with a 95% confidence interval was considered statistically significant.
Ethics Approval and Consent to Participate
The study protocol was evaluated and approved by the Department Research Ethics Review Committee (DRERC/17/18/02-L) of Addis Ababa University College of Health Sciences, and ethical clearance was obtained. Official cooperation letters for DRH and APHI were obtained from Addis Ababa University. Additionally, after explaining the importance, purpose, and procedure of the study briefly a written consent was obtained from study participants. A parent or legal guardian provided written informed consent for any participant under the age of 18 years. Any study participant who found to be infected with the bacteria was referred to a physician for treatment. Moreover, this study was conducted in accordance with the Declaration of Helsinki.
Results
Socio-Demographic Characteristics
In this study, a total of 336 diabetic patients, of which 39 (11.6%) with and 297 (88.4%) without symptoms of UTI were included. From the total study participants, 181 (53.9%) were males and the age of the study participants ranged from 15 to 80 years, with a mean age of 41.0 ±15.3 years. The majority of them (33.9%) lived in urban areas and 74 (22%) were farmers (Table 1).
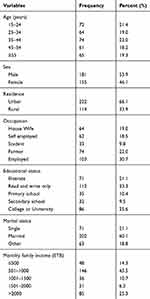 |
Table 1 Socio-Demographic Characteristics of the Study Participants (n=336) in DRH, Northeastern Ethiopia, 2018 |
Prevalence of Urinary Tract Infection and Bacterial Uropathogen Isolates
The overall prevalence of UTI was 11.6%. The prevalence of significant bacteriuria among asymptomatic and symptomatic diabetic patients was 28/297 (9.4%) and 11/39 (28.2%), respectively. Seven different bacterial species were isolated and there was no double bacterial infection. Gram-negative bacteria were more prevalent, 28 (71.8%), than Gram-positive bacteria, 11 (28.2%). Overall, the predominantly isolated bacteria were E. coli, 12 (30.8%) (Table 2).
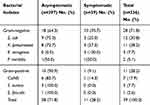 |
Table 2 Frequency of Bacterial Uropathogens of Symptomatic and Asymptomatic UTI Among Diabetic Patients at DRH, Northeastern Ethiopia, 2018 |
Among the three predominant uropathogens, K. pneumoniae was higher than E. coli only among DM patients whose age category is 36 to 45 years and E. coli was the only bacterial isolate in the age category of 46–55 years (Figure 1).
 |
Figure 1 Distribution of the three common bacterial uropathogens in each age category among diabetic mellitus patients at DRH, Northeastern Ethiopia, 2018. |
All bacterial uropathogens were detected among female DM patients whereas in male patients P. mirabilis and E. faecalis were not isolated. From the five bacterial isolates that were detected in both male and female, only S. aureus was more frequent in male patients (Figure 2)
 |
Figure 2 Distribution of bacterial uropathogens of symptomatic and asymptomatic UTI in relation to sex among diabetic patients at DRH, Northeastern Ethiopia, 2018. |
Antimicrobial Susceptibility Pattern of Bacterial Uropathogens
Gram-Negative Bacteria
In this study, besides the intrinsically resistant isolates of Klebsiella pneumoniae and Enterobacter aerogenes, the rest of the isolated Gram-negative bacterial uropathogens including Escherichia coli and Proteus mirabilis showed full acquired resistance rate (100%) for ampicillin. On the other hand, Gram-negative isolates showed high sensitivity to nitrofurantoin, 26 (92.9%), cefotaxime, 25 (89.3%), ciprofloxacin, 24 (85.7%), and amoxicillin-clavulanic acid, 24 (85.7%) (Table 3).
 |
Table 3 Antimicrobial Susceptibility Pattern of Gram-Negative Bacteria (n=28) Isolated from Urine Culture of Diabetic Patients (n=336) at DRH, Northeastern Ethiopia, 2018 |
Gram-Positive Bacteria
Gram-positive uropathogens showed a high level of resistance for penicillin and tetracycline. On the other hand, they showed high sensitivity to nitrofurantoin. Coagulase negative staphylococci (CoNS), which were the predominant isolates among the Gram-positive isolates, were resistant to penicillin, tetracycline, and norfloxacin, and sensitive to nitrofurantoin (Table 4).
 |
Table 4 Antimicrobial Susceptibility Pattern of Gram-Positive Bacteria (n=11) Isolated from Urine Culture Among Diabetic Patients (n=336) at DRH, Northeastern Ethiopia, 2018 |
Multidrug Resistance Patterns of the Isolates
Overall, 37 (94.9%) bacterial isolates were resistant to at least one antimicrobial agent, whereas 24 (61.5%) isolates were resistant to ≥2 antimicrobials. Multidrug resistance (MDR), which is defined as a bacterium that is non-susceptible to at least one agent in three or more antimicrobial categories,24 was seen in 18 (46.2%) of all isolated bacterial uropathogens (Table 5).
 |
Table 5 Multidrug Resistance Patterns of Bacterial Isolates (n=39) from Diabetic Patients (n=336) at DRH, Northeastern Ethiopia, 2018 |
ESBL-Producing Gram-Negative Uropathogens
Twenty-eight Enterobacteria were isolated from the total study participants. The isolates were E. coli, 12 (42.9%), K. pneumoniae, 11 (39.3%), E. aerogenes, 3 (10.7%), and P. mirabilis, 2 (7.1%). However, E. aerogenes, 3 (10.7%), and P. mirabilis, 2 (7.1%), were excluded from further screening for ESBL because the methods were not validated for these groups. As a result, out of the 23 Enterobacteria clinical isolates that were screened for ESBL production, E. coli, 5 (21.7%), and K. pneumoniae, 9 (39%), fulfilled the criteria for confirmatory test and 2 (8.7%) were positive for ESBL production. These ESBL producer isolates were K. pneumoniae.
Associated Risk Factors of UTI
In this study, 16 independent variables were considered during the bivariate analysis as risk factors for bacterial UTI. In multivariate analysis, educational status of illiterates (AOR = 7.226, 95% CI: (1.478, 35.340), p = 0.015), participants having high blood glucose level (AOR=2.940, 95% CI: (1.080, 8.005), p = 0.035) and participants showing current symptoms of UTI (AOR = 2.702, 95% CI: (1.102, 6.624), p = 0.030) were found to have statistically significant association with UTI (Table 6).
 |
Table 6 Bivariate and Multivariate Logistic Regression Analysis of Factors Associated with UTI Among Diabetic Patients (n=336) Attending in DRH, Northeastern Ethiopia, 2018 |
Discussion
In this study, the overall prevalence of urinary tract infection in both symptomatic and asymptomatic diabetic patients was 11.6%. Comparable findings have been reported in previous studies conducted in Addis Ababa (10.9%),15 Debre Tabor (10.9%),16 Hawassa (13.8%),25 and Romania (12.0%).9 However, this finding was relatively lower as compared to studies conducted in Harar (15.4%),14 Addis Ababa (14.9%),18 Gondar (17.8%),26 Metu, Ethiopia (16.7%),17 Nekemet, Ethiopia (16.5%),27 and Sudan (19.5%).13 It was much lower than reports from different parts of the world such as a study in Uganda (22.0%),28 Kuwait (35%),7 India (49.15%),29 and Nepal (54.76%).30 The variation might be explained by differences in geographical features, the host factor, and practices such as the social habits of the community, standards of personal hygiene, and health education practices.
The reported prevalence of symptomatic UTI among diabetic patients in this study was 28.2%. This was lower than studies conducted in Gondar (51.4%)26 and India (49.15%).29 However, it was relatively higher than the study performed in Addis Ababa (13.6%),15 Debre Tabor (19%),16 Hawassa (23.1%),25 Harar (20%),14 and Sudan (17.1%).13 On the other hand, the prevalence of asymptomatic UTI (9.4%) was comparable with studies conducted in Addis Ababa (10.4%),15 Hawassa (11.2%),25 and Harar (12.4%),14 but lower than similar study reports from Gondar (14.7%),26 Debre Tabor (80.9%),16 and Sudan (20.9%).13 Such variations might also be due to differences in risk factors with geographical areas, sample size, study population, and deployment of diverse methodologies.
Bacteriological studies usually revealed the involvement of Gram-negative enteric organisms such as E. coli, Klebsiella species, Enterobacter species, and Proteus species in causing UTI.17,31,32 Similarly, the predominant numbers of pathogens isolated in our study were Gram-negative bacilli (71.8%). Similarly, they were the dominant causative agent of UTIs in Harar, Gondar, rural South India, Iraq, Nepal, Sudan, and Kuwait.7,13,14,19,26,33,34 In the current study, E.coli was the most commonly grown organism, which is in agreement with other studies in different parts of the world.13,14,25,26,30,33 The high rate of E. coli might be due to the high abundance of E. coli in fecal flora, which ascends through genitalia to cause UTI.35 In addition, it might be due to numerous virulence factors used for colonization and invasion of the urinary epithelium such as P-fimbriae or pili adherence factors, which mediate the attachment of E. coli to uroepithelial cells.36 On the other hand, coagulase negative staphylococci was the third most common species identified in the present study whereas it was the predominant one in a study conducted in Nigeria.12
Gram-positive cocci play a lesser role in causing UTI. Among the patients infected with Gram-positive cocci in our study, CoNS, 7 (17.9%), was the predominant isolate, followed by S. aureus, 3 (7.7%). Similarly, other studies18,25 also indicated that CoNS species are more dominant than S. aureus.
Mainly due to the habit of empirical treatment, infrequent bacterial identification, and absence of susceptibility testing, antimicrobial resistance among bacterial uropathogens to the commonly used antibiotics has increased, leaving clinicians with very limited choices of drugs for the treatment of UTI. In this study, besides the intrinsically resistant isolates of Klebsiella pneumoniae and Enterobacter aerogenes, the rest of the isolated Gram-negative bacterial uropathogens including Escherichia coli and Proteus mirabilis showed the full acquired resistance rate (100%) for ampicillin. Similarly, a study conducted in Ethiopia indicated high bacterial resistance to the drug.18,25,27 This could be due to the overuse of the drug for many years and may be due to easy availability and low cost of the antibiotic. These factors are common in the study area where some patients buy drugs without prescription. On the other hand, higher rates of sensitivity were observed against nitrofurantoin, cefotaxime, ciprofloxacin, amoxicillin-clavulanic acid, norfloxacin, tetracycline, amikacin, and gentamycin. This higher resistance to antibiotics and even a high incidence of MDR Gram-negative bacterial isolates might be suggestive of developing chronic kidney diseases of DM patients.37
Gram-positive bacteria were relatively resistant to penicillin (63.6%) and tetracycline (36.4%). This might be due to the easy availability and indiscriminate use of commonly used drugs such as tetracycline and penicillin, which could lead to an increase in resistance. Conversely, most tested Gram-positive isolates showed sensitivity to nitrofurantoin (90.9%). This is comparable with other studies conducted in Hawassa, Addis Ababa, and in Arba Minch, Ethiopia.18,25,32 Our tested isolates also showed high sensitivity to trimethoprim-sulfamethoxazole (81.8%), which was not in agreement with studies conducted in Hawassa and in Arba Minch.25,32 Based on our findings, nitrofurantoin and trimethoprim-sulfamethoxazole can be drugs of choice for empiric treatment of UTIs, particularly among diabetic patients in the study area.
The high frequency of multiple antibiotic resistances might be a reflection of inappropriate use of antimicrobials, lack of laboratory diagnostic tests, and lack of guidelines for the selection of antibiotics. Multidrug resistance (non-susceptibility to at least one agent belonging to three or more antimicrobial categories) was observed in 18 (46.2%) of the total isolated bacteria in this study. This finding was lower than previous studies conducted in Debre Tabor (56.7%),16 Gondar (59.8%),26 Addis Ababa (71.7%)15 Harar (92.5%),14 and Hawassa (93.9%).25
In the present study, significant bacteriuria was significantly associated with current symptoms of UTI, but some previous studies in Gondar, Harar, Hawassa, and Debre Tabor reported no statistical association of this variable.14,16,25,26 The present study also revealed that hyperglycemia was positively associated with UTI. Up to 85% of the isolates were from participants with high blood glucose levels (≥126 mg/dL). This is supported by a previous study conducted in Gondar.26 The high UTI prevalence among hyperglycemic patients might be most likely due to poor contraction of a dysfunctional bladder leading to static urine pools; this, together with glycosuria, creates a suitable environment for bacterial growth. However, in some studies, the associations between UTI and blood glucose levels were not reported.38 This might be because a single blood glucose measurement may not represent glycemic control over time, which would predispose diabetic patients to UTI.
In this study, illiterate study participants had higher odds of getting UTI compared with those who are literate. Similar to our findings, other studies elsewhere have also reported lower levels of education as a risk factor for UTIs.27,39,40 This might be due to a lower level of awareness of illiterate diabetic patients on how to keep their general health, particularly their personal hygiene, and protect themselves from bacterial infection.39
The prevalence of Gram-negative ESBL producers in this study was 8.7%, which is observed only in K. pneumoniae. This might be due to K. pneumoniae being known to produce more ESBL gene than E. coli.37 Despite there being no documented reports on the occurrence of ESBL-producing bacterial uropathogens causing UTI, particularly among diabetic patients in Ethiopia, ESBL production was assessed in different setups and study groups. For instance, there were reports among pregnant women and among patients with urinary tract infection on studies conducted in Adama, Jimma, Mekelle, Addis Ababa, Ethiopia, and elsewhere like Tanzania and Nepal.22,41-46 In all these studies, similar to our study, Klebsiella species were the isolates that showed ESBL production. In addition, there is a study conducted among DM patients that showed K. pneumonia was the more dominant species in producing ESBL than E. coli.47 The ESBL-positive isolates in our study showed a high level of resistance to ampicillin (100.0%), trimethoprim-sulfamethoxazole (50%), ceftriaxone (50%), cefotaxime (100%), and ceftazidime (100%). This means that the use of these antibiotics for treatment of infection caused by ESBL-producing strains may result in treatment failure in a significant proportion of cases.48 Thus, the problem of ESBL-producing uropathogens is clinically important and yet remains relatively unappreciated by most clinicians. In the present study, E. coli does not produce ESBL, which is in contrast to a study conducted in India.8
Limitations of the Study
In this study, due to the unavailability of the test methods in the research laboratory setup, we were not able to further characterize the coagulase negative staphylococci up to species level. Moreover, the present study employed a health facility based convenient sampling technique which could not be generalizable for the total population in the area.
Conclusion
The overall prevalence of significant bacteriuria (11.6%) in this study was comparable with some studies in Ethiopia and relatively lower than others. The majority of the isolates were Gram-negative bacteria; E. coli and K. pneumoniae were identified as the two dominant isolates. Moderately higher rates of resistance to the commonly used antimicrobial agents were noticed for both Gram-negative and Gram-positive bacterial isolates. Moreover, a significant amount of ESBL producers and MDR among nearly half of the bacterial isolates has been indicated. Educational status, current symptoms of UTI, and blood glucose levels were significantly associated with the presence of UTI among diabetic patients. Health information dissemination should be given about UTI, glycemic control, and habit of drug use for DM patients. UTI screening for diabetic patients whose blood glucose level ≥126 mg/dL should be performed. UTI management among symptomatic DM patients should be supported by laboratory results of urine culture and AST. Further studies should be conducted by using highly sensitive and specific techniques such as PCR, including genotypic characterization in ESBL-producing bacteria causing UTI among diabetic patients in a larger sample size.
Abbreviations
AST, antimicrobial susceptibility testing; APHI, Amhara Public Health Institute; CLED, cysteine lactose electrolyte-deficient agar; CLSI, Clinical and Laboratory Standards Institute; CoNS, coagulase negative staphylococci; DM, diabetes mellitus; DRH, Dessie Referral Hospital; ESBL, extended spectrum beta-lactamase; MDR, multidrug resistance; SB, significant bacteriuria; UTI, urinary tract infection.
Acknowledgments
Our deepest appreciation and gratitude go to the friendly and welcoming staff members of APHI, Dessie branch for their unreserved expert support during the data collection and laboratory work. We would also like to thank all the data collectors and study participants for their cooperation during data collection; without their willingness, this thesis would not have been possible.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Federation ID, Atlas I; International Diabetes Federation. IDF Diabetes atlas.
2. World Health Organization. Global Report on Diabetes 2016. 2016. ISBN 978 92 4 156525 7.
3. Bishu KG, Jenkins C, Yebyo HG, Atsbha M, Wubayehu T, Gebregziabher M. Diabetes in Ethiopia: a systematic review of prevalence, risk factors, complications, and cost. Obes Med. 2019;15:100132. doi:10.1016/j.obmed.2019.100132
4. Walsh C, Collyns T. The pathophysiology of urinary tract infections. Surgery. 2017;35(6):293–298. doi:10.1016/j.mpsur.2017.03.007
5. Simkhada R. Urinary tract infection and antibiotic sensitivity pattern among diabetics. Nepal Med Coll J. 2013;15(1):1–4.
6. Bagir GS, Haydardedeoglu FE, Colakoglu S, Bakiner OS, Ozsahin KA, Ertorer ME. Urinary tract infection in diabetes: susceptible organisms and antibiogram patterns in an outpatient clinic of a tertiary health care center. Med Science. 2019.
7. Sewify M, Nair S, Warsame S, et al. Prevalence of urinary tract infection and antimicrobial susceptibility among diabetic patients with controlled and uncontrolled glycemia in Kuwait. J Diabetes Res. 2016.
8. Aswani SM, Chandrashekar U, Shivashankara K, Pruthvi B. Clinical profile of urinary tract infections in diabetics and non-diabetics. Australas Med J. 2014;7(1):29.
9. Chiţă T, Timar B, Muntean D, et al. Urinary tract infections in Romanian patients with diabetes: prevalence, etiology, and risk factors. Ther Clin Risk Manag. 2017;13:1.
10. Geerlings SE. Urinary tract infections in patients with diabetes mellitus: epidemiology, pathogenesis and treatment. Int J Antimicrob Agents. 2008;31:54–57. doi:10.1016/j.ijantimicag.2007.07.042
11. Shah MA, Kassab YW, Anwar MF, et al. Prevalence and associated factors of urinary tract infections among diabetic patients. Health Sci J. 2019;13(2):1–5.
12. Anejo-Okopi JA, Okojokwu OJ, Ramyil SM-C, et al. Bacterial and antibiotic susceptibility pattern of urinary tract infection isolated from asymptomatic and symptomatic diabetic patients attending tertiary hospital in Jos, Nigeria. Trends Med. 2017;17(1). doi:10.15761/TiM.1000108
13. Hamdan HZ, Kubbara E, Adam AM, Hassan OS, Suliman SO, Adam I. Urinary tract infections and antimicrobial sensitivity among diabetic patients at Khartoum, Sudan. Ann Clin Microbiol Antimicrob. 2015;14(1):26. doi:10.1186/s12941-015-0082-4
14. Abate D, Kabew G, Urgessa F, Meaza D. Bacterial etiologies, antimicrobial susceptibility patterns and associated risk factors of urinary tract infection among diabetic patients attending diabetic clinics in Harar, Eastern Ethiopia. East Afr Med J Health Biomed Sci. 2017;1(2):11–20.
15. Yeshitela B, Gebre-Selassie S, Feleke Y. Asymptomatic bacteriuria and symptomatic urinary tract infections (UTI) in patients with diabetes mellitus in tikur anbessa specialized University Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2012;50(3):239–249.
16. Worku S, Derbie A, Sinishaw MA, Adem Y, Biadglegne F. Prevalence of bacteriuria and antimicrobial susceptibility patterns among diabetic and nondiabetic patients attending at Debre Tabor Hospital, Northwest Ethiopia. Int J Microbiol. 2017;2017:1–8. doi:10.1155/2017/5809494
17. Gutema T, Weldegebreal F, Marami D, Teklemariam Z. Prevalence, antimicrobial susceptibility pattern, and associated factors of urinary tract infections among adult diabetic patients at Metu Karl Heinz Referral Hospital, Southwest Ethiopia. Int J Microbiol. 2018;2018:1–7. doi:10.1155/2018/7591259
18. Woldemariam HK, Geleta DA, Tulu KD, et al. Common uropathogens and their antibiotic susceptibility pattern among diabetic patients. BMC Infect Dis. 2019;19(1):43. doi:10.1186/s12879-018-3669-5
19. Al-Qaseer A, Abdul-wahab B, Abbas O. Bacteriological finding of urinary tract infection in diabetic patients. Int J Adv Res. 2014;2(10):274–279.
20. Krishnakumar S, Rajan RA, Babu MM, Bai VDM. Antimicrobial susceptibility pattern of extended spectrum of beta lactamase (ESBL) producing uropathogens from pregnant women. Indian J Med Healthcare. 2012;1(8):189–192.
21. Kateregga JN, Kantume R, Atuhaire C, Lubowa MN, Ndukui JG. Phenotypic expression and prevalence of ESBL-producing Enterobacteriaceae in samples collected from patients in various wards of Mulago Hospital, Uganda. BMC Pharmacol Toxicol. 2015;16(1):14. doi:10.1186/s40360-015-0013-1
22. Thapa R, Lamichhane P, Banjara MR, Acharya GP. Prevalence of extended spectrum beta lactamase producing uropathogens in pregnant women. Asian J Pharm Clin Res. 2015;8(1):207–210.
23. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 27th Ed. CLSI Supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2017.
24. Magiorakos AP, Srinivasan A, Carey R, et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
25. Nigussie D, Amsalu A. Prevalence of uropathogen and their antibiotic resistance pattern among diabetic patients. Turk J Urol. 2017;43(1):85. doi:10.5152/tud.2016.86155
26. Yismaw G, Asrat D, Woldeamanuel Y, Unakal CG. Urinary tract infection: bacterial etiologies, drug resistance profile and associated risk factors in diabetic patients attending Gondar University Hospital, Gondar, Ethiopia. Euro J Exp Bio. 2012;2(4):889–898.
27. Kebamo S, Dabso R, Deressa A, Gebrie M. Urinary tract infection: bacterial etiologies, drug resistance profile and associated risk factors among diabetic patients attending Nekemte Referral Hospital, Ethiopia. Am J Curr Microbiol. 2017;5(1):19–31.
28. Nabaigw BI, Mwambi B, Okiria J, Oyet C. Common uropathogens among diabetic patients with urinary tract infection at Jinja Regional Referral Hospital, Uganda. Afr J Lab Med. 2018;7(1):1–3.
29. Sharma V, Gupta V, Mittal M. Prevalence of uropathogens in diabetic patients and their antimicrobial susceptibility pattern. Natl J Lab Med. 2012;1(1):26–28.
30. Kumar Jha P, Baral R, Khanal B. Prevalence of uropathogens in diabetic patients and their susceptibility pattern at a tertiary care center in Nepal-a retrospective study. Int J Bio Lab Sci. 2014;3:29–34.
31. Janifer J, Geethalakshmi S, Satyavani K, Viswanathan V. Prevalence of lower urinary tract infection in South Indian type 2 diabetic subjects. Indian J Nephrol. 2009;19(3):107. doi:10.4103/0971-4065.57107
32. Mama M, Manilal A, Gezmu T, Kidanewold A, Gosa F, Gebresilasie A. Prevalence and associated factors of urinary tract infections among diabetic patients in Arba Minch Hospital, Arba Minch province, South Ethiopia. Turk J Urol. 2019;45(1):56. doi:10.5152/tud.2018.32855
33. Pragash DS, Girija S, Sekar U, Rayapu V, Sheriff D. Uropathogens and diabetes mellitus-a perspective. IOSR J Dent Med Sci. 2017;16(5):29–32. doi:10.9790/0853-1605052932
34. Kumar D, Singh AK, Ali MR, Chander Y. Antimicrobial susceptibility profile of extended spectrum β-lactamase (ESBL) producing Escherichia coli from various clinical samples. Infect Dis. 2014;7:S13820.
35. Wiles TJ, Kulesus RR, Mulvey MA. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp Mol Pathol. 2008;85(1):11–19. doi:10.1016/j.yexmp.2008.03.007
36. Mulvey MA. Adhesion and entry of uropathogenic Escherichia coli. Cell Microbiol. 2002;4(5):257–271. doi:10.1046/j.1462-5822.2002.00193.x
37. Majeed HT, Aljanaby AAJ. Antibiotic susceptibility patterns and prevalence of some extended spectrum beta-lactamases genes in gram-negative bacteria isolated from patients infected with urinary tract infections in Al-Najaf City, Iraq. Avicenna J Med Biotechnol. 2019;11(2):192.
38. Alebiosu C, Osinupebi O, Olajubu F. Significant asymptomatic bacteriuria among Nigerian type 2 diabetics. J Natl Med Assoc. 2003;95(5):344.
39. Alo M, Saidu A. Prevalence and antbiogram of bacterial isolates causing urinary tract infections at federal teaching hospital Abakaliki I (FETHA I). Microbiol Res J. 2015;403–417.
40. Mubarak AA, Ashraf AM, El-hag M, et al. Prevalence of urinary tract infections among diabetes mellitus and non-diabetic patients attending a teaching hospital in Ajman, UAE. Gulf Med J. 2012;1(S1):s228–s232.
41. Mulisa G, Selassie L, WT JG, Shiferew T, Zewdu A. Prevalence of extended spectrum beta-lactamase producing Enterobacteriaceae: a cross sectional study at Adama hospital, Adama, Ethiopia. J Emerg Infect Dis. 2016;1(1):1–6.
42. Mulualem Y, Kasa T, Mekonnen Z, Suleman S. Occurrence of extended spectrum beta (b)-lactamases in multidrug resistant Escherichia coli isolated from a clinical setting in Jimma university specialized hospital, Jimma, southwest Ethiopia. East Afr J Public Health. 2012;9(2):58–61.
43. Moyo SJ, Aboud S, Kasubi M, Lyamuya EF, Maselle SY. Antimicrobial resistance among producers and non-producers of extended spectrum beta-lactamases in urinary isolates at a tertiary Hospital in Tanzania. BMC Res Notes. 2010;3(1):348. doi:10.1186/1756-0500-3-348
44. Legese MH, Weldearegay GM, Asrat D. Extended-spectrum beta-lactamase-and carbapenemase-producing Enterobacteriaceae among Ethiopian children. Infect Drug Resist. 2017;10:27. doi:10.2147/IDR.S127177
45. Shakya P, Shrestha D, Maharjan E, Sharma VK, Paudyal R. ESBL production among E. coli and Klebsiella spp. causing urinary tract infection: a hospital based study. Open Microbiol J. 2017;11(1):23. doi:10.2174/1874285801711010023
46. Gebremariam G, Legese H, Woldu Y, Araya T, Hagos K, GebreyesusWasihun A. Bacteriological profile, risk factors and antimicrobial susceptibility patterns of symptomatic urinary tract infection among students of Mekelle University, northern Ethiopia. BMC Infect Dis. 2019;19(1):950. doi:10.1186/s12879-019-4610-2
47. Al Yousef SA, Younis S, Farrag E, Moussa HS, Bayoumi FS, Ali AM. Clinical and laboratory profile of urinary tract infections associated with extended spectrum β-lactamase producing Escherichia coli and Klebsiella pneumoniae. Ann Clin Lab Sci. 2016;46(4):393–400.
48. Lee SY, Kotapati S, Kuti JL, Nightingale CH, Nicolau DP. Impact of extended-spectrum β-lactamase–producing Escherichia coli and Klebsiella species on clinical outcomes and hospital costs: a matched cohort study. Infect Control Hosp Epidemiol. 2006;27(11):1226–1232.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.