Back to Journals » Clinical Interventions in Aging » Volume 15
Bacterial Flora of the Nose and Paranasal Sinuses Among Patients Over 65 Years Old with Chronic Rhinosinusitis Who Underwent Endoscopic Sinus Surgery
Authors Leszczyńska J, Stryjewska-Makuch G , Ścierski W, Lisowska G
Received 16 May 2019
Accepted for publication 4 January 2020
Published 14 February 2020 Volume 2020:15 Pages 207—215
DOI https://doi.org/10.2147/CIA.S215917
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Joanna Leszczyńska,1 Grażyna Stryjewska-Makuch,1 Wojciech Ścierski,2 Grażyna Lisowska2
1Department of Laryngology and Laryngological Oncology, Upper Silesian Medical Centre of Silesian Medical University, Katowice, Poland; 2Department of Otorhinolaryngology and Laryngological Oncology, Medical University of Silesia, Zabrze, Poland
Correspondence: Joanna Leszczyńska
Department of Laryngology and Laryngological Oncology, Upper Silesian Medical Centre of Silesian Medical University, ul. Ziołowa 45/47, Katowice 40-635, Poland
Tel/Fax +48 32 359 80 00
Email [email protected]
Purpose: Chronic rhinosinusitis (CRS) is one of the most common chronic diseases in the geriatric population. However, CRS inflammatory mechanisms in older people have not been thoroughly investigated. Our work aimed to analyze the bacterial flora of the nose and paranasal sinuses in patients with CRS over 65 years of age, including comorbidities, previously performed endoscopic sinus surgery (ESS), presence or absence of polyps and the extent of the inflammatory process.
Patients and Methods: The study involved 529 patients between 18 and 84 years of age with chronic rhinosinusitis who underwent endoscopic sinus surgery. There were 101 patients separated over 65 years of age (M = 52, K = 49, mean age 69 ± 0.7 years). The control group consisted of 168 patients aged 18– 40 years with CRS. The bacterial culture of material collected from the patients during ESS was analyzed.
Results: In the group of patients over 65 years of age, more frequent occurrence of Proteus spp. and Pseudomonas aeruginosa was found in comparison to younger patients. In older patients with bronchial asthma, the occurrence of S. aureus, Escherichia coli, and Citrobacter spp. was more frequent than in control group. Multiple sinus surgical procedures in older patients were associated with the dominance of Staphylococcus aureus and Escherichia coli, which was not demonstrated in the control group. There were no statistically significant differences between the occurrence of bacterial strain and the presence of polyps, both in the group of patients over 65 years of age as well as in the control group.
Conclusion: The bacterial flora of patients with CRS is different in older and younger patients. A different therapeutic approach should be considered in older patients with CRS, but this problem requires further studies.
Keywords: sinusitis in elderly, aging, sinus culture, bacterial sinusitis, endoscopic sinus surgery
Introduction
In developed countries, a constant aging process is observed. Also in Poland, there is a similar phenomenon resulting from the extension of life expectancy. Currently, the number of people over 65 years of age constitutes about 15% of the entire Polish population.1 Chronic rhinosinusitis (CRS) is one of the most common inflammatory diseases affecting about 5–15% of the general population in Europe and the USA.2 It is estimated that CRS is the sixth most common chronic disease in the geriatric population.3,4 A Korean epidemiological study involving 4098 patients showed the incidence of CRS increased dramatically between 50 and 59 years old, and then doubled after 60 years (compared to young patients between the ages of 19 and 39).5 This disease significantly reduces the quality of patients’ life by causing the deterioration of their overall health and fitness in comparison with the general population. This results in an increase in health-care costs. Despite the large group of patients and the interest of clinicians, still little is known about the etiology, course, and results of CRS treatment in patients over 65 years. According to the guidelines of the European position paper on rhinosinusitis and nasal polyps 2012, the occurrence of nasal blockage and/or nasal discharge, which may be accompanied by facial pain and/or reduction or loss of smell lasting at least 12 weeks—or along with other symptoms—is the basis for the diagnosis of CRS.2 The presence or absence of nasal polyps is a traditional method of phenotyping patients with CRS into two groups: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP). Polyps can arise via different pathophysiological mechanisms and may be primarily associated with eosinophil or neutrophil domination in inflammatory infiltration.6,7
When analyzing CRS in the elderly population, the natural aging of the immune system should be considered. The mechanisms of this process are complex and concern, among others, hemopoietic defects of the bone marrow, dysfunction of peripheral lymphocyte migration, maturation, and function. Due to thymic involution, the activity of T and B cells decreases, and the production of antibodies directed against their own tissues and proinflammatory cytokines increases. The activity of dendritic cells, neutrophil and macrophage decreases, which results in greater susceptibility to infections. In the population, an increased incidence of allergic rhinitis, bronchial asthma, and skin allergies in people over 60 years of age is observed. It is estimated that in patients over 65, there are, at least, three chronic diseases, and often five.8,9 In older people, reduced immunity to infections and the presence of comorbidities may result in a more severe clinical course of CRS. So far, the relationship between the type and amount of the microorganism and the severity of CRS has not yet been precisely defined.
The Aims
The aim of the study was to assess in patients with CRS after 65 years of age the following:
- Bacterial flora in patients who underwent endoscopic sinus surgery compared to a younger group of patients.
- Relationship between the type of bacterial flora, the severity of chronic inflammatory process and the extent of endoscopic surgery.
- Influence of comorbidities (bronchial asthma, IgE-dependent allergy, hypersensitivity to non-steroidal anti-inflammatory drugs [NSAIDs]) on bacterial flora
- Bacterial flora in patients operated for the first time and repeatedly.
- Bacterial flora in patients with CRSwNP and CRSsNP.
Patients and Methods
Clinical study covered 529 patients (M = 55%, K = 45%, age 18 to 84 years, mean age = 49.8 ± 14.6 years) with chronic rhinosinusitis who underwent ESS in the Department of Laryngology and Laryngological Oncology in Katowice and in the Department of Otorhinolaryngology and Oncology in Zabrze of the Silesian Medical University in Katowice. From this group, 101 patients after 65 years of age were distinguished (M = 52, K = 49, mean age 69 ± 0.7 years) where 82 patients had CRSwNP and 19 CRSsNP. The control group consisted of 168 patients aged 18–40 years (M = 95, K = 73 mean age 32.4 ± 5.7 years) with CRS (68 patients with CRSwNP, 100 patients with CRSsNP). The remaining patients (260 people from the age range 41–64) were excluded from further study. All participants provided written informed consent, and this study was conducted in accordance with the Declaration of Helsinki.
The exclusion criteria were as follows:
- Age under 18 years of age.
- Treatment with antibiotics and systemic steroids less than 4 weeks prior to the ESS.
- Active infection.
- Congenital and acquired immunodeficiency, cystic fibrosis.
- Neoplastic process of the nose and paranasal sinuses.
- Fungal sinusitis.
The patients were qualified for surgical treatment after laryngological examination, endoscopy and computed tomography of the paranasal sinuses. All patients had collected microbiological material from the medial nasal meatus during endoscopic sinus surgery.
The material was collected with a swab stick with AMIES substrate. For the growth of aerobic bacteria, the sheep blood base was used—agar Columbia (Gram-positive bacteria), MacConkey base (Gram-negative bacteria), Sabouraud base (fungi growth), chocolate in the environment with increased carbon dioxide content (Neisseria and Haemophilus cultures). Anaerobic bacteria were grown on Schedler’s base. Each culture was kept at 35–37°C for 24–48 hrs, except for fungi and bacteria on Schaedler’s medium, where cultivation was carried out for 7 days. During the culture, the material remaining on the stick for 7 days was stored in the propagating base (cardio-cerebral broth). Identification and susceptibility were assessed using the Vitek 2 compact. Mycosis of the sinuses was diagnosed on the basis of histopathological examination. Microbiological tests were performed by the laboratory of the Medical University according to accepted procedures common for Upper Silesian Medical Centre. Bacterial colonies were counted as follows. Massive confluent growth (uncountable colonies on an agar plate) was defined as +++, 100–50 colonies—determined as ++, <50 colonies were determined as +.
All samples with growth ≥50 colonies on an agar plate were included in the study and subjected to further statistical analysis.
The patient’s disease histories were analyzed for the presence of comorbidities: bronchial asthma, IgE-dependent allergy, and NSAIDs intolerance. Bronchial asthma in the examined group of patients was diagnosed in accordance with the guidelines of the Global Strategy for Asthma Management and Prevention 2018.10 In the perioperative period, all patients had well-controlled asthma regardless of its phenotype and severity. IgE-dependent allergy in the studied patients was diagnosed on the basis of confirmed positive prick test results for inhalation allergens and/or IgE serum antibody testing. Based on the history and the occurrence of adverse reactions (dyspnea-exacerbated respiratory disease [NERD], urticaria-exacerbated cutaneous disease [NECD]), after admission for one or several different NSAIDs, patients were classified as hypersensitive to NSAIDs. There were no provocative tests with these drugs in these patients. A group of patients operated on for CRS was identified for the first and the next time.
To unify the extent of the surgery, four types of ESS were distinguished. Type I includes antrostomy (enlargement of the natural ostium of the maxillary sinus) and uncinectomy (removal of the uncinate process), type II includes anterior and posterior ethmoidectomy (removal of the anterior and/or posterior ethmoid cells) with or without antrostomy. Type III is a frontoethmoidectomy (opening the frontal recess and/or frontal sinus and ethmoid), while type IV is each opening of the sphenoid sinus (sphenoidectomy).
Statistical analyses were performed in the Statistica 12 program (StatSoft., Inc.). All calculations were performed based on the Pearson chi-square test. The chi-square test results and the probability for each sample are summarized in the tables. The charts were made in MS Excel 2013 (Microsoft).
The protocol of the study was approved by the Local Bioethical Committee at the Medical University of Silesia.
Results
Ad 1) Evaluation of Bacterial Flora in Patients Over 65 Years of Age Who Underwent ESS Compared to a Younger Group of Patients (≤40 Years)
The analysis concerned 10 types of bacteria (Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Klebsiella spp., Coagulase-negative Staphylococci [CNS], Streptococcus spp. Citrobacter spp., Enterobacter spp., Proteus spp., Pseudomonas aeruginosa) Their percentage distribution for the whole group of 269 patients (study and control group) is shown in Figure 1. Due to the high number of Staphylococcus epidermidis in the examined material, this bacteria was isolated from the CNS group.
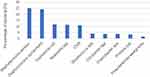 |
Figure 1 Percentage distribution of bacterial flora in study and control group in total. |
Then, separately in the study group (>65 years of age), as well as in the control group (≤40 years of age), the frequency of individual bacteria is calculated in Figure 2.
 |
Figure 2 Percentage distribution of bacterial flora separately in the study and control group. |
To determine if there are more frequent some types of bacteria in the studied groups, a statistical analysis based on the chi-square test is made in Table 1.
 |
Table 1 Determination of the Most Frequent Types of Bacteria in the Studied Group. Results of Statistical Analyses Were Based on the Chi-Square Test |
Only two types of bacteria are more common in patients over 65 years: Proteus spp. and Pseudomonas aeruginosa.
Ad 2) Determination of the Relationship Between the Type of Bacterial Flora and the Extent of Inflammatory Process and the Type of Endoscopic Surgery
Based on the chi-square test, whether some types of bacteria are associated with more extensive ESS procedures (types III and IV) in individual groups of patients is examined in Table 2.
 |
Table 2 Association Between Type III and IV ESS Procedures and Bacterial Flora. Results of Statistical Analyses Were Based on the Chi-Square Test |
In the group of patients >65 years in whom Citrobacter spp. was grown, more extensive ESS procedures (type III and IV) were performed. In the control group, there was no statistically significant relationship between the ESS types and bacterial flora.
Ad 3) Assessment of the Influence of Comorbidities (Bronchial Asthma, IgE-Dependent Allergy, Hypersensitivity to NSAIDs) on Bacterial Flora
In both groups, the most common comorbid disease was bronchial asthma (22%), followed by IgE-dependent allergy (13%) and the least common hypersensitivity to NSAIDs (9%). Patients aged up to 40 years are more likely to suffer from allergies than older people; in the case of hypersensitivity to NSAIDs, the relationship is reversed and these are statistically significant relationships (p ≪ 0.05). Among the elderly, bronchial asthma is more common—the result is of borderline statistical significance. To determine the relationship between comorbid disease and the type of bacteria, chi-square independence tests were performed (Table 3).
 |
Table 3 Influence of Comorbidities on Bacterial Flora. Results of Statistical Analyses Were Based on the Chi-Square Test |
In patients over 65 years of age with asthma, the occurrence of Staphylococcus aureus, Escherichia coli, and Citrobacter spp. was more frequent than in control group
In older patients with hypersensitivity to NSAIDs, the occurrence of Staphylococcus aureus was more frequent, compared to younger group.
Among the patients with IgE-dependent allergy, only in younger patients, the occurrence of Staphylococcus aureus and Staphylococcus epidermidis were more frequent.
Ad 4) Comparison of Bacterial Flora in Patients Operated for the First Time and Repeatedly
A statistical analysis based on the chi-square independence test was carried out for both groups - Table 4. In reoperated patients, over 65 years of age, the occurrence of Staphylococcus aureus and Escherichia coli was more frequent than in control group.
 |
Table 4 Comparison of Bacterial Flora in Patients Operated on for the First Time and Patients Operated on Repeatedly. Results of Statistical Analyses Were Based on the Chi-Square Test |
Ad 5) Comparison of Bacterial Flora in Patients with (CRSwNP) and (CRSsNP)
A statistical analysis (separate for each type of bacteria) was performed for both groups based on the chi-square independence test. There were no statistically significant differences between the occurrence of a certain type of bacteria and the presence of nasal polyps in both groups over 65 years of age as well as in the control group. The incidence of nasal polyps for the group up to 40 years and over 65 years was compared, and there were statistically significant differences between the groups: t (df = 267) = - 3.77 and p <0.05. In the elderly group, nasal polyps were more common.
Table 5 presents a summary of the most important obtained results.
 |
Table 5 Summary of the Most Important Results |
Discussion
The etiology, pathogenesis and role of bacterial infections in the development of CRS remain unclear. In the geriatric population, there is still little research on whether there are differences in the etiology and pathogenesis of CRS in this group of patients and whether the treatment requires a special therapeutic approach. This is particularly important due to the increased risk of complications after ESS as well as antibiotic and steroid therapy in older patients. The physiological process of aging the cells of the immune system is called immunosenescence. With age, there is a significant reduction in the ability to respond to antigens, as well as impaired shaping of congenital and acquired (cellular and humoral) responses. As a consequence, a subclinical, generalized, chronic inflammation process develops, which results in increased susceptibility to infections, neoplastic diseases, and autoimmunity.11
Recent studies have shown a reduced level of S100 protein in the mucosa of the sinuses of patients over 60 years of age. This protein mediates anti-inflammatory effects and affects the integrity of the epithelium, which is a key factor hindering the inhalation of pathogens and allergens.12,13 In the elderly, decreased mucociliary clearance and thinning of the nasal mucosa were found.13,14 Due to a reduction in the percentage of water in the body, a thicker mucus is observed.14 The weakening of these protective barriers in older people may result in easier colonization by microorganisms compared to the younger population.
In the whole material, the most common bacterial types were CNS (about 35%, among which Staphylococcus epidermidis dominated at 24%) and Staphylococcus aureus (25%). Similar observations are also described by other authors.2,15 Despite the suggestion that CNS are associated with contamination and not with infection of the sinus mucosa, in vivo studies documented the pathogenic effect of Staphylococcus epidermidis on the sinus mucosas.16
A comparable number of cultured Staphylococcus epidermidis and Staphylococcus aureus may be associated with the interaction phenomenon occurring between these bacteria. Epidemiological studies in the general population have shown that the presence of Staphylococcus epidermidis in the sinus mucosa correlates with the absence of Staphylococcus aureus. In vivo studies have shown that Staphylococcus epidermidis secretes serine protease (Esp), which inhibits biofilm formation and colonization by Staphylococcus aureus. Purified Esp destroys the previously existing biofilms Staphylococcus aureus and increases its sensitivity to mediators of the immune system.17,18 Also, in some studies of bacterial biofilm of the sinus mucosa, the dominance of Staphylococcus epidermidis over Staphylococcus aureus and other bacteria in the production of biofilm has been reported.19
In our study in the group of patients after 65 years of age (compared to younger patients), more frequent occurrence of two opportunistic Gram-negative bacteria was confirmed: Pseudomonas aeruginosa and Proteus spp. (p = 0.01). The reason for this phenomenon may be the fact that older patients in their lives have been treated with antibiotics more often and longer, which in turn, leads to the development of resistant bacterial strains. The process of reducing immunity related to age seems to be also an important argument.
So far, only one study has actually analyzed the problem of bacterial infections in patients with CRS after 60 years of age. As applied, the group of American researchers also confirmed the frequent occurrence of Pseudomonas aeruginosa in patients with CRS over 60 years of age compared to younger patients.20 In addition, neutrophils domination in inflammatory infiltrations and association bacterial infection with increased levels of IL-1β, IL-6, IL-8, and TNF-α cytokines are confirmed in older patients. These relationships were not observed in younger patients, which suggests a mechanism uniquely associated with aging.20 It has also been suggested that younger and older patients may have different immunological responses to colonizing bacteria. Clinically, in older patients with CRS, bacterial colonization is more frequent, with a poor long-term response to antibiotics.20
Predomination of neutrophil infiltrates over eosinophils in older patients with CRS compared to younger groups was also confirmed by the study of Cho SH et al.12 Molecular studies of the microbiome from recent years have disproved the myth that healthy sinuses are “sterile”, at the same time revealing the wide diversity of the microflora of this region. In both healthy people and patients with CRS, the presence of complex microbial microorganisms in the sinus cavity was confirmed, where the group with CRS was often characterized by reduced microflora diversity compared to the control group.21 The imbalance between microorganisms colonizing the sinus mucosa and reduced bacterial diversity may play a role in the pathogenesis of CRS. However, it is still unclear whether the presence of microbial dysbiosis in CRS is the cause or rather the relationship of the disease process.22 The few studies of sinus microflora in the elderly population show that advanced age is an independent predictor factor of the composition of the microbiome. In addition, these studies confirmed the more frequent occurrence of Pseudomonas species in older patients with CRS compared to younger patients.23
It is known that bronchial asthma, allergy, and hypersensitivity to NSAIDs are associated with CRS. Analyzing the bacterial flora of the sinuses in older patients with CRS, we decided to investigate whether there are significant relationships between these diseases and the cultured pathogen, given that such studies have not been presented so far.
In our study among elderly patients with CRS, bronchial asthma, and nasal polyps were more common than in the younger group of patients, which is consistent with the reports of other authors.14,24,25 Bronchial asthma was the most common comorbid disease in both groups of patients. Based on the common immune mechanisms and pathophysiology of inflammatory diseases of the upper and lower respiratory tracts, recent studies have shown that the composition and function of the upper airway microbiome may influence asthma pathogenesis. What is more, microbes can play different roles depending on the age group.25 Moreover, the results of microbiological studies carried out so far in patients with bronchial asthma are not consistent.25–27 Recent reports have confirmed the presence of a neutrophil asthma phenotype in older patients. It was also found that colonization of the airways by Haemophilus spp., Streptococcus spp. or Moraxella catarrhalis positively correlated with sputum neutrophilia in patients with severe refractory asthma.28
In our study of the group of patients >65 years of age with bronchial asthma, a statistically significant more frequent occurrence of bacteria was found: Staphylococcus aureus, Escherichia coli, and Citrobacter spp. compared to younger patients. At the same time, more frequent occurrence of Staphylococcus aureus was confirmed among patients >65 years of age, with CRS and hypersensitivity to NSAIDs than in the control group. In our opinion, this may be associated with chronic and long-term carrying of these pathogens and may have an adverse relationship with asthma associated with hypersensitivity to NSAIDs.
In our study of patients with CRS up to 40 years of age, IgE-related allergy was more frequent than in the elderly group. Some authors suggest the frequent occurrence of Staphylococcus aureus in patients with allergic sinusitis,29,30 which we also confirmed but only in the group of younger patients.
In the group of patients over 65 years of age, our analysis showed more frequent occurrence of Gram-negative bacteria (Staphylococcus aureus and Escherichia coli) in reoperated patients compared to the younger group. Such observations may be the result of the use of prolonged antibiotic therapy in reoperated patients. Interestingly, in the recent molecular studies of the sinus microbiome, in patients after the next ESS, no specific pathogen dominated, but significant reductions in bacterial diversity were found.21,31 We have also shown that, in the examined group of elderly patients, there is a strong relationship between the presence of Citrobacter spp. in the nasal mucosa, the extent of the inflammatory process and, consequently, the need to perform a more extensive surgery.
A huge therapeutic challenge is CRS with polyps (CRSwNP) associated with severe and uncontrolled asthma, which is more common in older people. For this reason, in our study, we have also subjected this problem to a deeper analysis. We confirmed the more frequent occurrence of polyps in older patients (99% of patients after 65 years had CRSwNP, 59% of patients <40 years of age had CRSwNP), which is consistent with previous studies.2,32 The most common type of bacterium among patients with CRSwNP with polyps after 65 years of age as well as in the control group was Staphylococcus aureus. However, this was not a statistically significant result. Finally, we did not show any relationship between the occurrence of a specific strain and the presence of polyps in both people over 65 years of age and the control group. Many researchers have postulated a theory where colonizing Staphylococcus aureus secretes a toxin antigen (sags), which enhances the inflammatory response with a predominance of infiltrates of eosinophilic and promotes the formation of polyps.2 However, due to the lack of evidence of the direct etiological role of this bacterium, Staphylococcus aureus is currently perceived as a factor modifying the course of the disease.2 What is more, the latest molecular studies of sinus microbiome did not show differences in microflora in patients with nasal polyps.23
CRS is one of the more frequent chronic diseases occurring in the elderly population, although the inflammatory mechanisms in this disease are not yet fully understood. Therefore, our work aimed to find a difference in this group of patients, especially in the field of sinus bacterial flora, which could also optimize the therapeutic process. Patients with CRS are often treated with extended cycles of antibiotics and steroids. Surgical intervention is recommended in patients who do not respond to treatment; however, age may be one of the risk factors for serious complications of sinus endoscopic surgery.33 Analyzing the results of our research and the few reports of other authors, we found that there are differences between the bacterial flora of the sinuses in older and younger patients with CRS. In addition, older patients are more susceptible to infection with opportunistic bacteria, which is associated with greater resistance to antibiotics and worse treatment outcome. What is more, according to the postulate about the predominance of neutrophilic type of inflammation in older patients with CRS, the use of corticosteroids may be less effective, leading to the search for alternative therapeutic approaches. Among them, probiotics deserve attention, which, according to recent studies, may restore the balance of the disturbed microbiome structure of the sinuses, resulting in lower susceptibility to infection.34
Our study undoubtedly draws attention to the role of Staphylococcus aureus, which is dominant in older patients with bronchial asthma and NSAID intolerance, as well as after previous surgical operations of the sinuses. Perhaps the use of probiotics would be effective in this group of patients, which could potentially reduce the number of pathogens and limit the remodeling of the upper respiratory tract mucosa, as suggested by some authors.32,34 Although probiotic therapies seem to be promising, further research is needed to establish their true role in the treatment of CRS. An undoubted limitation of our work was a study method based on the analysis of traditional bacterial cultures rather than a molecular study of the microbiome. Further research is necessary to better understand the pathomechanism and role of bacterial infections in older patients with CRS.
Conclusion
Based on our study, it can be concluded that the bacterial flora of the paranasal sinuses of patients with CRS is different in older and younger patients. Older patients are more susceptible to bacterial infections than younger ones. Some comorbidities (hypersensitivity to NSAIDs and bronchial asthma), as well as previous sinus surgical procedures in older patients with CRS, have an impact on bacterial flora. Therefore, a separate therapeutic approach in older patients with CRS seems to be justified, but this problem requires further research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. United Nations Statistics Division - Demographic and Social Statistics, Available from: unstats.un.org.
2. Fokkens WJ, Lund VJ, Mullol J, et al. European position paper on Rhinosinusitis and nasal polyps. Rhinol. 2012;Suppl 23:S1–S298.
3. Lee JY, Lee SW. Influence of age on the surgical outcome after endoscopic sinus surgery for chronic rhinosinusitis with Nasal Polyposis. Laryngoscope. 2007;117(6):1084–1089. doi:10.1097/MLG.0b013e318058197a
4. Jiang RS, Hsu CY. Endoscopic sinus surgery for the treatment of chronic rhinosinusitis in geriatric patients. Ear Nose Throat J. 2001;80:230–232. doi:10.1177/014556130108000411
5. Kim YS, Kim NH, Seong SY, Kim KR, Lee GB, Kim KS. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am J Rhinol Allergy. 2011;25:117–121. doi:10.2500/ajra.2011.25.3630
6. Zhang N, Van Zele T, Perez-Novo C, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122(5):9618. doi:10.1016/j.jaci.2008.07.008
7. Cope EK, Lynch SV. Novel microbiome-based therapeutics for chronic rhinosinusitis. Curr Allergy Asthma Rep. 2015;15(3):504. doi:10.1007/s11882-014-0504-y
8. Bogacka E, Mieszczak A, Groblewska A. Allergy in elderly- is it different than an allergy in adulthood? Pol J Allergol. 2014;1:94–101. doi:10.1016/j.alergo.2014.09.001
9. Bozek A. Immunotherapy in the elderly- efficacy vs. Risk? Pol J Allergol. 2015;2:70–72.
10. Global Strategy for Asthma Management and Prevention; 2018. Available from: http://www.ginasthma.org/2018.
11. Roży A. The immune system in the elderly. Alergia. 2016;1:29–33.
12. Cho SH, Hong SJ, Han B, et al. Age-related differences in the pathogenesis of chronic rhinosinusitis. J Allergy Clin Immunol. 2012;129(3):858–860. doi:10.1016/j.jaci.2011.12.002
13. Hsu DW, Suh JD. Rhinitis and sinusitis in the geriatric population. Otolaryngol Clin North Am. 2018;51(4):803–813. doi:10.1016/j.otc.2018.03.008
14. Ho JC, Chan KN, Hu WH, et al. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med. 2001;163:983–988. doi:10.1164/ajrccm.163.4.9909121
15. Busaba NY, Siegel NS, Salman SD. Microbiology of chronić ethmoid sinusitis. Americal J Otolaryngol. 2004;25(6):379–384.
16. Sachse F, von Eiff C, Becker K, Steinhoff M. Proinflammatory impact of Staphylococcus epidermidis on the nasal epithelium quantified by IL-8 and GRO-alpha responses in primary human nasal epithelial cells. Rudack CInt Arch Allergy Immunol. 2008;145(1):24–32. doi:10.1159/000107463
17. Iwase T, Uehara Y, Shinji H, et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465(7296):346–349. doi:10.1038/nature09074
18. Fredheim EGA, Flægstad T, Askarian F, Klingenberg C.Colonisation and interaction between S. epidermidis and S. aureus in the nose and throat of healthy adolescents. Eur J Clin Microbiol Infect Dis. 2015;34(1):123–129. doi:10.1007/s10096-014-2197-5
19. Marcinkiewicz J, Stręk P, Strus M, et al. Staphylococcus epidermidis and biofilm-associated neutrophils in chronic rhinosinusitis. A pilot study. Int J Exp Pathol. 2015;96(6):378–386. doi:10.1111/iep.12156
20. Morse JC, Li P, Ely KA, et al. Chronic rhinosinusitis in elderly patients is associated with an exaggerated neutrophilic pro-inflammatory response to pathogenic bacteria. J Allergy Clin Immunol. 2018;20:0091–6749.
21. Lee JT, Frank DN, Ramakrishnan V. Microbiome of the paranasal sinuses: update and literature review. Am J Rhinol Allergy. 2016;30(1):3–16. doi:10.2500/ajra.2016.30.4255
22. Psaltis AJ, Wormald PJ. Therapy of sinonasal microbiome in CRS: a critical approach. Curr Allergy Asthma Rep. 2017;17(9):59. doi:10.1007/s11882-017-0726-x
23. Mahdavinia M, Engen PA, LoSavio PS, et al. The nasal microbiome in patients with chronic rhinosinusitis: analyzing the effects of atopy and bacterial functional pathways in 111 patients. J Allergy Clin Immunol. 2018;142(1):287–290. doi:10.1016/j.jaci.2018.01.033
24. Dunn RM, Busse PJ, Wechsler ME. Asthma in the elderly and late onset adult asthma. Allergy. 2018;73:284–294. doi:10.1111/all.13258
25. Lee JJ, Kim SH, Lee MJ, et al. Different upper airway microbiome and their functional genes associated with asthma in young adults and elderly individuals. Allergy. Epub 2018 Nov 22.
26. Durack J, Lynch SV, Nariya S, et al. Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J Allergy Clin Immunol. 2017;140:63–75. doi:10.1016/j.jaci.2016.08.055
27. Simpson JL, Daly J, Baines KJ, et al. Airway dysbiosis: haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur Respir J. 2016;47:792–800. doi:10.1183/13993003.00405-2015
28. Green BJ, Wiriyachaiporn S, Grainge C Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One. Epub 2014 Apr 8. doi:10.1371/journal.pone.0100645
29. Mufarrih SH, Qureshi NQ, Sadruddin A, et al. Relationship between staphylococcus aureus carriage and surgical site infections following total hip and knee arthroplasty in the South Asian population: protocol for a prospective cohort study. JMIR Res Protoc. 2018;7(6):e10219. doi:10.2196/10219
30. Larson DA, Han JK. Microbiology of sinusitis: does allergy or endoscopic sinus surgery affect the microbiologic flora? Curr Opin Otolaryngol Head Neck Surg. 2011;19(3):199–203. doi:10.1097/MOO.0b013e328344f67a
31. Feazel LM, Robertson CE, Ramakrishnan VR, Frank DN. Microbiome complexity and Staphylococcus aureus in chronic rhinosinusitis. Laryngoscope. 2012;122(2):467–472. doi:10.1002/lary.22398
32. Tan BK, Chandra RK, Pollak J, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131(5):1350–1360. doi:10.1016/j.jaci.2013.02.002
33. Krings JG, Kallogjeri D, Wineland A, Nepple KG, Piccirillo JF, Getz AE, Complications of primary and revision functional endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2014;124:838–845. doi:10.1002/lary.24401
34. Cervin AU. The potential for topical probiotic treatment of chronic rhinosinusitis, a personal perspective. Front Cell Infect Microbiol. Epub 2018 Jan 12. doi:10.3389/fcimb.2017.00530
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
