Back to Journals » Infection and Drug Resistance » Volume 7
Bacterial bloodstream infections and antimicrobial susceptibility pattern in pediatric hematology/oncology patients after anticancer chemotherapy
Authors Al-Mulla N, Taj-Aldeen SJ, El Shafie S, Janahi M, Al-Nasser A, Chandra P
Received 2 July 2014
Accepted for publication 18 August 2014
Published 6 November 2014 Volume 2014:7 Pages 289—299
DOI https://doi.org/10.2147/IDR.S70486
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Naima A Al-Mulla,1,2 Saad J Taj-Aldeen,3 Sittana El Shafie,4 Mohammed Janahi,2,5 Abdullah A Al-Nasser,1 Prem Chandra6
1Hematology/Oncology, Department of Pediatrics, Hamad Medical Corporation, Doha, Qatar; 2Weill-Cornel Medical College, Ar-Rayyan, Qatar; 3Department of Laboratory Medicine and Pathology, Hamad Medical Corporation, 4Department of Laboratory Medicine and Infection Control, Aspetar Hospital, 5Infectious Disease division, Department of Pediatrics, 6Medical Research Center, Hamad Medical Corporation, Doha, Qatar
Purpose: Bloodstream infections in pediatric hematology and oncology represent a major problem worldwide, but this has not been studied in Qatar. In this study, we investigated the burden of infection and the resistance pattern in the bacterial etiology, in the only tertiary pediatric hematology and oncology center in Qatar.
Methods: All pediatric cancer patients (n=185) were evaluated retrospectively during the period 2004–2011; a total of 70 (38%) patients were diagnosed with bloodstream infections. Bacterial etiology was determined, along with their susceptibility patterns. Neutropenia, duration of neutropenia, fever, duration of fever, and C-reactive protein (CRP) were evaluated throughout the study.
Results: A total of 70 patients (38%) were diagnosed with acute leukemias, lymphomas, solid tumors, or brain tumors; those patients experienced 111 episodes of bacteremia. The most common Gram-positive (n=64 [55%]) isolates were Staphylococcus epidermidis (n=26), Staphylococcus hominis (n=9), and Staphylococcus haemolyticus (n=7), and the common Gram-negative (n=52 [45%]) isolates were Klebsiella pneumoniae (n=14), Pseudomonas aeruginosa (n=10), and Escherichia coli (n=7). There was a significant association observed between fever with positive blood culture and different types of cancer (P=0.035). The majority of bacteremia (n=68 [61.3%]) occurred in nonneutropenic episodes. Elevated values of CRP (≥5 mg/L) were detected in 82 (95.3%) episodes and were negatively correlated with absolute neutrophil count (ANC) (r=-0.18; P=0.248) among all cases. However, the infection-related fatality rate was 2.2% (n=4), with three caused by Gram-negative pathogens. Multidrug resistant organisms were implicated in 33 (28.4%) cases and caused three of the mortality cases.
Conclusion: Multidrug resistant organisms cause mortality in pediatric cancer patients. Investigation of antimicrobial susceptibility of these organisms may guide successful antimicrobial therapy and improve the surveillance and quality of pediatric malignancy care.
Keywords: risk factors, antibiotic susceptibility
Introduction
Intensive treatment for childhood cancer has led to improved survival rates and to an increase in the rate of acute complications and late effects. One of the most serious challenges of further increases in chemotherapy intensity is the bloodstream infection (BSI).1,2 Infection is one of the most important causes of morbidity and mortality in children with cancer.3 Neutropenia is a common complication for those patients following modern aggressive anticancer chemotherapy agents. Fever in such patients is often related to infection.4
The most common risk factors reported for BSI are: younger age at onset of bacteremia, absolute neutrophil count (ANC) <500 cells/µL, intravascular catheters, status of underlying disease, status of bone marrow (no recovery), high temperature, and more immunosuppressive antineoplastic regimens.5–7 Both severity and duration of neutropenia influence the risk for infection.7–10 Febrile neutropenia is a heterogeneous condition ie, neutropenic patients are not equally prone to infections, partly due to the underlying cancer, chemotherapy, and comorbidity factors.10–12 Pediatric patients with acute leukemias generally have a higher incidence of infectious complications than do those with other malignancies.13
There has been a dramatic shift in the pattern of infection in neutropenic patients, with 70% of bacteremias being due to Gram-positive organisms by the late 1980s. A few studies have reported an increase in the frequency of isolation of Gram-positive organisms (mainly Staphylococcus aureus and coagulase-negative Staphylococcus spp. [CoNSs]) and a decrease in Gram-negative bacteremias in cancer patients.5,14,15
Empirical treatment with broad-spectrum combinations of antibiotics against Gram-negative organisms has had a marked reduction in mortality.10,16 Numerous studies have demonstrated the activity of broad-spectrum β-lactams against Gram-negative aerobes.16 However, the standard management in cancer patients with fever and neutropenia is the prompt administration of empiric antibiotic parenteral therapy8,17–19 until the resolution of the fever and maintenance of immune integrity.20 Moreover, the rate of response to empirical therapy in neutropenic patients with fever ranges between 40% and 90%.21 Therefore, the development of an effective strategy for the management of BSIs requires careful analysis of the types of organisms and pattern of antimicrobial resistance of the isolates at each particular institution. This information will help in development of guidelines in management of patients with bacterial BSIs, including the choice of empiric antimicrobial therapy.
In this retrospective study, we characterize the bacterial BSIs, the probable risk factors and their association with different types of cancer, the pattern of resistance to antimicrobial agents, and the outcome, among pediatric hematology and oncology patients. This study aimed to provide the number and types of probable risk factors for infections in such enrolled patients in the only tertiary hospital, Hamad Medical Corporation (HMC), in Qatar.
Materials and methods
Patients
All pediatric malignant hematology and oncology patients in Qatar seen at the Pediatric Hematology/Oncology Unit of the HMC (age ≤15 years) who had positive blood culture between January 2004 and December 2011 were reviewed utilizing the microbiology and patients records database. All patients with BSI episodes who were followed for chemotherapy treatment during different phases, stages of disease and clinical indications for malignant disorders by the pediatric hematology/oncology unit at the HMC were included in this study. Clinical and laboratory parameters, such as fever, neutropenia, duration of neutropenia, and C-reactive protein (CRP) values, were used for identifying expected BSI patients. Patients in our unit routinely have their vital signs, including temperature, recorded every 4 hours and complete blood count (CBC) monitored every 24 hours or more often if clinically indicated. Febrile and afebrile patients and patients with or without neutropenia, who had hospital- and community-acquired bacterial infection, were assessed with special attention to oral mucosa, pharynx, periodontium, tissue around nails, peripheral and central venous lines (CVL), and perianal areas. Then they were fully investigated with CBC plus differential, renal and hepatic profiles, blood culture from each lumen of the CVL, if present, and peripheral vein. The HMC research and ethics committee (reference number RC/330/2005) granted approval to conduct this study.
Definitions
Fever was defined for our patients as a single oral temperature of ≥38.3°C for ≥1 hour or an axillary temperature of ≥37.8°C, or ≥37.5°C for >1 hour, not related to administration of pyrexial agents (blood; blood products; intravenous immunoglobulin [IVIG]; pyrogenic drugs, for example, ara-C; etc). Neutropenia was defined as an ANC <500 cells/µL, while counts <100 cells/µL were considered as “profound neutropenia”.7,22 CRP values of <5 mg/L were considered negative and ≥5 mg/L as positive, based on HMC laboratory protocol.
Patient’s management
Patients with positive blood culture were treated with antimicrobial agents based on the susceptibility results. Patients with isolated Gram-negative bacteria were continued treatments for 14 days after the first negative blood culture results, while patients with Gram-positive bacterial infections were treated for 10 days after the first negative culture results. All patients with positive culture results (either febrile or nonfebrile, neutropenic or nonneutropenic) were treated according to the type of bacterial species.
Microbiology
Two blood samples per patient were drawn from two separate sites (central venous line [CVL] and peripheral lines) and directly injected to Bactec™ bottles, and incubated in a Bactec incubator (BD Biosciences, Franklin Lakes, NJ, USA). Aerobic and anaerobic blood culture bottles were incubated for 7 days at 37°C. Positive cultures were Gram-stained and subcultured onto sheep blood agar, MacConkey agar, and chocolate agar. Isolates of bacteria were identified by biochemical methods, using an API® (BioMérieux, Craponne, France), Viteck® 2 (BioMérieux), or Phoenix™ (BD Biosciences) system. An episode was considered to be possible bacteremia if only a single blood culture was positive. With regards to CoNSs, Corynebacterium, and other skin contaminants, at least two sets of positive blood cultures were required to confirm bacteremia. Gram-negative organisms, Streptococcus pneumoniae, Enterococcus, and S. aureus were classified as high-risk bacteremias; the rest of the cultured organisms were classified as low-risk, based on a priori determination of organisms likely to cause sepsis.23,24
The antibiotic susceptibility for isolated pathogens was performed and interpreted according to Clinical and Laboratory Standards Institute guidelines.25 Antimicrobial susceptibility testing of isolated bacterial pathogens to clinically used antimicrobials was performed by using Etest® methods (BioMérieux) and automated Phoenix. The multidrug resistant (MDR) organism phenotype was defined as diminished susceptibility to three or more of the following: fluoroquinolones, aminoglycosides, and β-lactam (β-lactamase inhibitor combinations and carbapenems).7,26
Statistical analysis
Categorical and continuous data values were expressed as frequency (percentage) and mean ± standard deviation (SD), median, and range. Descriptive statistics were used to summarize all demographic and other clinical characteristics of the patients. Quantitative variable means between two and more than two independent groups were analyzed using unpaired Student’s t-test and one-way analysis of variance (ANOVA). Where an overall group difference was found statistically significant, pairwise comparisons were made using appropriate multiple comparison tests. The results were presented with the associated 95% confidence interval. Mann–Whitney U and Kruskal–Wallis tests were applied for nonnormal or skewed data.
Associations between two or more qualitative variables were assessed using chi-square test. For small cell frequencies, chi-square test with continuity correction factor or Fisher exact test was used. Relationships between two quantitative variables were examined using Pearson’s correlation coefficients. Pictorial presentations of the key results were made using appropriate statistical graphs. In statistical analysis, each bacteremic episode was considered as a separate and independent observation since we considered clinical and laboratory status at episodes as predictors of outcome. A two-sided P-value <0.05 was considered to be statistically significant. All statistical analyses were done using statistical packages SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient’s characteristics
During the study period, January 2004 to December 2011, a total of 185 pediatric cancer patients were admitted to the Pediatrics Department Hematology/Oncology Unit. Patients were hospitalized for different causes, including chemotherapy administration, radiation therapy and/or bacteremia. BSI was detected in 111 episodes, in 70 cancer patients. Acute leukemias, acute lymphoblastic leukemia (ALL) (n=58 [47%]) and acute myelocytic leukemia (AML) (n=11 [7%]), were the most common underlying malignancies with bacteremia, followed by lymphomas (n=18 [20%]), solid tumors (n=17 [17%]), and brain tumors (n=7 [9%]). There were nearly equal numbers of male (n=34 [49%]) and females patients (n=36 [51%]). The study included children younger than 15 years of age, and mean age at diagnosis was 6.4±4.2 years; as well, 26 (37%) were <4 years and 44 (63%) were ≥4 years (Table 1).
Clinical features
All cancer patients who underwent chemotherapy had CVLs (Hickmann or Port-A-Cath). The patient diagnosis and clinical features of the episodes are listed in Table 1. Of the 111 bacteremic episodes, 43 (38.7%) episodes were neutropenic. ANC and absolute monocyte count (AMC) were <500 (cells/µL), CRP >5mg/L, temperature >38.3°C, and patients with >7 days duration of neutropenia were more frequently detected in patients with bacteremia. Bacteremia was documented in 74.8% of episodes characterized with temperature ≥38.3°C and was significantly higher among ALL and AML compared with other types of cancer (P=0.035), while 28 (25.2%) episodes were afebrile during the BSI. The duration of fever >24 hours was seen in 26 (29.9%) patients.
Elevated values of CRP (≥5 mg/L) were detected in 82 (95.4%) episodes and were negatively correlated with ANC (r=−0.18; P=0.248) among ALL cases. The correlation between temperature and CRP was minimal and positive (r=0.11; P=0.50) (data not shown). Brain tumor patients with bacteremia showed the highest CRP average values, with median CRP of 67.6 mg/L (range 7–351) followed by solid tumors (36.0 [range 5–114]), and these seem to be much higher than for other types of cancers, as demonstrated by box and whisker plot (Figure 1). There was significant and negative correlation observed between duration of neutropenia and ANC (r=−0.39; P=0.002). Similarly, ANC and temperature were found to be negatively correlated (r=−0.25; P=0.055) (data not shown).
In neutropenic patients, the percentage of bacteremic episodes among all leukemic patients was observed to be significantly higher (n=33 [47.8%]) compared with lymphomas, and solid and brain tumors (n=10 [31.3%]) (P=0.012). Median duration of neutropenia was found to be higher among acute leukemias, lymphomas and brain tumors than among solid tumors; however, this difference was not statistically significant (P=0.761). Mean temperature was higher in the acute leukemia and brain tumor groups than in other types of cancers, but the difference was statistically insignificant (P=0.417). The median ANC values were observed to be less among acute leukemia cases than other types of malignancies (P=0.495) (data not shown).
The percentage of patients with bacteremic episodes was found to be significantly higher in ALL (50%) and AML (56%) in comparison with lymphoma (31%), solid tumors (25%), and brain tumors (40%) (P=0.038). Further statistical analysis showed that among ALL patients, the percentage of patients with bacteremic episodes was found to be significantly higher compared with patients with solid tumor (n=33 [50%] vs n=14 [25%]) (P=0.039). Similarly, bacteremic episodes among ALL were observed to be higher compared with both the lymphoma and brain tumor groups; however, the difference was not statistically significant (P>0.05). No statistically significant association was observed between age and sex, for bacteremic episodes (P>0.05) (Table 1).
Of the 111 bacteremic episodes, 68 (61.3%) had ANC ≥500 cells/µL, 82 (95.3%) had CRP ≥5 mg/L, 83 (74.8%) had temperature ≥38.3°C, 26 (29.9%) had duration of fever more than 24 hours, and 58 (72.5%) patients had duration of neutropenia >7 days. Among the ALL and AML cases, the percentage of episodes having temperature ≥38.3°C was significantly higher (81% and 81.8%; respectively) compared with lymphoma, solid tumor, and brain tumor (66.7%, 58.8%, and 71.4% respectively [P=0.035]) (Table 1).
Microbial etiology and susceptibility pattern
Gram-positive bacteria were more common than Gram-negative throughout the study period. The ratio of Gram-positive to Gram-negative bacteria among the study period of bacteremia (111 episodes, 116 organisms) was in the range of 1.4, in the years 2004–2005 to 1.15 in 2010–2011. The patterns of Gram-positive and Gram-negative bacteremia throughout the study period 2004–2011 are presented in Figure 2. Pathogen distribution among all isolates of the 70 pediatric bacteremia patients is shown in Table 2. A total of 52 (44.8%) of 116 recovered isolates were Gram-negative organisms, and 64 (55.2%) isolates of 116 were Gram-positive. Klebsiella pneumoniae was the most common Gram-negative pathogen, accounting for 14 (12%) of 116 isolates, followed by Pseudomonas aeruginosa (10 [8.6%]), Escherichia coli (7 [6%]), Acinetobacter baumannii (6 [5.1%]), and Enterobacter cloacae (5 [4.3%]). The most common Gram-positive microorganism was Staphylococcus epidermidis, in 26 (22.4%) of 116 isolates. Staphylococcus hominis was found in only 9 (7.7%) and S. haemolyticus in 7 (6%) cases.
  | Figure 2 Pattern of Gram-positive and Gram-negative bacteria in pediatric cancer patients (2004–2011). |
  | Table 2 Pattern of bacteria isolated from blood stream infections in pediatric oncology patients |
Table 3 reports the results of in vitro antibiotic susceptibility tests of the 116 isolated strains of Gram-positive and Gram-negative bacteria. Since not all the antibiotics were tested in all strains, the number of isolates tested was different for each antibiotic. K. pneumoniae revealed high susceptibility (100%) to imipenem, meropenem, and amikacin, and was less susceptible to ciprofloxacin (82%) and cephalosporins. P. aeruginosa isolates were 100% susceptible to all antipseudomonal antibiotics. A. baumannii isolates were 100% susceptible to all tested antibiotics except ceftriaxone. E. coli revealed high susceptibility (100%) to imipenem, meropenem, piperacillin/tazobactam, and amikacin, and less susceptible for gentamicin (55%) and ciprofloxacin (78%). All the tested Gram-positive isolates were 100% susceptible to linezolid and streptogramins. S. epidermidis revealed high susceptibility to erythromycin (100%) and less to vancomycin (96%). S. haemolyticus and S. hominis were (100%) susceptible to vancomycin but less susceptible to erythromycin and gentamicin.
  | Table 3 Susceptibility pattern of bloodstream bacteria |
MDR Gram-positive organisms accounted for 21/64 (32.8%), and Gram-negative organisms accounted for 12/52 (23%), which represents a total of 33/116 (28.4%).
Mortality
The total number of deaths was 20 (10.8%), of which four (2.2%) died due to bacterial infection, 13 died due to progressive diseases, and three due to other causes (Figure 3). Infection-related mortality occurred in a total of four (2.2%) deaths, due to bacterial infection among one each of ALL, lymphoma, solid tumor, and brain tumor patients. Deaths occurred within 30 days of a positive blood culture caused by infections with P. aeruginosa, Stenotrophomonas maltophilia, Enterococcus gallinarum, and E. coli, respectively. The last three of these isolates were MDR.
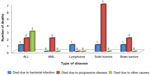  | Figure 3 Causes of mortality in pediatric cancer patients. |
Total BSI infections in neutropenic patients were 43 episodes. K. pneumoniae and S. epidermidis were the most common pathogens (n=10 each). Two neutropenic patients died from MDR infections, one with E. coli and the other with S. maltophilia (Table 4).
Discussion
Infections are the leading cause of morbidity and mortality in febrile patients with malignancy who become neutropenic due to chemotherapy.27 Our study confirms that pediatric cancer patients are at an increased risk of postchemotherapy infection and its adverse events. The onset and duration of neutropenia, fever, CRP, and type of cancer may be predicted for each patient.28,29 Some risk profiles have been tested, but very few have proposed a well-designed and statistically supported score to predict BSI in children who are treated for malignant diseases. BSI in this population should be closely monitored to improve clinical outcome and prevent complications.22 The risk of severe infection and eventual death varies according to different patient characteristics.30
In this study the total number of cancer patients analyzed was 185, with 111 BSI episodes. The number of patients with bacteremia was 70 (38.7%), which is slightly higher than other reported values of 11%–30%.28,31–34 Of the patients involved in the episodes, 49% were males. Although, other studies reported variability in sex predilection, our male patients percentage value fall within the reported range 45%–51%.28,29,34
Most enrolled patients were in good general condition, a significant proportion 68 (61.3%) of all episodes with BSI did not have neutropenia at the time of diagnosis, and 28 (25.2%) were initially afebrile at the time of diagnosis with severe BSI. This is in agreement with results from other groups who found severe BSI-related infections in pediatric cancer patients in the absence of neutropenia.35 In a recent study, BSI was reported in 6.3% of the pediatric patients with fever and nonneutropenia.29
Although, statistically nonsignificant, a large proportion of episodes had been evaluated (n=82 [95.4%]). Results on significance of CRP as predictor for bacteremia have been variable. Asturias et al28 reported no statistical relationship between CRP and bacteremia in children with cancer and febrile neutropenia. In adult cancer patients, CRP ≥10 mg/dL was a statistically significant predictor for bacteremia. Therefore, surveillance should consider all risk factors collectively as indicators of BSI.
The treatment outcome of bacteremia in pediatric oncology patients was successful in 107 episodes of positive blood cultures. It is not surprising that the attributable mortality in our study was very low (n=4 [2.2%]) – this was mainly due to close monitoring, rapid diagnosis, and prompt treatment in any suspected case of sepsis and due to advances in intensive care in this particular population.
In the current study, four patients died due to BSI, three mortality cases of febrile neutropenic patients who had BSI with Gram-negative organisms (two MDR cases), were characterized with temperature around 39.5°C. The fourth patient was afebrile and nonneutropenic and died due to an MDR strain of E. gallinarum BSI. MDR were previously found to be significantly associated with increased overall mortality in pediatric oncology patients.36 The reported mortality rate for patients with febrile neutropenia is <5%.37,38 This low incidence is similar to the results from our current study and makes it necessary to evaluate the present protocol to build up valid conclusions.
There is substantial evidence on a global scale for a significant shift toward more Gram-positive isolates from BSI.39,40 Bacteremia was reported in 13% of febrile neutropenic patients, and the Gram-positive cocci were found to be the most common organisms.9 A similar trend of Gram-positive (n=64 [55.2%]) organisms were isolated in the present study – none of BSI was caused by methicillin-resistant S. aureus (MRSA), but two species of Enterococcus were isolated; Enterococcus faecium and E. gallinarum. The latter was an MDR strain, a well-known resistant organism to vancomycin, and harbors the VanC resistance phenotype seen in motile species of Enterococcus like E. gallinarum.41
BSI in our studied population was caused in 44.8% of cases by Gram-negative bacteria. K. pneumoniae was the most commonly isolated pathogen (14/52 [21%]), and three isolates (3/14 [21%]) were extended-spectrum beta-lactamase (ESBL) strains, thus implicating these as hospital-acquired infections. It is well documented that 38% of K. pneumoniae is expressed an ESBL.39 A review by Zaidi et al40 demonstrated that in 11,471 bloodstream samples throughout the developing world, 60% of BSI was caused by Gram-negative bacteria, with K. pneumoniae accounting for 23%, Pseudomonas for 7%, and Acinetobacter for 3.5%. In the present study, the prevalence of P. aeruginosa was 10/52 (19%), with no MDR detected, and one ALL patient died due to a non-MDR P. aeruginosa BSI. MDR P. aeruginosa represents a challenging pathogen in critically ill pediatric patients – the 30-day mortality rates were 19.6% for all patients and were 35.8% for those with MDR BSI, and only the MDR showed a significant association with infection-related death.42 In a similar study with adult hematologic malignancies, deaths occurred in 31.6% of the patients and was almost significantly higher (40.1%) for those with MDR BSI.43
The pattern of resistance in Gram-positive bacteria is relatively high for clindamycin and gentamicin, whereas they were susceptible to linezolid and streptogramin. S. epidermidis was 96% susceptible to vancomycin. CoNSs are increasingly becoming important causes of health care-associated infections. Although these are perhaps the commonest isolates from clinical samples in any diagnostic bacteriology laboratory, it is in many cases difficult to assign clinical significance to them as they are also normal commensals found on the skin surface and elsewhere. The first clinically significant isolate of a CoNSs showing resistance to vancomycin was described by Schwalbe et al.44 Among the various species of CoNS, S. haemolyticus is the most common species associated with vancomycin resistance45 and was susceptible in this study. Other species that have been associated with vancomycin resistance include S. epidermidis, Staphylococcus warneri, and S. hominis.46
The pathogens causing bacteremia in neutropenic patients were approximately equally divided between Gram-positive and Gram-negative organisms (Table 4), but this trend may vary with the geographical location of the study.47 This study showed that 72% of the bacteremic episodes involved prolonged neutropenia >7 days. It is reported that patients with malignancy and prolonged neutropenia are considered to be at high risk for severe infectious complications.48
Although mortality rate due to bacteremia continues to decrease, new epidemiological risk factors and microbiological patterns continue to challenge physicians and health care providers. Therefore studies that assess the risk of major complications, and epidemiological and microbiological patterns carry significant weight as every patient is different and deserves focused attention to determine the best course of treatment. Our study is the first published study from Qatar to report the bacterial etiology of pediatric hematology/oncology patients and to identify antimicrobial susceptibility of these organisms to guide empiric antimicrobial therapy and to improve the quality of pediatric malignancy care. The present study evaluated some risk factors, neutropenia, duration of neutropenia, fever, duration of fever, and CRP ≥5mg/L in 111 episodes of 70 patients with various types of cancer. The median value of CRP in brain tumor patients with BSI was higher than those values from other patients. A significant relationship between fever and positive BSI culture was documented (P=0.035). Designing a practical and reliable surveillance system will be beneficial to predict BSI at time of onset and guide the proper antimicrobial therapy. Amikacin and carbapenems are characterized by high in vitro activity against Gram-negative organisms, whereas, linezolid and streptogramin have shown high in vitro activity against Gram-positive organisms. Further prospective study is needed that will incorporate our current predicted pattern of bacterial resistance and patients’ risk factors.
Acknowledgments
The authors would like to thank Mr Elias Mamo Alemayehu, the research assistant at the Hamad Medical Corporation Pediatric Hematology and Oncology unit, for his contribution with data collection from patient files. This research was supported by the Medical Research Center, Hamad Medical Corporation, Doha, Qatar.
Disclosure
The authors report no conflicts of interest in this work.
References
Castagnola E, Rossi MR, Cesaro S, et al. Incidence of bacteremias and invasive mycoses in children with acute non-lymphoblastic leukemia: results from a multi-center Italian study. Pediatr Blood Cancer. 2010; 55(6):1103–1107. | |
Katsibardi K, Papadakis V, Charisiadou A, Pangalis A, Polchronopoulou S. Blood stream infections through the entire course of acute lymphoblastic leukemia treatment. Neoplasma. 2011;58(4):326–330. | |
Quarello P, Saracco P, Giacchino M, et al. Epidemiology of infections in children with acquired aplastic anaemia: a retrospective multicenter study in Italy. Eur J Haematol. 2012;88(6):526–534. | |
Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer. 2013;60(6):923–927. | |
Simon A, Ammann RA, Bode U, et al. Healthcare-associated infections in pediatric cancer patients: results of a prospective surveillance study from university hospitals in Germany and Switzerland. BMC Infect Dis. 2008;8:70. | |
Ha YE, Song JH, Kang WK, et al. Clinical factors predicting bacteremia in low-risk febrile neutropenia after anti-cancer chemotherapy. Support Care Cancer. 2011;19(11):1761–1767. | |
Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2011;52(4):e56–e93. | |
Jones GR, Konsler GK, Dunaway RP, Pusek SN. Infection risk factors in febrile, neutropenic children and adolescents. Pediatr Hematol Oncol. 1996;13(3):217–229. | |
Chanock SJ, Pizzo PA. Infectious complications of patients undergoing therapy for acute leukemia: current status and future prospects. Semin Oncol. 1997;24(1):132–140. | |
Quadri TL, Brown AE. Infectious complications in the critically ill patient with cancer. Semin Oncol. 2000;27(3):335–346. | |
Paganini HR, Sarkis CM, De Martino MG, et al. Oral administration of cefixime to lower risk febrile neutropenic children with cancer. Cancer. 2000;88(12):2848–2852. | |
Crokaert F. Febrile neutropenia in children. Int J Antimicrob Agents. 2000;16(2):173–176. | |
Albano EA, Pizzo PA. Infectious complications in childhood acute leukemias. Pediatr Clin North Am. 1988;35(4):873–901. | |
Zinner SH. Changing epidemiology of infections in patients with neutropenia and cancer: emphasis on gram-positive and resistant bacteria. Clin Infect Dis. 1999;29(3):490–494. | |
Meir HM, Balawi IA, Meer HM, Nayel H, Al-Mobarak MF. Fever and granulocytopenia in children with acute lymphoblastic leukemia under induction therapy. Saudi Med J. 2001;22(5):423–427. | |
Maschmeyer G. Interventional antimicrobial therapy in febrile neutropenic patients. Diagn Microbiol Infect Dis. 1999;34(3):205–212. | |
Martino P, Girmenia C. The role of antimicrobial policies in neutropenic patients. Int J Antimicrob Agents. 2000;16(3):303–307. | |
Pizzo PA. Fever in immunocompromised patients. N Engl J Med. 1999;341(12):893–900. | |
Pizzo PA. Management of fever in patients with cancer and treatment-induced neutropenia. N Engl J Med. 1993;328(18):1323–1332. | |
Santolaya ME, Alvarez AM, Avilés CL, et al. Prospective evaluation of a model of prediction of invasive bacterial infection risk among children with cancer, fever, and neutropenia. Clin Infect Dis. 2002;35(6):678–683. | |
Kern WV. Modifications of therapy. Int J Antimicrob Agents. 2000; 16(2):139–141. | |
O’Grady NP, Alexander M, Dellinger EP, et al; Healthcare Infection Control Practices Advisory Committee. Guidelines for the prevention of intravascular catheter-related infections. Infect Control Hosp Epidemiol. 2002;23(12):759–769. | |
Mermel LA, Farr BM, Sherertz RJ, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001;32(9):1249–1272. | |
Overturf GD. American Academy of Pediatrics, Committee on Infectious Diseases. Technical report: prevention of pneumococcal infections, including the use of pneumococcal conjugate and polysaccharide vaccines and antibiotic prophylaxis. Pediatrics. 2000; 106(2 Pt 1):367–376. | |
Cockerill FR, Wickler MA, Alder J, et al. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. | |
Adams-Haduch JM, Paterson DL, Sidjabat HE, et al. Genetic basis of multidrug resistance in Acinetobacter baumannii clinical isolates at a tertiary medical center in Pennsylvania. Antimicrob Agents Chemother. 2008;52(11):3837–3843. | |
Auletta JJ, O’Riordan MA, Nieder ML. Infections in children with cancer: a continued need for the comprehensive physical examination. J Pediatr Hematol Oncol. 1999;21(6):501–508. | |
Asturias EJ, Corral JE, Quezada J. Evaluation of six risk factors for the development of bacteremia in children with cancer and febrile neutropenia. Curr Oncol. 2010;17(2):59–63. | |
Kelly MJ, Vivier PM, Panken TM, Schwartz CL. Bacteremia in febrile nonneutropenic pediatric oncology patients. Pediatr Blood Cancer. 2010;54(1):83–87. | |
Paesmans M. Risk factors assessment in febrile neutropenia. Int J Antimicrob Agents. 2000;16(2):107–111. | |
Okamoto Y, Ribeiro RC, Srivastava DK, Shenep JL, Pui CH, Razzouk BI. Viridans streptococcal sepsis: clinical features and complications in childhood acute myeloid leukemia. J Pediatr Hematol Oncol. 2003;25(9):696–703. | |
Viscoli C, Cometta A, Kern WV, et al; International Antimicrobial Therapy Group of the European Organization for Research and Treatment of Cancer. Piperacillin-tazobactam monotherapy in high-risk febrile and neutropenic cancer patients. Clin Microbiol Infect. 2006;12(3):212–216. | |
Duncan C, Chisholm JC, Freeman S, Riley U, Sharland M, Pritchard-Jones K. A prospective study of admissions for febrile neutropenia in secondary paediatric units in South East England. Pediatr Blood Cancer. 2007;49(5):678–681. | |
Pereira CA, Marra AR, Camargo LF, et al; Brazilian SCOPE Study Group. Nosocomial bloodstream infections in Brazilian pediatric patients: microbiology, epidemiology, and clinical features. PLoS One. 2013;8(7):e68144. | |
Aledo A, Heller G, Ren L, et al. Septicemia and septic shock in pediatric patients: 140 consecutive cases on a pediatric hematology-oncology service. J Pediatr Hematol Oncol. 1998;20(3):215–221. | |
El-Mahallawy HA, El-Wakil M, Moneer MM, Shalaby L. Antibiotic resistance is associated with longer bacteremic episodes and worse outcome in febrile neutropenic children with cancer. Pediatr Blood Cancer. 2011;57(2):283–288 | |
Orudjev E, Lange BJ. Evolving concepts of management of febrile neutropenia in children with cancer. Med Pediatr Oncol. 2002;39(2):77–85. | |
Paganini H, Bologna R, Debbag R, et al. Fever and neutropenia in children with cancer in one pediatric hospital in Argentina. Pediatr Hematol Oncol. 1998;15(5):405–413. | |
Ramphal R. Changes in the etiology of bacteremia in febrile neutropenic patients and the susceptibilities of the currently isolated pathogens. Clin Infect Dis. 2004;39 Suppl 1:S25–S31. | |
Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. Lancet. 2005;365(9465):1175–1188. | |
Cetinkaya Y, Falk P, Mayhall CG. Vancomycin-resistant enterococci. Clin Microbiol Rev. 2000;13(4):686–707. | |
Caselli D, Cesaro S, Ziino O, et al; Infection Study Group of the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP). Multidrug resistant Pseudomonas aeruginosa infection in children undergoing chemotherapy and hematopoietic stem cell transplantation. Haematologica. 2010;95(9):1612–1615. | |
Trecarichi EM, Tumbarello M, Caira M, et al. Multidrug resistant Pseudomonas aeruginosa bloodstream infection in adult patients with hematologic malignancies. Haematologica. 2011;96(1):e1–e3; author reply e4. | |
Schwalbe RS, Stapleton JT, Gilligan PH. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987; 316(15):927–931. | |
Biavasco F, Vignaroli C, Varaldo PE. Glycopeptide resistance in coagulase-negative staphylococci. Eur J Clin Microbiol Infect Dis. 2000;19(6):403–417. | |
Srinivasan A, Dick JD, Perl TM. Vancomycin resistance in staphylococci. Clin Microbiol Rev. 2002;15(3):430–438. | |
Miedema KG, Winter RH, Ammann RA, et al. Bacteria causing bacteremia in pediatric cancer patients presenting with febrile neutropenia – species distribution and susceptibility patterns. Support Care Cancer. 2013;21(9):2417–2426. | |
Basu SK, Fernandez ID, Fisher SG, Asselin BL, Lyman GH. Length of stay and mortality associated with febrile neutropenia among children with cancer. J Clin Oncol. 2005;23(31):7958–7966. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



