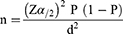Back to Journals » Infection and Drug Resistance » Volume 15
Bacterial and Fungal Profile, Antibiotic Susceptibility Patterns of Bacterial Pathogens and Associated Risk Factors of Urinary Tract Infection Among Symptomatic Pediatrics Patients Attending St. Paul’s Hospital Millennium Medical College: A Cross-Sectional Study
Authors Bitew A , Zena N, Abdeta A
Received 12 January 2022
Accepted for publication 28 March 2022
Published 6 April 2022 Volume 2022:15 Pages 1613—1624
DOI https://doi.org/10.2147/IDR.S358153
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Adane Bitew,1 Nuhamen Zena,2 Abera Abdeta3
1Department of Medical Laboratory Science, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 2Department of Microbiology, St Paul Hospital Millennium Medical College, Addis Ababa, Ethiopia; 3National Clinical Bacteriology and Mycology Reference Laboratory, Ethiopian Public Health Institute, Addis Ababa, Ethiopia
Correspondence: Abera Abdeta, National Clinical Bacteriology and Mycology Reference Laboratory, Ethiopian public health institute, P.O. Box:1242, Addis Ababa, Ethiopia, Tel +251911566420, Email [email protected]
Background: Urinary tract infection is a common infection in pediatrics. Understanding the common etiology of urinary tract infections, their antimicrobial susceptibility pattern, and associated risk factors in a particular setting can provide evidence for the appropriate treatment of the cases.
Purpose: The current study is aimed to determine the common etiology and prevalence of uropathogens associated with urinary tract infection, as well as the antibiotic susceptibility profile of bacterial isolates, and to identify risk factors associated with urinary tract infection among pediatric patients.
Materials and Methods: The study was conducted at St. Paul Hospital Millennium Medical College between October 2019 and July 2020. Urine was collected aseptically from patients, inoculated onto culture media, and incubated at 37 °C for 18– 48 hours. Bacteria and yeast were identified following standard procedures. Antibiotic susceptibility testing of bacterial pathogens was carried out by the Kirby Bauer disc diffusion method. Descriptive statistics and logistical regressions were used to estimate the crude ratio with a 95% confidence interval. P-value < 0.05 was considered significant.
Results: Significant bacterial/fungal growth was observed in 65 samples giving a prevalence of 28.6% of which 75.4% (49/65) and 24.6% (16/65) were bacterial and fungal pathogens, respectively. About 79.6% of bacterial etiology were Escherichia coli and Klebsiella pneumoniae. The highest resistance was observed against ampicillin (100%), cefazolin (92.1%), and trimethoprim-sulfamethoxazole (84.1%), both of which are commonly used for empirical treatment in Ethiopia. Length of hospital stay (P=0.01) and catheterization (P=0.04) were statistically associated with urinary tract infection.
Conclusion: The high prevalence of urinary tract infection was observed in our study. Enterobacteriaceae were the major cause of urinary tract infection. Length of hospital stay and catheterization were significantly associated with urinary tract infection. Both Gram-negative and Gram-positive bacteria were extremely resistant to ampicillin and trimethoprim-sulfamethoxazole.
Keywords: antibiotic susceptibility pattern, pediatrics, urinary tract infection, Ethiopia
A Letter to the Editor has been published for this article.
A Response to Letter has been published for this article.
Introduction
Urinary tract infection (UTI) caused by bacteria and yeast is one of the most common urinary tract system disorders in children.1 In developing countries, it is the third most common infection in the pediatric age group after respiratory and gastrointestinal tract infections.2 Urinary tract infection in children has been associated with short-term morbidity including fever, dysuria, urinary urgency, and flank pain. It also causes long-term renal damage, such as permanent kidney scarring and long-term problems including hypertension and renal failure.3 Wennerstrom et al 15 depicted that renal scarring was present in about 15% of children following a first UTI, highlighting the importance of prompt diagnosis and early treatment of UTI. In addition, healthcare-associated urinary tract management expenditure is considerably high.3,4 Many studies conducted on pediatric UTIs in different developing countries documented prevalence rates of UTI ranging from 16% to 34%.5–9 Moreover, up to 8% of children between the ages of 1 month and 11 years develop at least one UTI10 and up to 30% of infants and children are known to experience recurrent infections during the first 6–12 months after initial UTI.11
Both Gram-negative and Gram-positive bacteria, as well as certain Candida spp, can cause UTIs. E. coli is the most prevalent cause of UTIs, followed by K. pneumoniae.12 Studies have shown that Candida species, particularly Candida albicans remain the most common cause of candida urinary tract infections in children.13 Age, circumcision status, and indwelling catheters are risk factors for acquiring a UTI in children. Boys are more vulnerable during the first year of life, after which, due to differences in sex organs, the incidence is primarily higher in girls and uncircumcised male infants are at higher risk.1,33 The antibiotic susceptibility pattern of uropathogens varies with time, geographic location, demography, and clinical characteristics of patients.1
Infectious diseases such as UTIs are thought to be responsible for 26% of deaths worldwide, with 98% of them occurring in low-income nations.14 A study conducted in Nepal and India among pediatric patients reported an overall prevalence of UTIs of 57%15 and 48%,16 respectively. A hospital-based study in South African children revealed that urinary tract infection accounts for 11% of health-care infections.17 Another study in Kenya found that urinary tract infection contributed about 11.9% of the burden of febrile infections in young children.18
In Ethiopia, few studies were conducted to determine urinary tract infections among pediatric patients: the study at Hawassa referral hospital, Yekatit 12 hospital, Felege-Hiwot specialized, and Gondar University hospitals revealed 27.5%,19 15.9%,20 16.7%,21 and 26.45%,22 respectively. The lack of urine culture in health facilities with different levels in developing countries including Ethiopia remained impractical as they are resource-intensive. Consequently, the spectrum of the etiological agents of UTI and their drug susceptibility profile in Ethiopia is hardly known. To this end, the present study aimed to determine the prevalence of urinary tract infections, profiling of bacterial and fungal pathogens associated with UTI, determine the anti-microbial susceptibility profile of bacterial isolates, and identify major predisposing factors associated with UTI.
For this study, the following definitions were applied:
- Significant bacterial/yeast growth: Defined by the presence of a positive urine culture with no more than two pathogens and at ≥ 103 colony-forming units (CFU/mL) for catheterized patients and ≥105 Voided midstream urine for patients.23
- Community-acquired: positive urine cultures in the community setting or the hospital environment with less than 48 hours of admission.
- Ward-acquired UTI: Positive urine culture within the first 48 h of intensive care unit (ICU) stay.
- ICU-acquired UTI: Positive urine culture after 48 h of ICU stay.
Materials and Methods
Study Area, Design, and Period
A hospital-based cross-sectional study was conducted at St. Paul Hospital Millennium Medical College (SPHMMC) in the Department of Pediatrics, from October 2019 to July 2020, Addis Ababa, Ethiopia.
Population
Source of Population
All pediatric in-patients and out-patients attended the Department of pediatrics during the study period.
Study Population
All pediatric in-patients and out-patients, with signs and symptoms of UTI, attended the study site within the study period.
Inclusion and Exclusion Criteria
Inclusion Criteria
- Pediatric patients less than or equal to 16 years of age;
- Fever which was defined as the axillary temperature of 37.5°C or higher at presentation;
- At least two episodes of vomiting within two days of presentation at a study site.
- Dysuria as complained by older children or crying during urination for young children as reported by the mother.
- The urinary frequency which is defined as passing urine more frequently than that used to be normal for the child.
- Urinary urgency as complained in older children.
- Flank pain/tenderness or urinary color change.
Exclusion Criteria
- Pediatric patients who have received antibiotics in the last two weeks.
- Children that were not willing to complete consent.
Sample Size Determination
The sample size was determined by using the formula for a single proportion sample size calculation by taking a 95% confidence interval, a 5% margin of error, and a prevalence of UTI from earlier work [15.9% or P= 0.159)] Merga Duffa et al20 conducted at Addis Ababa as shown below.
Where n = required initial sample size;
Z α/2 = critical value for normal distribution at 95% confidence interval which is equal to 1.96 (Z value at alpha = 0.05);
P = prevalence of UTI (15.9%) from the earlier study;
D = margin of error which is equal to 5% and α =is the level of error one is willing to tolerate; substituting these into the formula, n= (1.96)2 0.159 (1–0.159)/(0.05)2=206 and assuming 10% non-respondents n =206+206/10 = 227.
Sampling Method
A convenient sampling method was used for this study. The data was collected until the required sample size was achieved.
Measurement and Data Collection
Data Collection Procedure
Data were collected after obtaining written informed consent from parents. Socio-demographic characteristics (age, sex, and residence) and associated risk factors (catheterization, previous history of UTI, the human immunodeficiency viruses (HIV) status, circumcision, and length of hospital stay) of study participants were collected by qualified nurses by employing a pre-tested structured questionnaire. Signs and symptoms of patients and underlying diseases were recorded by attending pediatricians.
Data Quality Assurance and Quality Control
Pre-analytical: Socio-demographic characteristics of study participants (age, sex, etc.) and clinical and treatment information were collected from the questionnaire.
Analytical: The performance of autoclaves, incubators, reagents, microscopes and the microbiological quality of culture medium (the sterility of culture media and growth performance of each culture medium) were evaluated as per standard procedures before they are used. Collection of clinical samples and transportations were carried out following an aseptic procedure. Inoculation of clinical samples was performed under a level II safety cabinet.
Post-analytical: All the extracted information such as laboratory findings were checked for eligibility, completeness, and consistency and recorded before entry into a statistical tool. The data were also kept in a secure location. Bacterial and yeast isolates were stored as per the standard operational procedure (SOP) of the St. Paul’s Hospital Millennium Medical College (SPHMMC).
Statistical Analysis
All data from the investigation were coded, double entered, and analyzed using Statistical Package for Social Sciences (SPSS) software version 23. Descriptive statistics and logistical regressions were used to estimate the crude ratio with a 95% confidence interval to the different variables. P-value < 0.05 was considered significant.
Laboratory Investigation
Sample Collection and Inoculation
Urine samples were collected from each pediatric patient with a sterile urine container. Study participants’ parents or guardians were given appropriate instructions on how to collect clean-catch midstream urine samples. Catheterized and suprapubic urine samples were collected by trained nurses and physicians. Immediately after collection, the samples were brought to the Microbiology laboratory of SPHMMC for further processing. Part of the sample was inoculated onto MacConkey agar plate (Oxoid, Basingstoke, and Hampshire, England) and blood agar (Oxoid, Basingstoke, and Hampshire, England) medium using a calibrated loop with a capacity of 1 μL in a safety cabinet. The remaining sample was inoculated onto Brain Heart infusion agar supplemented with chloramphenicol (100µgml−1) and gentamycin (50 µgml−1), (Oxoid, Basingstoke, and Hampshire, England).
Incubation
All inoculated plates were incubated at 37 °C for 18–48 h aerobically and inspected for the growth of bacteria and/or yeasts. Colony counts yielding bacterial or yeast growth of ≥105cfu/ mL of urine were regarded as significant growth. Urine samples that yielded three or more species were not considered for further investigation.
Bacterial Identification
Pure isolates of the bacterial pathogen were preliminarily characterized by colony morphology, Gram-stain. Gram-positive bacteria were further, characterized by using catalase, bile esculin agar, Pyrrolidonyl Aminopeptidase (PRY), and rabbit plasma. Gram-negative bacteria were identified to the species level by conventional biochemical tests like (urease test, indole test, citrate utilization test, triple sugar iron test, Hydrogen sulfide (H2S) production test, lysine Iron agar test, motility test, and oxidase test).
Yeast Identification
Yeasts were identified employing conventional routine diagnostic methods such as Gram stain, Germ tube test, carbohydrate fermentation, and assimilation test, and by employing chromogenic medium (CHROMagar Candida medium, bioM´erieux, France) as per the instruction of the manufacturer.
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility test was carried out by Kirby Bauer disc diffusion method as per Clinical Laboratory Standards Institute (CLSI) guidelines24 on Mueller Hinton agar (Oxoid, Basingstoke, England). A bacterial suspension of each isolate was prepared in 0.5 mL of nutrient broth medium and the turbidity was adjusted to match that of 0.5 McFarland standards to obtain approximately the organism number of 1×106 colony forming units (CFU) per mL. A sterile swab was dipped into the suspension and the excess material was removed by pressing it against the sides of the tube. Then, the swab was applied to the center of the Mueller Hinton agar plate and evenly spread on the medium. Antibiotic discs were placed within 15 min of inoculation to Mueller Hinton agar seeded with each isolate and were incubated for 24h at 35–37 °C. The diameter zone of inhibition was measured using a caliper. The diameter zone inhibitions were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guideline24 as susceptible (S), intermediate (I), or resistant (R). Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922), and Pseudomonas aeruginosa (ATCC 27853) were used as quality control strains to check the efficacy of the antibiotics.
For gram-negative isolates, we used antibiotic discs: amoxicillin/clavulanic acid (30μg); ciprofloxacin (5μg); nitrofurantoin (300μg); ampicillin (10μg); amikacin (30μg); meropenem (10μg); piperacillin-tazobactam (100/10μg); cefazolin (30μg); trimethoprim-sulfamethoxazole (1.25/23.75μg).
Antimicrobial disks used for gram-positive isolates were: penicillin (10 units); cefoxitin (30μg); nitrofurantoin (300μg); vancomycin (30μg); trimethoprim-sulfamethoxazole (1.25/23.75 μg); ciprofloxacin (5μg); doxycycline (30μg). All the antimicrobial disks used in our study were the products of Oxide, Basingstoke, and Hampshire, England.
Results
Patient Characteristics
As shown in Table 1, two hundred twenty-seven (227) pediatric patients presenting or highly suspicious of having UTI and met the selection criteria were recruited in the study. The number of male study participants (138; 60.8%) outnumbered that of female study participants (89; 39.2%) where the female–male ratio was 1.6:1. The number of study subjects across the age group was variable in which patients with an age group of ˂ 3 years was the highest (119; 52.4%) followed by age groups of 13–15 (37; 16.3%) and 3–6 years (31; 13.7%), respectively. The study subjects were urban-dominated, where the urban–rural ratio is 2.4:1 (Table 1).
 |
Table 1 Socio-Demographic Characteristics of Study Subjects and Frequency of Culture-Positive Samples (N= 227) |
Prevalence of UTIs
Out of two hundred twenty-seven (227), urine samples collected significant bacterial/yeast growth were observed in 65 samples giving an overall prevalence of 28.6% (65/227) of which 21.6% (49/227) were bacterial pathogens while 7% (16/227) were fungal pathogens. The prevalence of UTI was the highest in the age group of 13–15 years of age with 17/37 (46.0%) as compared to the lowest value of 2/21 (9.5%) in the age group of 10–12 years (Table 2). Urinary tract infection was higher in females with 30/89 (33.7%) as compared to 35/138 (25.4%) in males.
 |
Table 2 Prevalence of UTI Based on Patient’s Admission Source and Type of Pathogen |
Among 49 bacterial isolates, 79.6% (39/49) was Enterobacteriaceae in which E. coli was the most frequent bacterium accounting for 42.9% (21/49) of the total bacterial isolates followed by K. pneumoniae representing 34.6% (17/49) of the bacterial isolates. 4 (8.2%) isolates were represented by Acinetobacter spp, a non-fermentative Gram-negative bacillus. Gram-positive bacteria accounted for only 10.2% (5/49) bacterial isolates in which 3 (60.0%) isolates were Enterococcus species. Of 16 yeast isolates 6 (37.5%) were represented by C. albicans. Out of 26 community-acquired uropathogens, 76.9% (20/26) of the pathogens were E. coli and Klebsiella pneumoniae. Out of 20 ward-acquired uropathogens, 15/20 were bacterial pathogens. Out of 19 intensive care unit acquired uropathogens, 10/19 were yeast species. Out of 65 culture-positive urine samples, 39 (60.0%) were hospital-acquired and 26 (40.0%) were community-acquired (Table 3).
 |
Table 3 Logistic Regression Analysis of Risk Factors Associated Urinary Tract Infection Among Pediatric Patients at SPHMMC (n = 227) |
Out of 227 pediatric patients, 129 stayed less than 3 days at the hospital of which, 25 (19.4%) were culture positive, 120 were admitted to the outpatient department of which, 25 (20.8%) were culture positive, 63 had the previous history of urinary tract infection of which, 23 (37.70%) were culture positive, 38 were on indwelling catheters of which 20 (52.6%) were culture positive, 71 had a temperature of >37.5°C of which 21 (29.6%) were culture positive (Table 3).
Factors Associated with UTIs
The predictor variables for UTI were subjected to bivariate analysis, and they had the following logistic regression values: Length of hospital stay for 3–6 months (COR 2.122; 95% CI: 3.31–3.43; P= 0.002) and catheterization (COR = 3.56; 95% CI: 1.73–7.1; P = 0.001). The bivariate significant predictor variables for UTI were subjected to multiple regression analysis, and they had the following logistic regression values: Length of hospital stay for 3–6 months (AOR = 6.06, 95% CI: 1.99–18.4; P = 0.01) and catheterization (AOR = 0.28; 95% CI: 0.13–0.57, P =0.04). Length of hospital stay for 3–6 months had a statistically significant correlation with UTI (P= 0.01). The association of UTI with catheterization was also statistically significant (P= 0.04). However, residence, sex, age, admission source, previous history of UTI, HIV status, body temperature, and chronic infections were found to have no significant association with UTI (Table 3).
Antibiotic Susceptibility Profile Bacterial Isolates
The overall antimicrobial susceptibility pattern of both Gram-negative and Gram-positive bacteria against the nine antibiotics evaluated is depicted in Tables 4 and 5. Amikacin and meropenem were the most active drugs against Gram-negative bacteria tested with a resistance rate of 4.6% and 9.1%, respectively. Gram-negative bacteria were the most resistant against ampicillin, cefazolin, and trimethoprim-sulfamethoxazole amongst all the drugs tested with a resistance rate of 100%, 92.1%, and 84.1%, respectively. E. coli, the most commonly recovered species were more resistant to ampicillin (100%), cefazolin (90.5%), and trimethoprim-sulfamethoxazole (80.0%). K. pneumoniae, the second most commonly isolated bacterium revealed a resistance rate of 94.1% for cefazolin and 88.2% for trimethoprim/sulfamethoxazole Table 4. The highest overall resistance rate of Gram-positive bacteria was observed in trimethoprim/sulfamethoxazole (100%) but all isolates (100%) of Gram-positive bacteria were susceptible to oxacillin (Table 5).
 |
Table 4 Antimicrobial Susceptibility Pattern of Gram-Negative Bacteria |
 |
Table 5 Antimicrobial Susceptibility Pattern of Gram-Positive Bacteria |
Discussion
Urinary tract infection (UTI) remains one of the most common causes of morbidity in pediatric practice. Early diagnosis of UTI in children is central as it can be the indicator of renal abnormalities such as scarring, hypertension, and end-stage renal diseases. In our study, the prevalence of urinary tract infection was 28.6% of which 21.6% were due to bacterial pathogens while 7% was due to fungal pathogens. The magnitude of UTI due to bacteria in our study was higher than the prevalence rate of 15.9% reported from Ethiopia by Merga Duffa et al,20 but a relatively similar with a prevalence rate of 27.5% demonstrated by a recent study conducted in Ethiopia by Mitiku et al.19 As far as our reference goes the incidence of UTI due to yeast in Ethiopians particularly in children is unknown. This is because fungal diseases in general in Ethiopia are not considered as important as bacterial and viral diseases. Therefore, the prevalence rate of 7% UTI due to yeast in pediatric patients reported by our study was the first for the country. The prevalence rate of UTI caused by yeast reported in our study was consistent with a prevalence rate of 5.2% reported in a study conducted in children by Seifi et al.25 However, a prevalence rate of 16.5% and 19.0% have been reported by Zarei-Mahmoudabad et al26 and by Alkilani et al27 in Iran and Egypt, respectively. The higher prevalence rate in these two studies is not surprising, since the study subjects included were ICU patients without age preference. The disparity in the prevalence rates of UTI among studies could have emanated from the difference in study design, socio-demographic characteristics of study subjects, and comorbidities.
In the current study, 60% of UTIs were hospital-acquired (intensive care unit and ward acquired). A similar result (78.5%) was observed by Aubron et al28 although the prevalence rates of UTI in developing countries vary from study to study and from region to region there are no regional differences concerning bacterial and fungal pathogens causing UTI. The most common bacteria recovered from urine culture are Gram-negative bacilli with a preponderance of Escherichia coli followed by Klebsiella pneumoniae.6,29,30 In concordance with earlier similar studies,29,30 our study also revealed that E. coli was the most frequent bacterium accounting for 42.9% of the total bacterial isolates followed by K. pneumoniae representing 34.6% of the bacterial isolates. E. coli was the most common bacterial pathogen in both community and hospital-acquired UTI (57.1% and 42.9%, respectively). Multiple studies showed that within hospital settings at least 10–15% of hospital-acquired UTIs are caused by Candida species and candiduria is especially common in intensive care units.31–33 In our study, candiduria represented 7% UTIs and 94% were hospital-acquired in which 62.5% of them were observed in ICU patients. C. albicans was the leading cause of candiduria and 81.1% candida species were isolated from ward-acquired positive urine culture and ICU acquired positive urine culture samples. Our result is not surprising given that candida species are opportunistic pathogens and cause disease in immune-compromised patients like ICU patients.
In the present study, females were more affected by UTI as compared to males and patients in the age group of 12–15 are more affected. However, the difference in both cases was not statistically significant. The lack of association of UTI with sex and age may be described by the predominant age bracket of the recruited patients. Given the known epidemiological pattern of UTI, the magnitude commonly seems to be equal for both sex during infancy, with male predominance in the neonatal period and female predominance during early childhood and the period of toilet training. Among other statistically analyzing risk factors, length of hospital stay for 3–30 days was statistically associated with UTI (P=0.01). The association of the length of hospital stay with UTI was observed in other studies.34,35 UTI in our study was also significantly associated with catheterization (P= 0.04). According to Gokula et al35 and Saint et al36 catheterizations escalate the threat of UTI by 3% to 10% depending on the length of catheterization. The problem of aseptic precaution during catheter insertion, infrequent change of catheter, and improper catheter care may explain an increase in catheter-associated UTI.
During the study period, the number of pediatric patients under the age of three who were admitted to the hospital with urinary tract infection symptoms was higher than that of other age groups. This might be explained by the fact that this age is the age of toilet training, which is consistent with other studies.37–39
In the present study, Gram-negative bacteria were the most resistant to ampicillin and trimethoprim-sulfamethoxazole with a resistance rate of 100% and 84.1%, respectively. E. coli, and K. pneumoniae the most commonly recovered species were more resistant to ampicillin (100%), and trimethoprim-sulfamethoxazole (81.0%). Similarly, the highest overall resistance rate of Gram-positive bacteria was also observed in trimethoprim/sulfamethoxazole (100%). Ampicillin and trimethoprim-sulfamethoxazole have been widely used as first-line drugs for the empirical treatment of UTI in Ethiopia in all health facilities as per the recommendation of standard treatment guidelines (STGs) of the ministry health.40–42 This may explain the high resistance rates of both Gram-negative and Gram-positive bacteria against ampicillin and trimethoprim-sulfamethoxazole in the present study. Continued use of a drug in a community increases the likely hood of selection and maintenance of drug-resistant strains in that environment.43–45 On the other hand, our study demonstrated that amikacin and meropenem were the most active drugs against Gram-negative bacteria while Oxacillin was the most active drugs against Gram-positive bacteria. The data in this article was extracted from Nuhamen Zena’s unpublished thesis, which was uploaded to the Addis Ababa University institutional repository.46
Limitation
Due to resource restrictions, we were unable to conduct antifungal susceptibility testing for the fungal pathogens we identified in this study.
Conclusion
The overall prevalence of UTIs was 28.6% of which 75.4% (49/65) were bacterial-associated UTIs while 24.6% (19/65) were UTIs due to yeast. Enterobacteriaceae were the major cause of UTIs. Both C. albicans and non-albicans Candida species were involved in causing UTI due to yeast, especially in ICU patients. Length of hospital stay from 3 to 6 months and catheterization were significantly associated with UTI. Both Gram-negative and Gram-positive bacteria were extremely resistant to ampicillin and trimethoprim-sulfamethoxazole recommended for empirical treatment of UTI by Ministry Health. Further work should be carried out on pediatric UTI and ampicillin and trimethoprim-sulfamethoxazole as drugs of choice for the empirical treatment of UTI should be reconsidered.
Data Sharing Statement
Raw data are available upon reasonable request from the corresponding author.
Ethics Approval and Consent to Participate
The research was carried out in accordance with Declaration of Helsinki. All ethical considerations and obligations were duly addressed and the study was conducted after obtaining ethical clearance from the Internal Review Board of the Department of Medical Laboratory Science, College of Health Science, Addis Ababa University, and permission from SPHMMC. Since our study involved children (below 16 years of age) they were not able to give true written consent. Therefore, the assent form was completed by parents/guardians. Briefly, the aim of the work and its benefits were clearly described to each parent/guardian. Parents/guardians were informed that the personal information of each child will be treated confidentially. Parents/guardians were informed that if he/she did not agree to participate in the study his or her child is not obliged to be involved in the study. Once they are consented to participate in the study and are disinterested to continue, they are free to withdraw from the study at any time of the course of the study.
Acknowledgments
We would like to acknowledge the attending pediatrician at the study sites for critical review of patients from the viewpoint of clinical presentation. We are also very grateful to the patients who participated in the study. We’d also want to express our gratitude to Nuhamen Zena for allowing us to extract important data from her unpublished study, which was uploaded to the Addis Ababa university repository.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27:302. doi:10.1097/INF.0b013e31815e4122
2. Srivastava RN, Bagga A. Urinary Tract Infection. In: Srivastava RN, Bagga A, Editors. Pediatric Nephrology.
3. Wennerstrom M, Hansson S, Jodal U, Stokland E. Primary and acquired renal scarring in boys and girls with urinary tract infection. J Pediatr. 2000;136:30–34. doi:10.1016/S0022-3476(00)90045-3
4. Millner R, Becknell B. Urinary tract infections. Pediatr Clin North Am. 2019;66:1–13. doi:10.1016/j.pcl.2018.08.002
5. Rabasa AI, Shattima D. Urinary tract infection in severely malnourished children at the University of Maiduguri Teaching Hospital. J Trop Pediatr. 2002;48:359–361. doi:10.1093/tropej/48.6.359
6. Page A-L, de Rekeneire N, Sayadi S, et al. Infections in children admitted with complicated severe acute malnutrition in Niger. PLoS One. 2013;8:e68699. doi:10.1371/journal.pone.0068699
7. Uwaezuoke SN, Ndu IK, Eze IC. The prevalence and risk of urinary tract infection in malnourished children: a systematic review and meta-analysis. BMC Pediatr. 2019;19:261. doi:10.1186/s12887-019-1628-y
8. Thuo N, Ohuma E, Karisa J, Talbert A, Berkley JA, Maitland K. The prognostic value of dipstick urinalysis in children admitted to hospital with severe malnutrition. Arch Dis Child. 2010;95:422–426. doi:10.1136/adc.2009.168211
9. Caksen H, Cesur Y, Uner A, et al. Urinary tract infection and antibiotic susceptibility in malnourished children. Int J Urol Nephrol. 2000;32:245–247. doi:10.1023/A:1007104326689
10. Marild S, Jodal U. Incidence rate of first-time symptomatic urinary tract infection in children under 6 years of age. Acta Paediatr. 1998;87:549–552. doi:10.1111/j.1651-2227.1998.tb01502.x
11. Mangiarotti P, Pizzini C, Fanos V. Antibiotic prophylaxis in children with relapsing urinary tract infections: review. J Chemother. 2000;12:115–123. doi:10.1179/joc.2000.12.2.115
12. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–284. doi:10.1038/nrmicro3432
13. Behzadi P, Behzadi E, Ranjbar R. Urinary tract infections and Candida albicans. Cent Eur J Urol. 2015;68(1):96. doi:10.5173/ceju.2015.01.474
14. Gachuhi GT. Antibiotic susceptibility pattern of bacterial uropathogens isolated from patients in Nakuru level 5 hospital, Kenya. Kenyatta University; 2017.
15. Maharjan G, Khadka P, Siddhi Shilpakar G, Chapagain G, Dhungana GR. Catheter-associated urinary tract infection and obstinate biofilm producers. Can J Infect Dis Med Microbiol. 2018;2018:1–7. doi:10.1155/2018/7624857
16. Sargiary P, Baro L, Choudhry G, Saikia L. Bacteriological profile and antimicrobial susceptibility pattern of community acquired urinary tract infection in children: a tertiary care experience. J Dental Med Sci. 2016;15(6):61–65.
17. Dramowski A, Whitelaw A, Cotton MF. Burden, spectrum, and impact of healthcare-associated infection at a South African children’s hospital. J Hosp Infect. 2016;94(4):364–372. doi:10.1016/j.jhin.2016.08.022
18. Masika WG, O’Meara WP, Holland TL, Armstrong J, Arez AP. Contribution of urinary tract infection to the burden of febrile illnesses in young children in rural Kenya. PLoS One. 2017;12(3):e0174199. doi:10.1371/journal.pone.0174199
19. Mitiku E, Amsalu A, Tadesse BT. Pediatric urinary tract infection as a cause of outpatient clinic visits in southern Ethiopia: a cross sectional study. Ethiop J Health Sci. 2018;28(2):187–196. doi:10.4314/ejhs.v28i2.10
20. Merga Duffa Y, Terfa Kitila K, Mamuye Gebretsadik D, Bitew A. Prevalence and antimicrobial susceptibility of bacterial uropathogens isolated from pediatric patients at yekatit 12 hospital medical college, Addis Ababa, Ethiopia. Int J Microbiol. 2018;2018:1–8. doi:10.1155/2018/8492309
21. Fenta A, Dagnew M, Eshetie S, Belachew T. Bacterial profile, antibiotic susceptibility pattern and associated risk factors of urinary tract infection among clinically suspected children attending at Felege-Hiwot comprehensive and specialized hospital, Northwest Ethiopia. A prospective study. BMC Infect Dis. 2020;20(1):1.
22. Ayelign B, Abebe B, Shibeshi A, et al. Bacterial isolates and their antimicrobial susceptibility patterns among pediatric patients with urinary tract infections. Turk J Urol. 2018;44(1):62. doi:10.5152/tud.2017.33678
23. Leber AL. Clinical Microbiology Procedures Handbook.
24. Performance CLSI. Standards for Antimicrobial Susceptibility Testing.
25. Seifi Z, Azish M, Salehi Z, Zarei Mahmoudabadi A, Shamsizadeh A. Candiduria in children and susceptibility patterns of recovered Candida species to antifungal drugs in Ahvaz. J Nephropathol. 2013;2:122–128. doi:10.12860/JNP.2013.20
26. Zarei-Mahmoudabadi A, Zarrin M. Ghanatir F Vazirianzadeh B. Candiduria in hospitalized patients in teaching hospitals of Ahvaz. Iranian J Microbiol. 2012;4:198–203.
27. Alkilani AA, El Shalakany AH, El-Masry EA, Awad ET, Mohamad EA. Nosocomial candiduria in critically Ill patients admitted to intensive care units in Menoufia University Hospitals, Egypt. Br J Med Med Res. 2016;15:1–15. doi:10.9734/BJMMR/2016/25940
28. Aubron C, Suzuki S, Glassford NJ, et al. Epidemiology of bacteriuria and candiduria in critically ill patients. Epidemiol Infec. 2015;143:655–662. doi:10.1017/S0950268814000934
29. Pradeep N, Shetty PN, Prashanth S, Jagadeeshwara S. Prevalence of urinary tract infection among preschool febrile children attending the pediatric OPD. Int J Contemp Pediatr. 2017;4:561–567. doi:10.18203/2349-3291.ijcp20170710
30. Gangina S, Akula S, Naik DR, Chandra TJ. Prevalence of urinary tract infection in febrile children between one to five years of age. Int J Pediatr Res. 2019;6:542–546. doi:10.17511/ijpr.2019.i10.08.:
31. Kauffman CA, Vasquez JA, Sobel JD, Jack D, Sobel D. Prospective multicenter surveillance study of funguria in hospitalized patients. Clin Infect Dis. 2000;30:14–18. doi:10.1086/313583
32. Alvarez-Lerma F, Nolla-Salas J, Leon C, et al. Candiduria in critically ill patients admitted to intensive care medical units. Intensive Care Med. 2003;29:1069–1076. doi:10.1007/s00134-003-1807-y
33. Kauffman CA. Candiduria. Clin Infect Dis. 2005;41:S371–6. doi:10.1086/430918
34. Prudhon C, Prinzo ZW, Briend A, Daelmans BM, Mason JB. Proceedings of the WHO, UNICEF, and SCN informal consultation on community-based management of severe malnutrition in children. Food Nutr Bull. 2006;27:S99–104. doi:10.1177/15648265060273S307
35. Gokula RR, Hickner JA, Smith MA. Inappropriate use of urinary catheters in elderly patients at a midwestern community teaching hospital. Am J Infect Cont. 2004;32:196–199. doi:10.1016/j.ajic.2003.08.007
36. Saint S, Lipsky BA, Goold SD. Indwelling urinary catheters: a one-point restraint? Ann Intern Med. 2002;137:125–127. doi:10.7326/0003-4819-137-2-200207160-00012
37. Karmazyn BK, Alazraki AL, Anupindi SA, et al. ACR appropriateness criteria® urinary tract infection—child. J Am Coll Radiol. 2017;14(5):S362–71. doi:10.1016/j.jacr.2017.02.028
38. Korbel L, Howell M, Spencer JD. The clinical diagnosis and management of urinary tract infections in children and adolescents. Paediatr Int Child Health. 2017;37(4):273–279. doi:10.1080/20469047.2017.1382046
39. Clark CJ, Kennedy WA, Shortliffe LD. Urinary tract infection in children: when to worry. Urol Clin. 2010;37(2):229–241. doi:10.1016/j.ucl.2010.03.009
40. Food, Medicine, and Health Care Administration and Control Authority. Standard Treatment Guideline for Health Centers.
41. Food, Medicine, and Health Care Administration and Control Authority. Standard Treatment Guidelines for Primary Hospitals. Addis Ababa: Food, Medicine, and Health Care Administration and Control Authority; 2010.
42. Food, Medicine, and Health care Administration and control authority. Standard Treatment Guidelines for General Hospitals.
43. Edlin RS, Copp HL. Antibiotic resistance in pediatric urology. Ther Adv Urol. 2014;62:54–61. doi:10.1177/1756287213511508
44. Adjei O, Opoku C. Urinary tract infections in African infants. Int J Antimicrob Agents. 2004;1:32–34. doi:10.1016/j.ijantimicag.2004.02.007
45. Paschke AA, Zaoutis T, Conway PH, Xie D, Keren R. Previous antimicrobial exposure is associated with drug-resistant urinary tract infections in children. Pediatrics. 2010;125:664–672. doi:10.1542/peds.2009-1527
46. Zena N. Profiling of bacterial and fungal uropathogens, antimicrobial susceptibility pattern of bacterial isolates, and associated risk factors among pediatric patients at St Paul Hospital Millennium Medical College (Master’s thesis, Addis Ababa University, Addis Ababa, Ethiopia); 2021. Available from: http://etd.aau.edu.et/handle/123456789/26591.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.