Back to Journals » Open Access Emergency Medicine » Volume 14
Back Plate Marking of a Mechanical Chest Compression Device to Reduce the Duration of Chest Compression Interruptions
Authors Khunpanich S, Pethyabarn W
Received 29 March 2022
Accepted for publication 5 July 2022
Published 2 August 2022 Volume 2022:14 Pages 405—412
DOI https://doi.org/10.2147/OAEM.S368510
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Hans-Christoph Pape
Sireethorn Khunpanich, Wasuntaraporn Pethyabarn
Department of Emergency Medicine, Songklanagarind Hospital, Faculty of Medicine, Prince of Songkhla University, Hat Yai, Songkhla, Thailand
Correspondence: Wasuntaraporn Pethyabarn, Email [email protected]
Objective: To compare the effectiveness of applying the back plate marking method vs the standard method, to a mechanical chest compression device, in regards to reducing the duration of chest compression interruptions during a simulated cardiac arrest.
Methods: An experimental study, one group pretest posttest design, conducted in a university-based hospital from November 2020 to October 2021. The study recruited 20 participants including emergency medical residents and paramedics. The participants were randomized into three-person teams and applied the device in both standard and back plate marking methods in sequential order. Teams were required to use a mechanical chest compression device in a manikin-based OHCA simulation to assess performance.
Results: The median time pause for the deployment of the upper part of the device was significantly reduced (16 vs 21s, P < 0.01) in the back plate marking method, as was the total pause for device deployment (31.5 vs 38.75s, P = 0.03) and the proportion of total hands-off time attributable to device application interruption (43.08% vs 49.18%, P = 0.02). There was no difference between groups in the duration of all compression interruptions (70.5 vs 82.75s, P = 0.20) and compression fractions (77.85 vs 76.91%, P = 0.19).
Conclusion: The back plate marking method was a significantly reduced time of the deployment of the upper part of the device and in regards to the overall pause for device deployment, but there was no difference in CPR quality between the two methods.
Keywords: high performance CPR, mechanical chest compression, cardiopulmonary resuscitation, chest compression interruption, compression fraction
Introduction
Cardiac arrest is one of the critical conditions that requires knowledge, experience and a variety of procedural skills to resuscitate patients. Mechanical chest compression devices have played a role both in-hospital cardiac arrests (IHCA) and out-hospital cardiac arrests (OHCA) especially during the COVID19 pandemic to reduce risk of exposure to virus aerosolization, or in specific settings where the delivery of high-quality manual compressions may be challenging or dangerous for the provider (eg, limited rescuers available, prolonged CPR, in a moving ambulance).1,2
The mechanical chest compression device (LUCAS-3) has been used at the emergency department of Songklanagarind hospital since October 2018. There were a total 147 cases of cardiac arrest (trauma 79 cases, non-trauma 68 cases) in 2019, for which LUCAS-3 was deployed; accounting for 50% of non-traumatic patients, of which 67% are out-hospital cardiac arrests (OHCA).
Mechanical chest compression devices can consistently deliver high-quality chest compressions. This results in similar survival rates and neurological outcomes to manual CPR in OHCA.3–5 The key risk associated with the use of mechanical chest compression devices is the pause in chest compressions associated with a reduction in coronary pressure during the early part of a cardiac arrest; and this may offset the potential benefit of improved chest compression delivery associated with these devices. The American Heart Association recommends minimizing CPR interruptions and maintaining a chest compression fraction, which is defined as the total time spent performing chest compressions divided by the total time taken for the complete resuscitation, of at least 60%.6 According to a study by Dana Yost et al,7 the median chest compression pause in connection to LUCAS deployment was 32.5s (IQR 25–61). Nevertheless, each compression pause should not take longer than 10 seconds, in order to achieve a compression fraction that is greater than 60%.8
Due to long chest compression interruption, subsequent research discovered that re-training, incorporating mechanical CPR into clinical practice, and pit crew training are all contributing factors to improved compression fraction9–11 but no studies have defined how to apply mechanical compression devices to reduce the duration of chest compression pauses.
We also assessed the chest compression pauses, associated with device deployment, in a manikin study which was conducted by emergency care medical residents at Songklanagarind Hospital; and this discovered that the median time was 20 seconds (IQR 14.75–33). Adjusting the position of the suction cup is one of the reasons for the long pause associated with mechanical chest compression devices.
Improving the mechanical deployment process could be an effective strategy for reducing the time required to adjust a mechanical chest compression device and improving CPR quality. This study aimed to compare the effectiveness of the standard method vs the back plate marking method.
Methods
Study Design
This research was an experimental study, one group pre-test post-test design, conducted in a university hospital from November 2020 to October 2021.
Ethics approval was obtained from the Institutional Ethics Committee Board of the Faculty of Medicine at University Hospital. According to our institutional review board protocol, all research information was kept as confidential data in an encrypted file with password protection and limited data access by only the researcher and assistant. The ethical registration number was REC 63–543-20-4.
Study Setting and Population
The study recruited emergency medical residents and paramedics who worked at the University Hospital between November 2020 and June 2021. Informed consent was obtained from all the participants. In addition, if there are any situations where they did not wish to participate, they had the option to terminate their involvement at any time, and without any impact on their training program or work assessments. Individual clinicians were required to have Advanced Life Support (ALS) certification during the previous three years and to have prior mechanical chest compression device experience. Participants were excluded if they suffered an injury that made them unable to use or handle the device, or had respiratory or contact transmission disease, eg, influenza infection, chicken pox disease, tuberculosis, and/or were unable to participate until the completion of the study.
Sample Size Calculation
The sample size was calculated by two dependent means using the formula  where
where 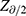 was 99% confidence,
was 99% confidence, 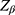 was set at 10% for type II error,
was set at 10% for type II error, 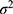 was the variance of the study outcome, and
was the variance of the study outcome, and  was the difference of the data between the two groups, as per Couper K et al.12 The minimum sample size, in accordance with the aforementioned calculation, was 12 participants. However, when a 20% dropout was added, then the calculation indicated that the sample size should be 15 participants for each group.
was the difference of the data between the two groups, as per Couper K et al.12 The minimum sample size, in accordance with the aforementioned calculation, was 12 participants. However, when a 20% dropout was added, then the calculation indicated that the sample size should be 15 participants for each group.
Study Protocol
We publicized the study at the emergency department through an invitation letter and face-to-face conversation. All participants provided written informed consent prior to receiving any study intervention. The LUCAS-3 mechanical chest compression device was used in this study. Participants were given one hour of instruction with manikin drills on proper device application and were required to pass an 80% post-test assessment. The participants were then divided into three-person teams. Team members were assigned roles with specific tasks, using a randomization system, including device deployment, chest compression, assisted breathing and data collection only conducted by the person who assembles the device. The teams undertook a manikin-based simulation scenario. The team acted as advance EMS and arrived first on the scene. The length of the section was approximately 6 min (three cycles of CPR). Two digital video recorders were employed to reduce the possibility of data loss and any potential camera obstruction by the participants.
The LUCAS-3 mechanical chest compression device consists of a back plate and an upper part that carries a suction cup (Figure 1). All participants must apply the device in both standard and back plate marking methods in sequential order, at separate times and without fixing an interval, depending on the availability of individual team members. Furthermore, the device must be applied by different, and randomly selected, team members. This process consists of three main components. First, the teams must complete a full cycle of manual CPR before transitioning to mechanical CPR. The device must be deployed in two stages including back plate and upper part of device in a set order. After performing a full cycle of manual CPR, the back plate is deployed, followed by the restart of full cycle CPR and then the deployment of the upper part of the mechanical device. The back plate marking method requires checking the placement of the back plate before applying the upper part of the device and the labeling tape in the center of the back plate is aligned with the nipple line (Figure 2). If the location of the back plate is incorrect, it is adjusted after the next full cycle of manual CPR. Second, the method of applying the upper part of the mechanical chest compression device was standardized into a two-step sequence as recommended by the manufacturer for minimally interrupted chest compression during transition. Third, the position of the suction cup is checked. If the suction cup’s location is improper, the device is immediately stopped and the suction cup’s location is adjusted. After completing each section, participants must switch tasks for one more time in order to deploy the device using each technique.
 |
Figure 1 The components of The LUCAS-3 mechanical chest compression device. |
 |
Figure 2 Back plate marking (A) aligned at the nipple line (B). |
Measures
The primary objective was to compare the effectiveness of applying the back plate marking method vs the standard method, to a mechanical chest compression device, in regards to reducing the duration of chest compression interruptions during a simulated cardiac arrest. The secondary objective was to evaluate the impact of CPR quality between the two methods, including the metrics of compression fraction and longest chest compression interruption.
Data Analysis
Two researchers reviewed videos independently. Both reviewers were not blinded to training allocation. Videos were viewed using software that enabled timings to be derived to the nearest one-tenth of a second. We assessed agreement between video reviewers by computing the average mean ± SD for difference data of two viewers. The average difference data between reviewers was 1.05 seconds (SD = 0.84). Differences in data of less than 2 seconds (2SD) were permitted and the analysis would take the mean value of the two reviewers; if the difference is greater than 2 seconds, then a repeat assessment by both researchers was required.
Statistical analysis was conducted using R software version 4.1.0. The descriptive data were outlined either by the mean ± standard deviation (SD) for continuous variables with normal distribution, or the median with interquartile range (IQR) for variables with non-normal distribution. Categorical data were expressed as numbers and percentages. According to the data distribution, continuous variables were examined using Wilcoxon rank-sum test and dependent t-test. Statistical significance was determined by a p-value of less than 0.05.
Results
Baseline Characteristic
Twenty-two clinicians consented to participate in the study (Figure 3). Twenty of them were assigned to randomized teams. Due to inability to participate in the trial, two subjects were eliminated after giving consent to participate; prior to randomization.
 |
Figure 3 Study flow diagram. |
Demographic data for the individual participants are shown in Table 1. The participants were emergency medical residents (n = 18, 90%) and paramedics (n = 2, 10%). The median duration using a mechanical compression device was 24 months; 50% of participants had used the device 11 to 20 times in clinical practice and the median post-test score was 92.8%. Because all of the participants in the study were from the same group, there were no differences between groups.
 |
Table 1 Individual Participant Characteristics |
Chest Compression Pauses Related with Device Deployment
A comparison of pauses related with device deployment outcomes is shown in Table 2. We found no difference in the chest compression pause followed by deployment of back plate between both groups (15.3 vs 14.5s, P = 0.40). While the median time pause for deployment of the upper part of the device was significantly decreased for the back plate making method (16 vs 21s, P=<0.01). In two of the 20 participants using the back plate marking method, the time necessary to deploy the upper part of the device was less than or equal to 10 seconds, while none could do it using the standard method. The total pause for device deployment, if the device gets adjusted multiple times, was a median 31.5s (27.62, 35.88) in the back plate marking method and 38.75s (31.12, 43.12) in the standard approach, with a significant difference between groups (p = 0.03). Back plate marking approach significantly reduced the proportion of total hands-off time attributive to device application interruption (43.08% vs 49.18%, P = 0.02).
 |
Table 2 Outcome Measurement |
CPR Metrics
The longest chest compression interruption was a median of 18s (15.62, 20.50) in back plate marking method and 18.75s (17, 21.25) in standard method, with no significant difference between groups (p = 0.11). All of the longest pauses in the study were caused by device application, either the back plate or the upper part of the device. The duration of all compression interruptions, defined as the total time of compression interruptions during the total time of the resuscitation, did not differ significantly between the two groups (p = 0.20). The median time for the back plate marking method was 70.5s (65.38 to 82.50) and 82.75s (70.50 to 88.12) for the standard method. The compression fraction was not significantly difference between the study groups. Mean compression fraction was 77.85% (2.13) in back plate marking method and 76.91% (3.06) in the standard approach (p = 0.19).
Discussion
This study aimed to assess the effectiveness of the back plate marking method vs the standard approach. We found that the back plate marking method significantly reduces the time for the deployment of the upper part of the device. This may be due to the person deploying the device not needing to adjust the upper part too much, because the position was corrected according to the back plate that was applied. The overall pause for the device deployment and interruption for the mechanical CPR device application, as a proportion of the total “hands off” time, was also significantly reduced, which may be due to a reduction in the time it took in order to deploy the upper part. However, there was no difference in CPR quality between the two methods. This included the compression fraction and the longest chest compression interruption. In contrast to the study by Levy M. et al,9 there was large significant improvement in mechanical CPR device application efficiency and overall high-performance CPR process after a one year quality improvement implementation. The median duration of the pause interval for device application decreased from 21 to 7s (p=<0.001), but the previous studies tended to combine the device application process with other interventions such as real-time feedback, extra training and case debriefing. As a result, the outcomes may reflect the entire impact of the intervention package. In addition, the short interval between performing the two methods during our study may affect the result. Furthermore, since the length of CPR is only 3 cycles, or approximately 6 minutes, the compression fraction may be affected. Therefore, further studies on using the back plate marking method may be required, especially with a longer training period and with a longer CPR duration.
There has been no research existing which provides an effective device deployment procedure for reducing the time of chest compression interruption due to device application.10 This is the first study showing that using other methods significantly reduces device insertion time and the proportion of chest compression interruption caused by device insertion. The compression fraction of the back plate marking method is higher than the standard method, despite the fact that the CPR quality is not significantly decreased. This might be because they are inexperienced with the new technique and/or due to the process of confirming the position with the labeling tape, which may cause a longer duration of interruption regarding chest compressions. This could lead to further research, such as practicing inserting to improve proficiency and the checking of back plate positioning before proceeding on to the upper part, in order to avoid interruptions in chest compressions and improving the overall CPR quality.
Limitations
This study has some limitations. A manikin study might not be able to completely substitute a patient study such as a smaller weight or to limit the vertical movement of the manikin’s shoulder. It also has the tendency to affect the time for a device deployment. Furthermore, the interval between performing both methods varied from 1 to 7 days, depending on the availability of individual team members. As a result, this can have an impact on reproduction performance.
Conclusions
The back plate marking method significantly reduced the time of deployment for the upper part of the device as well as the overall pause in regards to device deployment. The CPR quality of back plate marking method was higher but there was no statistically significant difference found between the two methods.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Brooks SC, Anderson ML, Bruder E, et al. Part 6: alternative techniques and ancillary devices for cardiopulmonary resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):S436–S43. doi:10.1161/CIR.0000000000000260
2. Slattery DE, Silver A. The hazards of providing care in emergency vehicles: an opportunity for reform. Prehosp Emerg Care. 2009;13:388–397. doi:10.1080/10903120802706104
3. Couper K, Smyth M, Perkins GD. Mechanical devices for chest compression: to use or not to use? Curr Opin Crit Care. 2015;21(3):188–194. doi:10.1097/MCC.0000000000000200
4. Rubertsson S, Lindgren E, Smekal D, et al. Mechanical chest compressions and simultaneous defibrillation vs conventional cardiopulmonary resuscitation in out-of-hospital cardiac arrest: the LINC randomized trial. JAMA. 2014;311(1):53–61. doi:10.1001/jama.2013.282538
5. Carron PN, Pantet R, Pasquier M, Hugli O. Mechanical chest compression in the PARAMEDIC trial. Lancet. 2015;386(9988):26. doi:10.1016/S0140-6736(15)61196-5
6. Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142(16 Suppl 2):S366–468. doi:10.1161/CIR.0000000000000916
7. Yost D, Phillips RH, Gonzales L, et al. Assessment of CPR interruptions from transthoracic impedance during use of the LUCAS™ mechanical chest compression system. Resuscitation. 2012;83(8):961–965. doi:10.1016/j.resuscitation.2012.01.019
8. Laerdal. What is chest compression fraction (CCF)? [Internet]; 2021. Available from: https://laerdal.force.com/HelpCenter/s/article/What-is-chest-compression-fraction-CCF.
9. Levy M, Yost D, Walker RG, Scheunemann E, Mendive SR. A quality improvement initiative to optimize use of a mechanical chest compression device within a high-performance CPR approach to out-of-hospital cardiac arrest resuscitation. Resuscitation. 2015;92:32–37. doi:10.1016/j.resuscitation.2015.04.005
10. Spiro JR, White S, Quinn N, et al. Automated cardiopulmonary resuscitation using a load-distributing band external cardiac support device for in-hospital cardiac arrest: a single centre experience of AutoPulse-CPR. Int J Cardiol. 2015;180:7–14. doi:10.1016/j.ijcard.2014.11.109
11. Ong ME, Quah JL, Annathurai A, et al. Improving the quality of cardiopulmonary resuscitation by training dedicated cardiac arrest teams incorporating a mechanical load-distributing device at the emergency department. Resuscitation. 2013;84(4):508–514. doi:10.1016/j.resuscitation.2012.07.033
12. Couper K, Velho RM, Quinn T, et al. Training approaches for the deployment of a mechanical chest compression device: a randomised controlled manikin study. BMJ Open. 2018;8(2):e019009. doi:10.1136/bmjopen-2017-019009
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
