Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Awareness and Knowledge of Adverse Effects of Topical Corticosteroids Among the General Population in Jeddah, Saudi Arabia
Authors Roblah TM , Baabdullah AM
Received 7 July 2023
Accepted for publication 14 October 2023
Published 26 October 2023 Volume 2023:16 Pages 3065—3075
DOI https://doi.org/10.2147/CCID.S421282
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Tala Musa Roblah,1 Ahmed Mohammed Baabdullah1,2
1Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia; 2Department of Dermatology, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
Correspondence: Tala Musa Roblah, King Abdulaziz University Hospital, Prince Majid Road, Al Sulaymaniyah, Jeddah, Saudi Arabia, Tel +966560969166, Email [email protected]
Purpose: Corticosteroids are naturally produced in the adrenal cortex and regulate a variety of physiological processes, including but not limited to, stress homeostasis, metabolism, and inflammation. As synthetic steroids exert the same effects as natural steroids, they are used in the treatment of various skin conditions. Synthetic steroids are delivered in a variety of forms, such as topical preparations, oral preparations, and injections. Topical steroids are one of the most commonly prescribed medications in dermatology clinics. Although topical steroids were thought to provide maximum benefit with no side effects, many side effects have been reported by topical corticosteroid users. Therefore, this study examined awareness and knowledge regarding topical steroids among the general population in Jeddah, Saudi Arabia.
Methodology: This was a cross-sectional study involving 426 subjects in Jeddah, Saudi Arabia. Subjects completed a self-administered questionnaire, and data were collected from February to April 2023. The questionnaire assessed age, sex, education level, occupation, and general knowledge regarding the side effects of topical steroids.
Results: The study cohort was predominantly female (77%). Half of the participants were aged 21– 39 years. Most of the subjects (70%) were steroid users, and the most common reported indication for steroid use was undetermined (37.6%). Nearly half of the participants did not know whether steroids were dangerous, and 59.4% did not know that topical steroids can cause systemic effects.
Conclusion: This study assesses knowledge regarding topical steroids among the general population in the western region of Saudi Arabia. The results revealed a lack of knowledge regarding the side effects of topical steroids. Awareness of potential topical steroid side effects should be increased among the general population to minimize the occurrence of serious side effects.
Keywords: topical steroids, awareness, knowledge, general population
Introduction
Corticosteroids, which are naturally produced in the adrenal cortex, can be divided into two categories: glucocorticoids and mineralocorticoids.1 Glucocorticoids regulate a variety of physiological processes, including stress homeostasis, inflammation, and metabolism.2–5 As synthetic steroids exert the same effects as natural corticosteroids,6,7 they are used to treat a variety of inflammatory, autoimmune, and lymphoproliferative disorders, ranging from brain tumors to simple skin lesions.2–4,6,7 Synthetic steroids are also used to prevent graft rejection and for substitution purposes in adrenal insufficiency states.2–4 Synthetic steroids are delivered in a variety of forms, such as topical preparations, oral preparations, and injections.1
Topical corticosteroids (TCs) are one of the most commonly prescribed medications in dermatology clinics, and they serve as the cornerstone of therapy for numerous dermatoses, including, but not limited to, atopic dermatitis, contact dermatitis, psoriasis, seborrheic dermatitis, and intertrigo.6,8,9 Although it was thought that TCs provide the maximum therapeutic benefit with minimal systemic side effects,10 a wide array of local and systemic side effects have been reported by topical steroid users.11,12 These undesirable effects can result from the misuse of TCs as fairness creams and from the unrestricted availability of TCs, which has led to their use for non-labeled indications.13–16 For instance, a study conducted at a tertiary hospital in India showed that 28% of 500 prescriptions were for TCs, and in 85% of these cases, the reason for the prescription could not be established.14 Another study conducted in India found that 14.8% of the study cohort with facial dermatoses used TCs, and the primary reason for using TCs was fairness (29%).10 A study conducted in Saudi Arabia reported that 31.75% of subjects used TCs without a medical prescription.17 Similarly, a study conducted in Rajasthan, India, reported that 65.4% of subjects obtained a TCs without a prescription.18
Potential side effects of TCs include atrophy of the skin, hirsutism, perioral dermatitis, acneiform eruption, telangiectasia, and hypertrichosis.15,17,19 The off-label and prolonged use of steroids without medical supervision can result in topical steroid–dependent face (TSDF).15 TSDF is a semi-permanent or in some cases permanent condition of the face characterized by erythema, blistering, and scaling upon attempting to stop TCs after prolonged use.14 In a study conducted in New Delhi, India, the most common side effect was tinea incognito.16 Similarly, in a study conducted in South Rajasthan, India, the most common side effect of TCs use was fungal infection.18 In contrast, acne was the most common side effect in a study conducted in Saudi Arabia.17
The systemic side effects of steroids are adrenal suppression and cushingoid appearance.20 These systemic side effects are mostly reported among children due to their thin skins and higher systemic absorption of the drug.11,12 Despite the fact that the use TCs is accompanied by a multiple side effects, the general population lacks awareness about topical steroids side effects.17 For instance, in a study conducted in Saudi, only 8% of the participants knew that TCs may have systemic side effects.17
The aforementioned studies show the importance of raising public awareness regarding the uses of TCs, local side effects of TCs, and systemic side effects of TCs in order to lower rates of side effects among the general population. Moreover, to our knowledge, the extent of public knowledge regarding topical steroids among the general population in the western region of Saudi Arabia has not been investigated to date. Hence, in this study, we assessed the awareness and depth of knowledge regarding topical steroids among the general population in Jeddah, Saudi Arabia.
Materials and Methods
Subject Recruitment and Data Collection
This was a cross-sectional study that included 426 subjects. In this study, the snow sampling method was utilized to recruit potential participants.The exclusion criteria were age less than 18 years and rejection of participation. After the exclusion criteria were applied, subjects who met the inclusion criteria and accepted invitation to the study successfully completed the questionnaire.Data collection was carried out using an online questionnaire distributed via Whatsapp From February 2023 to April 2023. The questionnaire was titled “Awareness and knowledge of topical corticosteroids among the general population in Jeddah, Saudi Arabia”, and it was taken from a research titled “Awareness and Knowledge about Topical Steroid among Population in Arar, Saudi Arabia”.17 The questionnaire was translated and cross checked to Arabic by two translators who are fluent in Arabic and English.Furthermore, both translators discussed and resolved discrepancies to finalize the translation.Once the participant clicked on the survey link, the purpose of the study and the time estimated to complete the survey appeared. If the participant then refused to participate, the questionnaire automatically closed. The first section of the questionnaire included socio-demographic data such as age, gender, education level, and occupation. The second part was designed to ask whether the participant had used steroids before, and if so, for what reason. The third section of the survey included 10 closed-ended questions to assess the participant’s awareness regarding side effects of TCs (is acne a side effect of topical steroid use?; are bacterial and fungal infections side effects of topical steroid use?; is hirsutism a side effect of topical steroid use?; is photosensitivity a side effect of topical steroid use?; is purpura a side effect of topical steroid use?; is skin atrophy a side effect of topical steroid use?; is skin folliculitis a side effect of topical steroid use?; is skin pigmentation a side effect of topical steroid use?; and can topical steroids cause systemic effects?). The last question assessed the participant’s point of view regarding topical steroids (are topical steroids dangerous or not?).
Statistical Analyses
Statistical analyses were conducted using IBM SPSS version 23 (IBM Corp., Armonk, NY, USA). Categorical variables are presented using simple descriptive statistics as counts and percentages. The chi-square test for goodness-of-fit was used to examine the equal distribution of sampling and subject answers. The chi-square test for independence or Fisher’s test, when appropriate, was used to test the associations between multiple variables, as listed in the Results section. Post hoc examination of the adjusted standardized residuals or partitioned data was used to pinpoint the exact source of significance at a Bonferroni or Bonferroni-Holm adjusted alpha. Cramer’s V was calculated to indicate the strength of the association. The significance threshold was set at P<0.05.
Ethical Approval
This study was conducted at the Department of Dermatology and received approval from the Research Ethics Committee of King Abdulaziz University Hospital (reference no. 86–23). The study was conducted in accordance with the tenets of the Declaration of Helsinki, and all participant records and data generated in the study were managed under strict confidentiality.
Results
Demographic Characteristics
A total of 426 participants agreed to participate in the study and completed the questionnaire. There was a female predominance among participants (77.7%). Moreover, half of the participants were aged 21–39 years, and most of the females were in this age group (65%). In contrast, 78.9% of males in the study group were 40 years of age or older. Nearly 80% of the subjects were university graduates, and nearly half of the subjects were unemployed. The demographic characteristics are shown in Table 1.
 |
Table 1 Demographics |
Steroid Use and Indications
Most of the subjects in the sample were steroid users (70%) with varying indications, as shown in Figure 1.
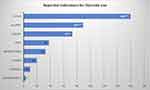 |
Figure 1 Reported indications for steroid use. Significantly higher reported indications for steroid use are shown in white numbers. ***Extremely significant (<0.001), **Very significant (<0.01). |
Awareness of TCs Side Effects
Regarding awareness of TCs side effects among the participants, purpura and skin pigmentation were the most-known side effects, reported by 31% of the participants, whereas folliculitis was the least-common known dermatological side effect of TCs(9%). Nearly half of the participants did not know whether TCs could be dangerous. Also, 59.4% of the participants did not know that TCs use can lead to systemic side effects, as shown in Table 2
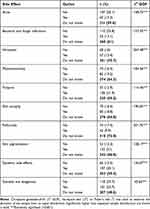 |
Table 2 Reported Awareness Regarding TCs Side Effects |
Effect of Gender on Awareness of TCs Side Effects
A significantly greater proportion of female subjects were aware that acne, photosensitivity, purpura, and skin pigmentation are side effects of TCs use as compared with male subjects. In addition, a significantly larger proportion of females were aware that hirsutism is a side effect of steroid use as compared with male subjects (16.0% vs 4.2%). Significance and chi-square values are listed in Table 3. Gender and hirsutism, photosensitivity, purpura, and skin pigmentation exhibited a significant association. Moreover, gender-related awareness of acne as a side effect exhibited an extremely significant moderate association (P<0.001).
 |
Table 3 Effect of Gender on Awareness of TCs Side Effects |
Effect of Age on Awareness
A significantly large proportion of subjects ≥40 years of age did not know that acne, hirsutism, and skin pigmentation are side effects of TCs use as compared to the other age groups. Interestingly, a significantly larger proportion of subjects in the 21 to 39 years of age group were aware that hirsutism, photosensitivity, purpura, and skin pigmentation are side effects of TCs use. Significance and chi-square scores are listed in Table 4. All age groups and hirsutism, photosensitivity, purpura, and skin pigmentation exhibited a significantly weak association (P<0.01).
 |
Table 4 Effect of Age on Awareness of TCs Side Effects |
Effects of Educational Level and Employment Status on Awareness of TCs Side Effects
Educational level had no effect of the subjects’ awareness of steroid-associated side effects as shown in Table 5 and Table 6. Likewise, no apparent associations were detected between the employment status of the subjects and their awareness of the side effects. However, employment was significantly associated with a lack of knowledge of acne as a side effect of TCs.
 |
Table 5 Effect of Education Level on Awareness of TCs Side Effects |
 |
Table 6 Effect of Employment Status on Awareness of TCs Side Effects |
Discussion
TCs are highly efficacious drugs used in the treatment of various dermatological conditions.21 Nevertheless, TCs are one of most abused classes of drugs.22 In this study, which is the first to assess awareness regarding TCs use in the western region of Saudi Arabia, most of the participants reported having used a TCs. Similarly, previous studies have reported that TCs are the most commonly used dermatologic medications worldwide.23,24 Studies conducted in Iraq, Iran, and Saudi Arabia identified acne as the most common indication for TCs use.10,17,25,26 Meena et al reported that half of the participants in their study used TCs for treating fungal infections.18 In addition, Sheth et al reported that 85% of subjects in their study used a TCs for treating superficial fungal infections.21 However, in our study, the most common dermatologic indications for TCs use were allergy and eczema. Elmorsy et al reported that 14% of subjects in their study knew that TCs use could lead to purpura.17 Surprisingly, only 31% of participants in our study knew that purpura is a potential side effect of TCs use. A study conducted in Arar, Saudi Arabia found that only 2% of respondents were aware of the potential systemic side effects of TCs.17 Similarly, Seo et al reported that among 3000 participants surveyed, only 2% were aware of the potential systemic side effects of TCs use.27 By comparison, 15.5% of the participants in our study were aware of the potential systemic side effects of TCs use. Hence, providing information on the potential systemic side effects of TCs is crucial in order to ensure public safety with regard to TCs use.27
There is variation regarding knowledge of side effects of TCs among different studies.17,28 For instance, Chhabra et al reported that 18.7% of recruited subjects knew that TCs can cause skin infection, 16% knew that TCs can cause acne, and 10.7% knew that TCs can cause photosensitivity.28 In contrast, Elmorsy et al reported that 42.25% of participants in their study knew that skin infections are a side effect of TCs use, and 74.5% of participants knew that acne is a side effect of TCs use, while 25.5% of participants knew that photosensitivity is a side effect of TCs use.17 In our study, 13.1% of the participants were aware that TCs can cause bacterial and fungal skin infection, 15.3% were aware that TCs can cause acne, and 19.2% were aware that TCs can cause photosensitivity. This variation in awareness among different populations further highlights the need for increasing awareness about potential TCs side effects among the public.
In agreement with the study conducted in Arar, our study found that females were more aware of most side effects than males.17 This could be attributed to the generally greater interest in cosmetic skin care among women than men.29 With respect to age, knowledge regarding the side effects of TCs was highest among subjects in the age range of 21–39 years. In contrast, a study carried out in India reported that knowledge regarding TCs side effects was greatest among those <25 years old (22%).28 The study done in Arar reported the highest awareness level among participants at 40 years of age.17 One reason for the age discrepancy in awareness could be related to the observation that as people age, they are more likely to use TCs for treating medical conditions. Hence, knowledge is gained via exposure and experience.30 Unexpectedly, no apparent associations were detected in our study between employment and educational status and the level of awareness, yet Alperstein and Peyrot reported that a greater level of awareness would be expected with higher level of education and among medical health care staff.31
Our study had some limitations, such as the small sample size. As there is very little literature available regarding awareness of TCs side effects among the general population, our findings need to be confirmed in larger-scale studies with more inclusive samples such as a large sample size. Second, as this was a drug-related study, some medical terminology was included in the questionnaire. Thus, it might have been difficult for some members of the general public to completely comprehend the questions. Awareness of TCs side effects should be increased among the general population, and information should be appropriately disseminated through medical conferences, articles, social media posts, and symposia.
One of the biggest strengths of this study is that it is the first study to assess the awareness of TCs side effects among the general population in the western region of Saudi Arabia. Previous studies were conducted on patients visiting dermatology outpatient clinics. However, the present study was conducted via a survey that was distributed electronically.
Conclusion
In summary, the majority of subjects included in our study used TCs. Moreover, the most common dermatological indications for the use of TCs were allergy and eczema. The majority of subjects were not aware of the various potential side effects of TCs, potential systemic side effects, or whether TCs use can be dangerous. Hence, awareness of risks associated with TCs use should be increased by focusing on the indications, side effects, and misconceptions. This could be achieved in various ways, such as healthcare professionals explaining this information to patients or through social media, campaigns, lectures, and seminars. Several side effects could be prevented when the public’s awareness increases.
Data Sharing statement
The data is available with the authors and can be shared.
Ethical Approval and Informed Consent
This study was conducted at the Department of Dermatology and received approval from the Research Ethics Committee of King Abdulaziz University Hospital (reference no. 86-23). The study was conducted in accordance with the tenets of the Declaration of Helsinki, and all participant records and data generated in the study were managed under strict confidentiality. All participants provided informed consent prior to filling the questionnaire. If the participant refused to participated, the online questionnaire automatically closed.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors didn’t receive any funding for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ahadian FM, McGreevy K, Schulteis G. Lumbar transforaminal epidural dexamethasone: a prospective, randomized, double-blind, dose-response trial. Regional Anesthesia &. Pain Med. 2011;36(6):572–578.
2. Simpson E, Waterman M, et al. Steroid biosynthesis in the adrenal cortex and its regulation by adrenocorticotropin. In: Degroot LJ, Besser M, Burger HG, editors. Endocrinology. Philadelphia: WB Saunders; 1995:1630–1641.
3. Lazar M. Mechanism of action of hormones that act as transcription-regulatory factors. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams’ Textbook of Endocrinology.
4. Chrousos G. Endocrinology and Metabolism. Felig P, Frohman L, eds. New York: McGraw-Hill; 2001.
5. Dreyfuss P, Baker R, Bogduk N. Comparative effectiveness of cervical transforaminal injections with particulate and nonparticulate corticosteroid preparations for cervical radicular pain. Pain Med. 2006;7(3):237–242. doi:10.1111/j.1526-4637.2006.00162.x
6. McClain RW, Yentzer BA, Feldman SR. Comparison of skin concentrations following topical versus oral corticosteroid treatment: reconsidering the treatment of common inflammatory dermatoses. J Drugs Dermatol. 2009;8(12):1076–1079.
7. Somayaji HS, Saifuddin A, Casey AT, Briggs TW. Spinal cord infarction following therapeutic computed tomography-guided left L2 nerve root injection. Spine. 2005;30(4):E106–E108. doi:10.1097/01.brs.0000153400.67526.07
8. Giannotti B, Pimpinelli N. Topical corticosteroids. Drugs. 1992;44(1):65–71. doi:10.2165/00003495-199244010-00006
9. Lee NP, Arriola ER. Topical corticosteroids: back to basics. West J Med. 1999;171(5–6):351.
10. Saraswat A, Lahiri K, Chatterjee M, et al. Topical corticosteroid abuse on the face: a prospective, multicenter study of dermatology outpatients. Indian J Dermatol Venereol Leprol. 2011;77(2):160–166. doi:10.4103/0378-6323.77455
11. Coondoo A, Chattopadhyay C. Use and abuse of topical corticosteroids in children. Indian J Paediatr Dermatol. 2014;15(1):1. doi:10.4103/2319-7250.131826
12. Coondoo A, Phiske M, Verma S, Lahiri K. Side-effects of topical steroids: a long overdue revisit. Indian Dermatol. 2014;5(4):416. doi:10.4103/2229-5178.142483
13. Nnoruka E, Okoye O. Topical steroid abuse: its use as a depigmenting agent. J Natl Med Assoc. 2006;98(6):934.
14. Rathi S. Abuse of topical steroid as cosmetic cream: a social background of steroid dermatitis. Indian J Dermatol. 2006;51(2):154. doi:10.4103/0019-5154.26949
15. Kumar S, Goyal A, Gupta YK. Abuse of topical corticosteroids in India: concerns and the way forward. J Pharmacol Pharmacother. 2016;7(1):1–5. doi:10.4103/0976-500X.179364
16. Mahar S, Mahajan K, Agarwal S, Kar HK, Bhattacharya SK. Topical corticosteroid misuse: the scenario in patients attending a tertiary care hospital in New Delhi. J Clin Diagnostic Res. 2016;10(12):FC16.
17. Elmorsy E, Alanazi YMA, Alenazy AES. Awareness and knowledge about tropical steroid among population in arar, Saudi Arabia. J Med Health Sci. 2019;13:1048
18. Meena S, Gupta LK, Khare AK, et al. Topical corticosteroids abuse: a clinical study of cutaneous adverse effects. Indian J Dermatol. 2017;62(6):675. doi:10.4103/ijd.IJD_110_17
19. Gupta M. Topical corticosteroid abuse among pediatric population–a prospective study. Our Dermatol Online. 2020;11(2):194–196. doi:10.7241/ourd.20202.24
20. Böckle BC, Jara D, Nindl W, Aberer W, Sepp NT. Adrenal insufficiency as a result of long-term misuse of topical corticosteroids. Dermatology. 2014;228(4):289–293. doi:10.1159/000358427
21. Sheth NK, Nair PA. Topical steroids: awareness and misuse among patients, pharmacists and general medical practitioner. Indian J Dermatol Venereol Leprol. 2021;87(1):54–59. doi:10.4103/ijdvl.IJDVL_84_18
22. Agarwal S, Mittal A, Rathore PK. Level of awareness and attitude towards topical steroids among medical interns. J Evol Med Dent Sci. 2020;9(33):2356–2361. doi:10.14260/jemds/2020/511
23. Stern RS. The pattern of topical corticosteroid prescribing in the United States, 1989–1991. J Am Acad Dermatol. 1996;35(2):183–186. doi:10.1016/S0190-9622(96)90319-9
24. Kumar M, Noushad P, Shailaja K, Jayasutha J, Ramasamy C. A study on drug prescribing pattern and use of corticosteroids in dermatological conditions at a tertiary care teaching hospital. Int J Pharm Sci Rev Res. 2011;9(2):132–135.
25. Nagesh T, Akhilesh A. Topical steroid awareness and abuse: a prospective study among dermatology outpatients. Indian J Dermatol. 2016;61(6):618. doi:10.4103/0019-5154.193666
26. Al Dhalimi M, Al Jawahiry N. Misuse of topical corticosteroids: a clinical study in an Iraqi hospital. Eastern Med Health J. 2006;12(6):847–852.
27. Seo H, Song SY, Kim D, et al. General public knowledge regarding topical corticosteroids: a nationwide survey in South Korea. Korean Journal of Clinical Pharmacy. 2022;32(2):84–92. doi:10.24304/kjcp.2022.32.2.84
28. Chhabra N, Sachdev D, Shukla AK. Knowledge, attitude and practice regarding topical steroids in dermatology outpatients: a cross-sectional study from a tertiary care hospital in Raipur, Chhattisgarh. Indian J Dermatol Venereol Leprol. 2021;87(3):429–432. doi:10.25259/IJDVL_582_19
29. Warowna M, Tokarczyk K, Hordyjewska A, Kręcisz B. Physiological dermatological changes in women’s female and skin care in a beauty salon. EJMT. 2019;2:23.
30. Howell RA, Allen S. Significant life experiences, motivations and values of climate change educators. Environ Educ Res. 2019;25(6):813–831. doi:10.1080/13504622.2016.1158242
31. Alperstein NM, Peyrot M. Consumer awareness of prescription drug advertising. J Advertising Res. 1993;33(4):50–57.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
