Back to Journals » International Journal of General Medicine » Volume 15
Autopsy Findings and Inflammatory Markers in SARS-CoV-2: A Single-Center Experience
Authors Cut TG , Ciocan V, Novacescu D, Voicu A , Marinescu AR, Lazureanu VE, Muresan CO, Enache A, Dumache R
Received 9 September 2022
Accepted for publication 18 December 2022
Published 28 December 2022 Volume 2022:15 Pages 8743—8753
DOI https://doi.org/10.2147/IJGM.S389300
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Talida Georgiana Cut,1– 5 Veronica Ciocan,4,6 Dorin Novacescu,3,5 Adrian Voicu,7 Adelina Raluca Marinescu,1,2 Voichita Elena Lazureanu,1,2 Camelia Oana Muresan,4,6 Alexandra Enache,4,6 Raluca Dumache4,6
1Department of Infectious Diseases, Victor Babes University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 2Victor Babes Clinical Hospital of Infectious Diseases and Pneumophtisiology Timisoara, Timisoara, Romania; 3Doctoral School Victor Babes University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 4Center for Ethics in Human Genetic Identifications, Victor Babes University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 5Academy of Romanian Scientists, Bucharest, Romania; 6Department of Forensic Medicine, Bioethics, Deontology and Medical Law, Victor Babes University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 7Department of Medical Informatics and Biostatistics, Victor Babes University of Medicine and Pharmacy Timisoara, Timisoara, Romania
Correspondence: Veronica Ciocan, Department of Forensic Medicine, Bioethics, Deontology and Medical Law, Victor Babes University of Medicine and Pharmacy Timisoara, Timisoara, Romania, Tel +40722944453, Email [email protected]
Purpose: The systemic inflammatory response related to COVID-19 can be easily investigated in living patients. Unfortunately, not every biomarker is suitable for postmortem analysis since several factors may interfere. The aim of this study was to summarize key histopathological findings within each organ system due to COVID-19 and to assess if serological inexpensive and widely available biomarkers such as CRP, IL-6, fibrinogen and d-Dimers, associated with adverse outcomes in COVID-19, can be implemented in a post-mortem assessment.
Patients and Methods: A total of 60 subjects divided in 2 groups were included. All subjects died outside a hospital setting and therefore did not receive specific or symptomatic therapies that could have modulated the inflammatory response. The first group included 45 subjects in which mandatory autopsy was performed in order to establish the cause of death and macroscopic examination of the lungs was highly suggestive of SARS-CoV-2 infection. As controls (Group 2), 20 subjects who died from polytrauma in high velocity car accidents and suicide were selected. Bronchial fluids collected during the autopsy procedure were used for the RT-PCR diagnosis of SARS-CoV-2 and serum samples were sent for analysis of IL-6, CRP, d-Dimers and fibrinogen.
Results: Compared with the control group, the subjects of the COVID-19 group were older (59± 19.5 vs.38± 19.15 years, p=0.0002) and had more underlying comorbidities such as hypertension (60% vs 35%, p=0.06) or were overweight (53.3% vs 30%, p=0.08). The levels of CRP, IL-6, fibrinogen and d-Dimers in postmortem plasma samples were significantly higher in COVID-19 subjects than in control group (p< 0.0001). Moreover, the level of IL-6 was significantly higher in overweight patients (r=0.52, P< 0.001). In all COVID-19 subjects, the histological examination revealed features corresponding to the exudative and/or proliferative phases of diffuse alveolar damage. Large pulmonary emboli were observed in 7 cases. Gross cardiac enlargement with left ventricular hypertrophy was observed in 19 cases. The most frequent pathological finding of the central nervous system was acute/early-subacute infarction.
Conclusion: Due to the complexity of the inflammatory response, we postulate that a combination of biomarkers, rather than a single laboratory parameter, might be more effective in obtaining a reliable postmortem COVID-19 diagnosis.
Keywords: COVID-19, postmortem, diffuse alveolar damage, interleukin-6, C reactive protein
Introduction
Since the first reports from Wuhan (China) at the end of 2019, the zoonotic infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread rampantly, raising major public health concerns and applying continuous strain on the global medical infrastructure.1 SARS-CoV-2 is an enveloped, positive-sense RNA single-stranded virus of β-coronavirus genus responsible for a broad spectrum of clinical manifestations raging from asymptomatic or mild flu-like forms to acute respiratory distress syndrome (ARDS) with multiorgan failure, particularly in elderly and severe-critically ill patients.2–5 The systemic inflammatory response to SARS-CoV-2 infection is a hallmark of 2019 coronavirus disease (COVID-19). It is initiated when the viral Spike glycoprotein (S) binds to the Angiotensin-Converting Enzyme 2 (ACE2).6 ACE2 is highly expressed on endothelial cells and epithelial cells of the respiratory tract.7 The subsequent fusion of the viral envelope with the host cell membrane leads to virus entry, replication and cell lysis which in turn activates biomolecular events such as an increase in production of C-reactive protein (CRP), interleukins 1β, 6, 10 (IL-1β, IL-6, IL-10), tumor necrosis factor-α (TNF-α), various glycoproteins and other acute phase reactants such as fibrinogen and procalcitonin.8,9
The corresponding histological findings are represented by diffuse alveolar damage in exudative or proliferative stage, inflammatory cell infiltrates, squamous metaplasia, type 2 pneumocyte activation and fibrin microthrombi formation within pulmonary, cardiac, renal and hepatic circulation.10–13
Postmortem examinations are the gold standard for understanding the pathophysiological pathways of diseases and the prime objective of the forensic autopsy is to provide answers about the cause of death.14 In order to predict the prognosis of COVID-19 in a personalized approach, a set of time and cost-efficient biomarker signatures must be selected. Unfortunately, not every biomarker is suitable for postmortem analysis since several factors such as time elapsed from death, possible supravital reactions, leakage from cell deterioration, blood hemolysis, diffusion dependent on concentration gradients, may interfere with a correct interpretation of the results and for these reasons postmortem biochemical investigations are limited.15–19
COVID-19 diagnosis may be challenging in the medico-legal setting especially if clinical data or antemortem laboratory results are not available. Moreover, a reliable post-mortem diagnosis is of importance for establishing the medico-legal implications of professional liability when COVID-19 related death is ascribed to malpractice (eg, nosocomial infection, low hygienic standards, violation of the epidemiological regulations).
The aim of this study was to summarize key histopathological findings within each organ system due to COVID-19 and to assess if serological inexpensive and widely available biomarkers such as CRP, IL-6, fibrinogen and d-Dimers, associated with adverse outcomes in COVID-19, can be implemented in a post-mortem assessment. The spectrum of our findings is presented in the context of existing literature.
Materials and Methods
Case Selection
The Romanian Ministry of Health did not recommend autopsies for Covid-19 confirmed deaths mainly because of legitimate concerns about infection risk and limited personal protective equipment (PPE) but according to Article 185 of the Code of Criminal Procedure, mandatory forensic autopsies are performed in all cases of suspicious or violent death, at the request of the prosecutor or criminal investigation bodies.20 A total of 45 COVID-19-positive subjects were included (Group 1); the demographic data is presented in Table 1. All subjects died outside a hospital setting and therefore did not receive specific or symptomatic therapies that could have modulated the inflammatory response. As controls (Group 2), we selected a total of 20 subjects who died from polytrauma in high velocity car accidents (n=10), and suicide (jumping from height n=5, hanging n=4, intoxication n=1). The case exclusion criteria was the presence of concomitant known infectious lung diseases (for example history or active pulmonary tuberculosis).
 |
Table 1 Demographics and Clinical Characteristics of COVID-19 Positive Cases and Control Cases |
Autopsies and Tissue Processing
Complete diagnostic autopsies were performed at the Institute of Forensic Medicine, Timisoara, Romania in 24 to 200 hours following death (postmortem interval). To have a better examination of the pathological changes, we tried to prevent the process of autolysis which refers to the postmortem autodigestion of tissue by endogenous cytoplasmic enzymes of predominantly lysosomal origin by minimizing the time between autopsy and PCR sampling.21 Swabs of the lower respiratory tract (trachea and primary bronchi) were taken during the autopsy. Serum samples were collected and sent for analysis of IL-6, CRP, D-dimers and fibrinogen. The analysis was performed on Multiskan FC Microplate Photometer (ThermoScientific, USA) using the ELISA sandwich (enzyme-linked immunosorbent assay) principle. IL-6 ELISA Human kit (Invitrogen, USA), CRP Human ELISA kit (Invitrogen, USA) and D-Dimer Human ELISA Kit (Invitrogen, USA) were used for the analysis. Biosafety Level 3 laboratory practices were adhered to.
Histological samples obtained after the autopsy were fixed in 10% buffered formalin for 48 h. Paraffin-embedded sections of 5-μm thickness were stained with hematoxylin and eosin.
SARS-CoV-2 RNA Detection
The molecular analysis was performed on an ABI 7500 real-time PCR System (Thermo Fisher, USA), using the genesig® Real-Time PCR assay (Primer Design™, UK). RNA extraction started with the manual pre-processing part obtaining a lysis solution composed of 200 µL lysis buffer, 20 µL proteinase K solution and 20 µL of internal extraction control (IEC). Further step was to incubate the samples in a thermo block Eppendorf Max C (Eppendorf, Germany) at T=56°C for 10 minutes. During this step the cartridges for the automate RNA extraction were prepared.
In order to complete the RNA extraction, the automate system Maxwell 48 RSC System (Promega, USA) with the Maxwell RSC Viral Total Nucleic Acid Purification kit (Promega, USA) kit were used. After RNA extraction, the samples were amplified on a 7500 ABI real-time PCR (Thermo Fischer Scientific, USA) using the genesig SARS-CoV-2 RT-PCR assay (Primer Design, UK). According to the World Health Organization’s (WHO) recommendations, 45 amplification cycles were used. Because the system used in this study expresses only the threshold cycle (CT) values (the limit of detection is 100 copies/reaction) and does not provide the quantitative value of the viral load, the samples were labeled as positive when specific SARS-CoV-2 genes were detected.
Statistical Analysis
Normally distributed values were presented as mean ± standard deviation, a comparative analysis of this data was performed between group 1 and 2 using unpaired 2-sided Student’s t-test. Categorical variables were described as percentages, and the significance was tested by the chi-square test. The data was analyzed for normality using the Shapiro–Wilk test, and the comparison of the quantitative variables of the two groups was performed using U de Mann–Whitney test. A p value of < 0.05 was considered statistically significant. The Odds Ratio (OR) with their confidence intervals 95% (95% CI) were calculated by Fisher’s exact test. The correlations between interleukin-6 and other variables were analyzed by the Spearman correlation analysis. All these statistical calculations were performed using MedCalc 20.015 software.
Results
Using RT-PCR, all swabs from Group 1 collected from lower respiratory tract during the autopsies were positive for SARS-CoV-2. The control group was always negative on the swab result.
Among the 45 deceased COVID-19 subjects (group one), 25 (55.60%) patients were male.
In the control group, 7 (35%) subjects were female. Death occurred over almost every decade of life in COVID-19 group: 1 (0–9); 8 (30–39); 7 (40–49); 5 (50–59); 7 (60–69); 8 (70–79); 8 (80–89); 1 (90–99). The time from death to complete diagnostic autopsy, also known as postmortem interval (PMI), was longer in COVID-19 group (48.86±32.48 hours vs 28.49±8.45 hours, p=0.007).
Compared with the control group, the subjects of the COVID-19 group were older (59±19.5 vs.38±19.15 years, p=0.0002) and had more underlying comorbidities such as hypertension (60% vs 35%, p=0.06) or were overweight (53.3% vs 30%, p=0.08).
We next compared the levels of CRP, IL-6, fibrinogen and d-Dimers in postmortem plasma samples between COVID-19 group and control group. The values of the above-mentioned parameters were significantly higher in COVID-19 subjects than in control group (p< 0.0001) (Figure 1). The Spearman rank correlation between IL-6 and other inflammatory parameters is presented graphically in Figure 2, in the form of scatter-plots with a Local Regression Smoothing (LOESS) trend-lines for each association. No significant positive correlations were found between the plasmatic level of IL-6 and other immune-inflammatory parameters: CRP (r=0.22, P=0.14), d-Dimers (r=0.023, P=0.88), fibrinogen (r=0.119, P=0.435). Moreover, the level of IL-6 was significantly higher in overweight patients (Figure 3, r=0.52, P<0.001).
 |
Figure 1 Statistically significant difference in the group of COVID-19 positive cases compared to the control group for the laboratory findings. |
 |
Figure 2 The Spearman rank correlation between IL-6 and other inflammatory parameters. Note: r=Spearman’s rank correlation coefficient. |
 |
Figure 3 The Spearman rank correlation between IL-6 and comorbidities. |
Upon microscopic examination of the COVID-19 group, the lungs of all subjects were heavy, congested, generally with abundant edema fluid with patchy involvement. In all the cases of group one, histological examination revealed features corresponding to the exudative and/or proliferative phases of diffuse alveolar damage (Table 2, Figure 4). Of these, 32 were in acute organizing phase and 13 showed more extensive organization. Hyaline membranes, edema and congestion were present to some extent in all cases. The interstitium contained varying amounts of lymphocytic inflammation, tended to more diffuse in cases with extensive disease. Histological examination of the main bronchi and bronchiolar branches revealed mild non-specific alterations, focal squamous metaplasia and transmural lymphocytic infiltrates. Intravascular fibrin thrombi were observed in all cases within small arteries or arterioles. Large pulmonary emboli were observed in 7 cases. The examination of the cardiovascular system revealed that 19 hearts had gross cardiac enlargement, with the majority having left ventricular hypertrophy and moderate to marked coronary atherosclerosis (Figures 5 and 6). All but one case revealed varying degrees of interstitial fibrosis, consistent with preexisting atherosclerotic disease. Steatosis was observed in the livers of 13 subjects. This could be explained by concomitant obesity, diabetes mellitus, and/or hyperlipemia. One case showed evidence of chronic liver disease with cirrhosis of undetermined etiology. The most frequent pathological findings of the central nervous system were of acute/early-subacute infarction, observed in 14 of 45 cases, and presented as small and patchy microinfarcts located within the peripheral neocortex and the deeper gray matter structures. Widespread microthrombi were noted in 17 of 45 cases. Histological analysis of the kidneys reported acute tubular injury (ATI) in 8 cases. ATI consisted in loss of brush border, vacuolar degeneration, dilatation of the tubular lumen with cellular debris, and detachment of epithelium from the tubular basement membrane. COVID-19-related findings in the gastrointestinal and endocrine systems were less striking.
 |
Table 2 Histological Data Summary |
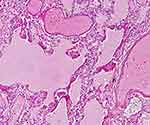 |
Figure 4 Lung tissue with edema, DAD with hyaline membranes and microvascular thrombi (H&E,x40). |
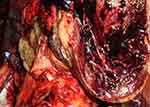 |
Figure 5 Macroscopic examination of heart – multiple white nodular areas highly suggestive of microinfarcts; postmortem clots intermingled with chordae tendineae. |
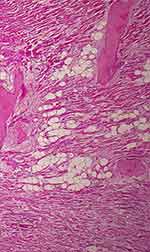 |
Figure 6 Inflammatory infiltration, necrosis and microvascular thrombi in myocardium (H&E,x40). |
Discussion
COVID-19 primarily affects the respiratory system and early autopsy studies document respiratory failure due to ARDS as predominant cause of death, frequently accompanied by capillary microthrombosis, superimposed bronchopneumonia, pulmonary thromboembolism, and signs of multi-organ failure.22 The worldwide mortality rate for COVID-19 in 2021 was 2% with a 0.06% of severe cases.23 Romania recorded, in October 2021, 110% more deaths than the average of Octobers in the years preceding the pandemic, namely 2017–2019.24 It is the largest proportion of excess mortality among those during the entire period of the pandemic, from the entire European Union.25 A study by Gebhard et al states that although the overall number of confirmed COVID-19 cases across all age groups is sex balanced in the European countries, the mortality rate is higher in men.26 Similarly, in our study, the male gender is dominant in both of our groups. In a study on 80 cases published by Edler et al the average age was reported to be 79 and 76 in the study of Menter et al27,28 In our study, the average age for group I was 59 with the highest number of cases (n=8) reported for the third, seventh and eighth decade. The fact that traumatic deaths accompanied by SARS-CoV-2 positivity were included in the study may be the reason for the lower average age compared to other studies.
The inflammatory response plays a central role in COVID-19 pathogenesis and clinical studies have reported a correlation between elevated serum levels of proinflammatory cytokines, such INF-γ, TNF-α, IL-6 and lung injury and poor prognosis.29 A combination of three fundamental mechanisms has been proposed for the pathogenesis of pulmonary changes induced by COVID-19: severe endothelial injury associated with intracellular SARS-CoV-2 virus and disrupted endothelial cell membranes, vascular thrombosis with microangiopathy and occlusion of alveolar capillaries and new vessel growth via intussusceptive angiogenesis.30 Postmortem specimens of SARS-CoV-2 infected lungs exhibited histological features of DAD with necrosis of the alveolar lining, hyperplasia of type II pneumocytes, intraalveolar fibrin deposition, infiltration of lymphocytes in the perivascular space and interstitial edema.1,13,31–34 Our results confirm the presence of these histological findings in all cases examined.
The immunohistochemical evaluation of proinflammatory cytokines performed by Frisoni et al on 60 cases showed a high and wide-spread lung expression of IL-6 in deaths caused by severe COVID-19 pneumonia.29 The postmortem serological investigations carried out in our study revealed higher serum expression of IL-6 for the COVID-19 group however this difference does not reflect magnitude and duration of IL-6-mediated signaling, which is dependent on the complex interplay of membrane-bound (cis-signaling, classically anti-inflammatory) and soluble IL-6 receptors (trans-signaling, pro-inflammatory) as well as soluble inhibitors.35–37 Furthermore, Bösmüller et al noted in some patients a drop in serum IL-6 levels before death.22
Tsokos et al engaged in a series of works aimed to assess the value of several molecules as postmortem markers of sepsis and concluded that IL-6 and CRP are good markers for the postmortem diagnosis of sepsis since the antemortem and postmortem levels were highly elevated in all individuals included in the sepsis group.37
Due to its rapid response, short half-life, and high magnitude of increase, CRP is a useful indicator of infections (bacterial, viral, fungal, and mycobacterial), inflammatory diseases, necrosis such as myocardial infarction or pancreatitis, trauma, and malignancy.38,39 Deng et al and Shi et al found that higher levels of CRP and IL-6 in SARS-CoV-2 patients were associated with myocardial injury, ARDS development, multiorgan failure and death.40,41 In addition, Stassi et al observed a decrease in CRP levels with increasing PMI.18 Astrup et al found that antemortem CRP levels were higher than postmortem concentrations in septic patients, and likely suggesting molecule proteolysis due to decompositional changes along with irrelevant postmortem molecule release from hepatocytes in the early postmortem period.42 Similar to our findings, Maeda et al reported low postmortem CRP levels in infantile and elderly cases of pneumonia.43
A common feature of severe COVID-19 is massive hypercoagulability with prominent elevation of d-Dimers, fibrin and fibrin degradation products.44,45 SARS-CoV-2 infection may be a trigger for venous thromboembolism with the involvement of several pathogenetic mechanisms such as endothelial dysfunction, characterized by increased levels of von Willebrand factor; systemic inflammation, and a procoagulant state induced by tissue factor pathway activation.46,47
Zhou et al observed that D-dimer levels above 1000 µg/L associate a fatal outcome.48 Our study findings support this contention since d-Dimers and fibrinogen postmortem serum levels were increased in COVID-19 group and in 7 cases pulmonary thromboembolism was present. In one of the first Romanian postmortem reports published by Dumache et al in 2021, it was presented the case of a COVID-19 patient which developed disseminated intravascular coagulation, increased d-Dimer levels and upon autopsy examination thrombi in lung vessels, findings that were also reported by Carsana et al, Edler et al and Remmelink et al2,13,27,32 In 30% of the subject form COVID-19 group, endotheliitis with microthrombus formation and involvement of medium-sized vessels leading to cerebral infarcts was prominent. Solomon et al reported histopathological changes in autopsies of 18 patients with COVID-19 with no suggestive changes of acute stroke on gross examination and no findings of thrombosis or vasculitis on histopathological examination, only changes of acute hypoxia in the cerebrum and cerebellum with neuronal loss.49 In another series of postmortem analysis by von Weyhern, CNS involvement occurred in form of pan-encephalitis, meningitis and brainstem damage but the authors reported no endotheliitis in the brain.50 Overall, the increased incidence of multifocal acute infarcts and numerous microthrombi observed in our study, could explain some of the clinical neuropsychiatric abnormalities seen in COVID-19 patients, such as mental status change or confusion. The liver damage observed in our patients is most likely a combination of metabolic, hypoxic and cytokine-induced damage, as evidence by centroacinar necrosis.
Limitations
Postmortem studies are prone to limitations, the most important of which consists in the inability to assess illness dynamics, as the evaluation takes place at the end of the disease course. This study is limited due to the small sample size and population distribution. Complete medical records for every patient were not available, leading to difficulties in correlating the clinical course with pathological findings. Additionally, CPR, IL-6, d-Dimes and fibrinogen measured from postmortem samples could not be compared with the values present before death because all the subjects included in the study died in out-of-hospital settings.
Conclusion
COVID-19 is a far more complex multiorgan and heterogenous illness than initially anticipated and further autopsy studies are needed to expand this evidence. Unfortunately, postmortem biochemical investigations are limited to few markers that are relatively stable in peripheral blood. Although CRP, IL-6, d-Dimers and fibrinogen measured from postmortem samples exhibited higher concentrations in COVID-19 group we consider that it is not advisable to reach the diagnosis of COVID-19-related death based on laboratory investigations only, especially when biochemical analyses are limited to a single parameter.
Continued autopsy studies correlated with immunostaining and electron microscopy are needed further in order to truly establish the role of these biomarkers in forensic settings.
Ethics and Consent Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Victor Babes University of Medicine and Pharmacy Timisoara, Nr. 29/15.06.2021. Written informed consent has been obtained from the next of kin to publish this paper.
Funding
The authors received no specific funding for this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Enache A, Ciocan V, Muresan CO, et al. Postmortem documentation of SARS-CoV-2 in utero and postpartum transmission, through amniotic fluid, placental, and pulmonary tissue RT-PCR. Appl Sci. 2021;11(20):9505. doi:10.3390/app11209505
2. Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. doi:10.1016/S1473-3099(20)30434-5
3. Cut TG, Tudoran C, Lazureanu VE, Marinescu AR, Dumache R, Tudoran M. Spontaneous pneumomediastinum, pneumothorax, pneumopericardium and subcutaneous emphysema—not so uncommon complications in patients with COVID-19 pulmonary infection—a series of cases. JCM. 2021;10(7):1346. doi:10.3390/jcm10071346
4. Calabrese F, Pezzuto F, Fortarezza F, et al. Pulmonary pathology and COVID-19: lessons from autopsy. the experience of European pulmonary pathologists. Virchows Arch. 2020;477(3):359–372. doi:10.1007/s00428-020-02886-6
5. Lupariello F, Godio L, Di Vella G. Immunohistochemistry patterns of SARS-CoV-2 deaths in forensic autopsies. Leg Med. 2021;51:101894. doi:10.1016/j.legalmed.2021.101894
6. Smilowitz NR, Kunichoff D, Garshick M, et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. 2021;42(23):2270–2279. doi:10.1093/eurheartj/ehaa1103
7. Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS‐CoV‐2 and mechanisms of immunopathological changes in COVID‐19. Allergy. 2020;75(7):1564–1581. doi:10.1111/all.14364
8. Marinescu AR, Lazureanu VE, Musta VF, et al. Severe thrombocytopenic purpura associated with COVID-19 in a pediatric patient. IDR. 2022;15:3405–3415. doi:10.2147/IDR.S363716
9. Cao W, Li T. COVID-19: towards understanding of pathogenesis. Cell Res. 2020;30(5):367–369. doi:10.1038/s41422-020-0327-4
10. Stassi C, Mondello C, Baldino G, Cardia L, Asmundo A, Ventura Spagnolo E. An insight into the role of postmortem immunohistochemistry in the comprehension of the inflammatory pathophysiology of COVID-19 disease and vaccine-related thrombotic adverse events: a narrative review. IJMS. 2021;22(21):12024.
11. Maiese A, Manetti AC, La Russa R, et al. Autopsy findings in COVID-19-related deaths: a literature review. Forensic Sci Med Pathol. 2021;17(2):279–296. doi:10.1007/s12024-020-00310-8
12. Aguiar D, Lobrinus JA, Schibler M, Fracasso T, Lardi C. Inside the lungs of COVID-19 disease. Int J Legal Med. 2020;134(4):1271–1274. doi:10.1007/s00414-020-02318-9
13. Dumache R, Daescu E, Ciocan V, et al. Molecular testing of SARS-CoV-2 infection from blood samples in disseminated intravascular coagulation (DIC) and elevated D-dimer levels. Clin Lab. 2021;67. doi:10.7754/Clin.Lab.2020.200704
14. Bryce C, Grimes Z, Pujadas E, et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol. 2021;34(8):1456–1467. doi:10.1038/s41379-021-00793-y
15. Saberi-Movahed F, Mohammadifard M, Mehrpooya A, et al. Decoding clinical biomarker space of COVID-19: exploring matrix factorization-based feature selection methods. Comput Biol Med. 2022;146:105426. doi:10.1016/j.compbiomed.2022.105426
16. Rostami M, Oussalah M. A novel explainable COVID-19 diagnosis method by integration of feature selection with random forest. Inform Med Unlocked. 2022;30:100941. doi:10.1016/j.imu.2022.100941
17. Cuț T, Enache A, Novacescu D, et al. Postmortem MicroRNA signatures as predictive markers in SARS-CoV-2 infection. Clin Lab. 2022;68. doi:10.7754/Clin.Lab.2021.210626
18. Stassi C, Mondello C, Baldino G, Ventura Spagnolo E. Post-mortem investigations for the diagnosis of sepsis: a review of literature. Diagnostics. 2020;10(10):849. doi:10.3390/diagnostics10100849
19. Tsokos M. Postmortem diagnosis of sepsis. Forensic Sci Int. 2007;165(2–3):155–164. doi:10.1016/j.forsciint.2006.05.015
20. Autopsy regulations in COVID-19 pandemic. Legislative Portal. Available from: https://legislatie.just.ro/Public/DetaliiDocumentAfis/224715.
21. Elsoukkary SS, Mostyka M, Dillard A, et al. Autopsy findings in 32 patients with COVID-19: a single-institution experience. Pathobiology. 2021;88(1):56–68. doi:10.1159/000511325
22. Bösmüller H, Traxler S, Bitzer M, et al. The evolution of pulmonary pathology in fatal COVID-19 disease: an autopsy study with clinical correlation. Virchows Arch. 2020;477(3):349–357. doi:10.1007/s00428-020-02881-x
23. Bugra A, Das T, Arslan MN, Ziyade N, Buyuk Y. Postmortem pathological changes in extrapulmonary organs in SARS-CoV-2 rt-PCR–positive cases: a single-center experience. Ir J Med Sci. 2022;191(1):81–91. doi:10.1007/s11845-021-02638-8
24. National Institute of Public Health. The coronavirus disease 2019 (COVID-19) treatment guidelines. Available from: https://www.cnscbt.ro/index.php/informatii-pentru-personalul-medico-sanitar/1525-metodologia-de-supraveghere-a-covid-19-actualizare-16-03-2020/file.
25. Excedentul de mortalitate COVID-19 în România [COVID-19 mortality in Romania]. Available from: https://monitorsocial.ro/indicator/excedentul-de-mortalitate-covid-19-in-romania/.
26. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. doi:10.1186/s13293-020-00304-9
27. Edler C, Schröder AS, Aepfelbacher M, et al. Dying with SARS-CoV-2 infection—an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134(4):1275–1284. doi:10.1007/s00414-020-02317-w
28. Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID‐19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. doi:10.1111/his.14134
29. Frisoni P, Neri M, D’Errico S, et al. Cytokine storm and histopathological findings in 60 cases of COVID-19-related death: from viral load research to immunohistochemical quantification of major players IL-1β, IL-6, IL-15 and TNF-α. Forensic Sci Med Pathol. 2022;18(1):4–19. doi:10.1007/s12024-021-00414-9
30. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383(2):120–128. doi:10.1056/NEJMoa2015432
31. Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396(10247):320–332. doi:10.1016/S0140-6736(20)31305-2
32. Remmelink M, De Mendonça R, D’Haene N, et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24(1):495. doi:10.1186/s13054-020-03218-5
33. Duarte-Neto AN, Caldini EG, Gomes-Gouvêa MS, et al. An autopsy study of the spectrum of severe COVID-19 in children: from SARS to different phenotypes of MIS-C. EClinicalMedicine. 2021;35:100850. doi:10.1016/j.eclinm.2021.100850
34. Hooper JE, Padera RF, Dolhnikoff M, et al. A postmortem portrait of the coronavirus disease 2019 (COVID-19) pandemic: a large multi-institutional autopsy survey study. Arch Pathol Lab Med. 2021;145(5):529–535. doi:10.5858/arpa.2020-0786-SA
35. Chen LYC, Biggs CM, Jamal S, Stukas S, Wellington CL, Sekhon MS. Soluble interleukin-6 receptor in the COVID-19 cytokine storm syndrome. Cell Rep Med. 2021;2(5):100269. doi:10.1016/j.xcrm.2021.100269
36. Schultheiß C, Willscher E, Paschold L, et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. 2022;3(6):100663. doi:10.1016/j.xcrm.2022.100663
37. Tsokos M, Reichelt U, Jung R, Nierhaus A, Püschel K. Interleukin-6 and C-reactive protein serum levels in sepsis-related fatalities during the early postmortem period. Forensic Sci Int. 2001;119(1):47–56. doi:10.1016/S0379-0738(00)00391-1
38. Palmiere C, Augsburger M. Markers for sepsis diagnosis in the forensic setting: state of the art. Croat Med J. 2014;55(2):103–114. doi:10.3325/cmj.2014.55.103
39. Schrag B, Iglesias K, Mangin P, Palmiere C. Procalcitonin and C-reactive protein in pericardial fluid for postmortem diagnosis of sepsis. Int J Legal Med. 2012;126(4):567–572. doi:10.1007/s00414-012-0692-8
40. Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J. 2020;133(11):1261–1267. doi:10.1097/CM9.0000000000000824
41. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi:10.1001/jamacardio.2020.0950
42. Astrup BS, Thomsen JL. The routine use of C-reactive protein in forensic investigations. Forensic Sci Int. 2007;172(1):49–55. doi:10.1016/j.forsciint.2006.10.021
43. Maeda H, Zhu BL, Bessho Y, et al. Postmortem serum nitrogen compounds and C-reactive protein levels with special regard to investigation of fatal hyperthermia. Forensic Sci Med Pathol. 2008;4(3):175–180. doi:10.1007/s12024-008-9029-9
44. Spiezia L, Boscolo A, Poletto F, et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(06):998–1000. doi:10.1055/s-0040-1714350
45. Jiang T, Lv B, Liu H, et al. Autopsy and statistical evidence of disturbed hemostasis progress in COVID-19: medical records from 407 patients. Thrombosis J. 2021;19(1):8. doi:10.1186/s12959-020-00256-5
46. Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi:10.1016/j.jcv.2020.104362
47. Tudoran C, Tudoran M, Abu-Awwad A, Cut TG, Voiță-Mekereș F. Spontaneous hematomas and deep vein thrombosis during the recovery from a SARS-CoV-2 infection: case report and literature review. Medicina. 2022;58(2):230. doi:10.3390/medicina58020230
48. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
49. Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological Features of Covid-19. N Engl J Med. 2020;383(10):989–992. doi:10.1056/NEJMc2019373
50. von Weyhern CH, Kaufmann I, Neff F, Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395(10241):e109. doi:10.1016/S0140-6736(20)31282-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
