Back to Journals » OncoTargets and Therapy » Volume 13
Autophagy-Related Beclin 1 and Head and Neck Cancers
Authors Hu YJ, Zhong JT, Gong L, Zhang SC, Zhou SH
Received 30 March 2020
Accepted for publication 10 June 2020
Published 30 June 2020 Volume 2020:13 Pages 6213—6227
DOI https://doi.org/10.2147/OTT.S256072
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Carlos E Vigil
Yang-Jie Hu,1,2 Jiang-Tao Zhong,2 Liang Gong,1 Si-Cong Zhang,1 Shui-Hong Zhou2
1Department of Otolaryngology, The Affiliated Cixi Hospital of Wenzhou Medical University, Cixi 315300, Zhejiang, People’s Republic of China; 2Department of Otolaryngology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, People’s Republic of China
Correspondence: Shui-Hong Zhou
Department of Otolaryngology, The First Affiliated Hospital, College of Medicine, Zhejiang University, 79 Qingchun Road, Hangzhou City 310003, People’s Republic of China
Tel +86-13868060120
Fax +86-571-87236895
Email [email protected]
Abstract: Beclin 1, a positive regulator of autophagy, behaves as a double-edged sword in tumorigenesis. Beclin 1 contributes to tumor suppression by removing defective or damaged organelles and other cellular components; however, its activity can also stimulate cancer initiation and progression. In head and neck cancer, Beclin 1 overexpression promotes autophagy, which limits DNA damage and chromosomal instability and increases necrosis and inflammation by impacting apoptotic and autophagic pathways. This paper reviews the relationship between Beclin 1, carcinogenesis and head and neck cancer prognosis.
Keywords: head and neck cancer, autophagy, Beclin 1, carcinogenesis
Introduction
Head and neck cancers are common malignant tumors, with most tumors being squamous cell carcinomas (SCCs); however, their pathogenesis, occurrence, development, and optimal treatment remain unclear. Tumor development is a multi-step, multi-gene mutation process. Multiple factors are also involved in the chemoradiotherapy resistance of malignant tumors.
Autophagy, a new focus of tumor research, plays an important role in tumorigenesis, development, and sensitivity to chemoradiotherapy.1–3 Autophagy has a dual role; it is a barrier to prevent cancer invasion and inhibit cancer growth, and it is an adaptive response to the relatively harsh environment of cancer in promoting cancer progression.4 Basic autophagy can maintain the survival of cancer cells, but excessive and continuously activated autophagy can activate self-degradation of cancer cells to lead to death4 (Figure 1).
Beclin 1 is an essential autophagy protein, which was shown using a yeast two-hybrid screen to interact directly with the Bcl-2 protein. Beclin 1 was named in 1998 and is encoded by the Beclin 1 autophagy-related gene (BECN1).5 Beclin 1 plays an important role in tumorigenesis.1 However, Beclin 1 may be either a suppressor or supporter under different conditions. This article reviews the significance of Beclin 1 in head and neck cancers.
The Autophagy BECN1 Gene and Beclin 1 Protein
The human BECN1 gene is located on chromosome 17q21 and is highly homologous with the yeast autophagy gene, Atg-6. It was the first mammalian autophagy gene to be discovered and it was identified as a haploid-insufficient tumor suppressor.6 BECN1 is important for the activity of the Class III phosphatidylinositol 3-kinase (P13K III)/Beclin 1/JNL/VPS34 signaling pathway, and the encoded Beclin 1 protein functions as the central adapter module within the PI3K-III complex.6
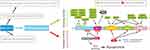
Beclin 1 is a 60-kDa protein of 450 amino acids. It has three different functional domains.7 The B cell CLL/lymphoma-2 (Bcl-2) homology 3 (BH3, amino acids 105–130) domain is located in a disordered N-terminal region and is sufficient for interaction with the anti-apoptotic family of proteins (Bcl-XL and Bcl-20).8–16 The central coiled-coil domain (CCD) is a flexible helix domain containing amino acids 141–171 which binds to the protein encoded by the UV irradiation resistance-associated gene (UVRAG) and to PI3-III.8–16 The central CCD can produce an inactive Beclin 1 homodimer or promote complex formation with AuTophaGy-related genes (ATG) 14 or UVRAG, which forms an active PI3K-III complex. The Beclin 1 BH3 domain interacts with BCL2/BCL-XL, which may stabilize the CCD-mediated Beclin 1 dimers, resulting in effective suppression of autophagosome biogenesis.17 The third domain, an evolutionarily conserved domain (ECD, amino acids 175–264), also termed a β-α repeated autophagic-specific domain (BARA; amino acids 265–450), binds to PI3K-III and takes part in the binding of lipid membranes of cell organelles.8–17 Beclin 1 may suppress cancer by coupling with lipid kinase rather than associating with BCL2. In addition, the ECD domain is essential for recruitment of VPS34, which not only induces autophagy but also leads to inhibition of tumors.18 Bax-interacting factor-1 is also a positive regulator of Beclin 1 through interaction with UVRAG18 (Figure 2).
Beclin 1 plays multifunctional roles in the cell (Figure 3). The main function of Beclin 1 is to regulate autophagy. It is involved in the initial stage of autophagolysosome formation, which means that Beclin 1 plays a crucial role in many biological cellular processes, including development, endocytosis, response to stress, aging, and cell death. Beclin 1 dysfunction may therefore cause many diseases.7–9,19,20 There are two interesting paradigms regarding the function and mode of regulation of Beclin 1, involving the promotion of autophagy and suppression of autophagy.21 Beclin 1 regulates autophagy through its phosphorylation.9,19,20 Bcl-2 interacting mediator (Bim) is a member of the Bcl-2 family that only contains BH3, which interacts with Beclin 1 and binds Beclin 1 to dynein light chain 1 (DLC1; also called LC8), which is a component of microtubules. Once Bim is phosphorylated, Bim and Beclin 1 dissociate from DLC1 to induce autophagy.22 Phosphorylation of Beclin 1 occurs at serine 15 in the N-terminal region through the action of kinases including unc-51-like kinase 1 (ULK1), phosphoglycerate kinase 1 (PGK1), death-associated protein kinase (DAPK3), mitogen-activated protein kinase-activated protein kinase 2/mitogen-activated protein kinase-activated protein kinase 3 (MAPKAPK2/MAPKAPK3), calcium/calmodulin-dependent protein kinase II (CaMKII), and adenosine 5ʹ-monophosphate-activated protein kinase (AMPK); at serine 30 via ULK1/2; in the BH3 domain via serine/threonine kinase-4 (STK4), DAPK1, DAPK2, and Rho associated coiled-coil containing protein kinase 1 (ROCK1); in the CCD domain via FAK; in the CCD, ECD-BARA, epidermal growth factor receptor (EGFR) domains via serine-threonine kinase (AKT) 1; in the CCD and BARA domains via breakpoint cluster region gene (BCR)-Abelson-leukemia-virus gene (ABL); and in the BARA domain via AMPK and Casein kinase 1 gamma 2 (CSNK1G2).19,23
Paradoxically, Beclin 1 phosphorylation also inhibits autophagy.20 Maejima et al found that the proapoptotic kinase, Mst1, may inhibit autophagy by phosphorylating the BH3 domain of Beclin 1. Mst1 also induces apoptosis by enhancing its interaction with Bcl-2/Bcl-xl, which phosphorylates Beclin 1, leading to Bax activation.24 Akt phosphorylates Beclin 1, leading to Beclin 1 sequestration to the cytoskeleton and suppression of its function in autophagy.25 Some metabolites and metabolic stimuli also regulate autophagy, including nutrient starvation, glucose, amino acids, glutamine, lipids and free fatty acids, hypoxia, and reactive oxygen species.26
Beclin 1 and Tumorigenesis
As previously mentioned, autophagy deficiency may be involved in a large number of diseases, including tumors. Dysfunctional Beclin 1 plays an important role in autophagy deficiency. Similar to the dual effects of autophagy, Beclin 1 has dual functions in tumorigenesis.7 It may impede tumor growth and progression by removing defective or damaged organelles and other cellular compounds, or it may play a role in cancer initiation and progression by regulating autophagy.7
Beclin 1 Affects Tumorigenesis and Tumor Development by Regulating Autophagy
In 1999, Liang et al first found that Beclin 1 may inhibit the proliferation of breast cancer MCF7 cells to prevent tumorigenesis.27 Beclin 1 may be a haploid-insufficient tumor suppressor. Thus, defective Beclin 1 plays a role in tumorigenesis.27
Beclin 1 as a Tumor Oncosuppressor
Many studies have shown that increased Beclin 1 expression in a number of cancers may activate autophagy and inhibit tumorigenesis.5,27-31 Beclin 1 plays a role in the induction of autophagy. Monoallelic loss of BECN1 may cause the occurrence of some cancers, including breast, ovarian, and prostate cancer. In BECN11+/– mice, lymphoma, lung carcinoma, and liver carcinoma may occur spontaneously.5,28-31 Haploinsufficiency in Beclin 1 impairs autophagy and promotes tumorigenesis in mice, and Beclin 1 is monoallelically deleted in many breast, prostate, and ovarian tumors, indicating an effect on autophagy in tumor suppression.5,28,29,32 In a study of mammary tumorigenesis, allelic loss of BECN1 was involved in breast tumorigenesis by increasing DNA damage and genomic instability.33 In immortalized baby mouse kidney epithelial (iBMK) cells, autophagy in Beclin+/+ and Beclin+/– iBMK cells was reduced, even under normal conditions.32 Autophagy induced in Beclin+/+ iBMK cells temporarily maintained homeostasis under stress in the background of a defect in apoptosis, and defective autophagy induced by Beclin 1+/– promoted tumorigenesis in iBMK cells. These results suggested that autophagy deficiency acts through a separate pathway to alleviate harsh stress in accordance with apoptosis to prevent death by necrosis.32
Some interactors of Beclin 1 may induce dissociation of the Beclin 1-Bcl-2 complex to reduce the Beclin 1/PI3K-III complex, leading to promotion of autophagy. The Autophagy and Beclin 1 Regulator 1 (AMBRA1) gene, which encodes a Beclin 1 interactor (Ambra1), enhances autophagy in a positive feedback mechanism by promoting autophagosome formation by activating the PI3K-III complex.34 In mice, spontaneous tumors occur in the liver and lung after a monoallelic deletion of AMBRA1, with the absence of a well-structured fibrous capsule, which suggests tumor aggressiveness. High mobility group box 1 (HMGB1) may inhibit formation of the Beclin 1/Bcl-2 complex to activate autophagy, which exerts multiple functions in various cancers including lung cancer, hematological malignancies, breast cancer, and osteosarcomas.35 Mitosis (NIMA)-related kinase 2 (NEK2) may bind and stabilize Beclin 1 to enhance autophagy, and NEK2 combined with chloroquine (CQ) and chemotherapeutic bortezomib may prevent NEK2-induced drug resistance in multiple myeloma.36 Some glycolytic enzymes also regulate autophagy. Phosphoglycerate kinase 1 (PGK1) may phosphorylate Beclin 1 to induce autophagy. Under hypoxia or glutamine deprivation, PGK1 may cause the interaction between PGK1 and Beclin 1 to result in phosphorylation of Beclin 1 at serine 30, which activates the ATG14-associated PIk3C3 vacuolar protein sorting 34 (VPS34)-BECN1-PIK3R4/VPS15 complex to generate the initiation stage of autophagy, and support cell proliferation and survival during stress.37 In nucleophosmin (NPM1)-mutated acute myeloid leukemia (AML), pyruvate kinase isoenzyme M2 may increase the phosphorylation of Beclin 1 to activate autophagy, leading to cell survival and poor survival of patients with NPM1-mutated AML.38 Chen et al found that knockdown of Beclin 1 inhibited cisplatin-induced autophagy and increased cisplatin-induced apoptotic cell death in A549lung cancer cells.39 It is also suggested that Beclin 1 is involved in the degradation of oncogenic proteins such as P53. Wild-type P53 protein interferes with oncosuppressive functions. Cancer cells with Beclin 1 depletion tend to accumulate increased amounts of mutant P53, whereas overexpression of Beclin 1 results in mutant P53 depletion. Wild-type P53 may inhibit malignant transformation.40
Beclin 1 expression in human solid cancers is decreased, which is associated with progression, invasion, and metastasis. Enhanced efficacy of anticancer therapies has been demonstrated by upregulating Beclin 1 expression to trigger autophagosome formation and accumulation.41 In some malignant tumors, decreased expression of Beclin 1 may enhance the therapeutic effect, although the underlying mechanism remains unclear.42,43 In the SGC-7901 gastric carcinoma, Beclin 1 binds to the N- and C-termini of lysosome-associated 4β (LAPTM4B) transmembrane protein, which competes with EGFR for LAPTM4B. Using this process, Beclin 1 can inhibit gastric cancer cell growth.42 In MDA-MB-231 triple negative breast cancer cells, Beclin 1 promotes cell proliferation and colony formation, while knockdown of Beclin 1 inhibits the migration and invasion of MDA-MB-231 cells.43
Beclin 1 Is Also an Oncosupportive Protein
Some studies have shown the opposite result that Beclin 1 acts as an oncosupportive component in tumorigenesis. The expression of Beclin 1 in some cancers, including colorectal cancers and gastric carcinomas, is higher while there is little or no expression in normal mucosal cells. The expression of Beclin 1 shows no significant correlation with clinicopathological characteristics.44 These results suggest that high Beclin 1 expression plays a role in the progression of tumors. In this regard, Jung et al suggested that Beclin 1 plays an oncosupportive role by increasing cell proliferation under harsh conditions such as hypoxia and nutrient starvation.45 Because Beclin 1 plays a role in protein sorting, in an oncosupportive role, Beclin 1 might mediate the evasion of specific receptors from lysosomal degradation, thus downregulating receptor degradation.46
In some systems, the role of Beclin 1 in tumorigenesis may be independent of autophagy.34 Beclin 1 may bind to UVRAG, which is monoallelically mutated in a high percentage of gastric and colonic tumors, while UVRAG overexpression and Beclin 1 interdependently activate autophagy, and UVRAG increases Beclin 1-PI3K III interaction and PI3KC3 enzymatic activity.47,48 In addition, UVRAG contributes to genomic stability in an autophagy-unrelated manner.49 Although Bcl-2 has an anti-apoptotic effect, Oh et al found that Bcl-2 binding to Beclin 1 was independent of its interaction with proapoptotic Bcl-2 protein in MCF7 breast cancer cells, and Bcl-2 mutants inhibited Beclin 1-dependent autophagy to induce tumorigenesis.50 In normal NCM-460 colon epithelial cells, 0.2 mM deoxycholic acid induced a significant decrease in the levels of Beclin 1 protein expression mediated through activated oxidative stress, which induced autophagy that activated a prosurvival pathway to play a role in colon carcinogenesis.51
Beclin 1 Affects Tumorigenesis and Tumor Development by Regulating Apoptosis
Beclin 1 also has non-autophagy functions. These include endocytosis, protein targeting/protein sorting, cytokinesis, and cell death.45 Beclin 1 may therefore regulate cell death in an autophagy-independent manner, such as by apoptosis. Apoptosis regulated by Beclin 1 may also play an important role during tumorigenesis and tumor development.
It has been suggested that mutual interaction between apoptosis and autophagy plays a novel role in tumorigenesis and progression.52 Beclin 1 plays an important role in this mutual process. Crosstalk between autophagy and apoptosis is complicated. Beclin 1 is a bridge linking autophagy, apoptosis, and differentiation. The BH-3 domain in Beclin 1 binds to both Bcl-2 and Bcl-xL.12 Once the BH-3 domain interacts with Bcl-2, the binding impedes Beclin 1-mediated autophagy, which might be considered as a convergence of apoptosis and autophagy. Simultaneous dysfunction of apoptosis and autophagy may cause accelerated DNA damage and genomic instability that promotes tumorigenesis.53 In gastric carcinomas, Zhou et al found that Beclin 1 showed low expression in four gastric carcinoma cell lines (MKN-45, MKN-28, BGC-823, and SGC-7901), while Bcl-xL showed high expression. They also found that Beclin 1 was moderately expressed in 153 gastric carcinoma tissues but was strongly expressed in the normal adjacent gastric mucosal tissues, as detected by immunohistochemistry (IHC) and Western blotting. However, the expression of Bxl-xL was high in these gastric carcinoma tissues compared to the normal adjacent gastric mucosal tissues.52 Low Beclin 1 expression was associated with poor differentiation and poor prognoses of gastric carcinomas. It has been shown that Beclin 1 overexpression in human gastric cancer (MKN28) enhances apoptosis triggered by cis-diaminedichloroplatinum (CDDP).54 Oxaliplatin and bortezomib significantly inhibit the growth of colorectal cancer by activating apoptosis by inducing the JNK-Bcl-Xl-Bax pathway and dissociating Beclin 1 from Bcl-xL.55 In gastric carcinomas, Wang et al used IHC to show that Beclin 1 expression in tissues of gastric carcinomas was significantly decreased when compared to paracarcinoma tissues.56 Beclin 1 expression was negatively associated with the TNM stage and invasion status. In MKN-45 gastric carcinoma cells, they also found that high expression of Beclin 1 might enhance apoptosis. They suggested a possible mechanism where Beclin 1 may inhibit the anti-apoptotic factor Bcl-xL to induce the apoptosis pathway, as well as the mutual effect of autophagy and apoptosis on gastric tumorigenesis.56 Shi et al detected Beclin 1 expression in seven hepatocellular carcinoma (HCC) cell lines (Hep G2, Hep3B, SMMC-7721, MHCC97-l, MHCC97-H, HCCLM3, and GCCLM6).57 They found that Beclin 1 expression in all HCC cell lines was low, and Beclin 1 expression was significantly lower in high-grade malignant HCCs. They also found that Beclin 1 expression was absent in 68.3% (205/300) of HCCs and patients with positive Beclin 1 expression had a good prognosis. Further study showed that the prognostic value of Beclin 1 expression was only significant in the Bcl-XL positive expression background. Thus, they suggested that there was a relationship between the autophagy defect and poor prognosis and apoptosis.57 In A549 human lung cancer cells, Li et al reported that cadmium may lead to both autophagy and apoptosis. This process was facilitated by Atg 4B binding to Bcl-2 to cause Beclin 1 release from the Bcl-2/Beclin 1 complex and thereby complete the Cd-induced switch from apoptosis to autophagy.58
However, Beclin 1 small interfering RNA (siRNA) decreases CDDP cytotoxicity. This mechanism is related to caspase activity.54 Caspases are cysteine aspartyl proteases, which play a key role in apoptosis. Caspases cleave Beclin 1 during apoptosis, resulting in destruction of its pro-autophagic activity.59 Beclin1 siRNA markedly decreased apoptosis in cervical HeLa cells, while overexpression increased apoptosis and autophagy. Beclin 1 also regulates caspase-960 and overexpression of Beclin 1 can result in tumor regression in vivo.60 It has been suggested that Beclin 1 may be the key molecular switch involved in mutual tuning of autophagy and apoptosis by modulation of caspase-9, which is an important mechanism in tumorigenesis of cervical carcinomas.
Beclin 1 and Head and Neck Cancers
Head and neck cancers are heterogenous tumors that are considered to be promoted by excessive alcohol and cigarette consumption. Although advances in therapeutic methods have been improved, the outcomes regarding novel approaches have been relatively limited. As previously mentioned, Beclin 1 plays an important role in tumorigenesis; however, studies of Beclin 1 involvement in head and neck cancers are relatively limited. From the results of these studies, the key roles of Beclin 1 in head and neck are summarized as follows.
Beclin 1 Is Involved in Carcinogenesis of Head and Neck Cancers
Beclin 1 Overexpression Inhibits Carcinogenesis of Head and Neck Cancers
In many cancers, overexpression of Beclin 1 plays an oncosuppressive role. In esophageal adenocarcinoma (EAC), Beclin 1 expression in normal HET-1A esophageal epithelium cells (derived from normal squamous epithelium) and nondysplastic Barratts’s esophagus (BE) cells detected by IHC, immunoblotting, and real-time polymerase chain reaction (RT-PCR), was high, whereas Beclin 1 expression was low in EACs. Acute bile acid upregulates Beclin 1 expression and chronic bile acid stimuli decrease Beclin 1 expression.61 These results suggested that Beclin 1-mediated autophagy was initially upregulated during premalignant stages of esophageal carcinogenesis and then decreased during the EAC transition.61 In thyroid cancer, another study showed that Beclin 1 had autophagy-independent anticancer effects on the exposure of thyroid cancer cells (FRO82-1, KTC1, KTC3, 8305C, and 8505C) to the proteasome inhibitor, MG132.62 Beclin 1 is also a marker of papillary thyroid carcinoma (PTC).63 Beclin 1 expression was positive in 98.9% of 97 PTC cases, whereas Beclin 1 expression was positive in 57.1% and 21.4% of follicular carcinomas and follicular adenomas, respectively.63 Cancer-associated fibroblasts (CAFs) play an important role in carcinogenesis, progression, and metastasis of cancers.64 CAFs induced resistance to cisplatin in SCC9 and CAL27 tongue squamous cell carcinoma (TSCC) cells. The mechanism involved the cisplatin-induced overexpression of Beclin 1 and microtubule-associated protein light chain 3-II (LC3-II) after SCC9 or CAL27 cells were co-cultured with CAFs.64 Inhibition of Beclin 1 by siRNA or CQ reduced the level of autophagy, resulting in the promotion of cytotoxicity of cisplatin on TSCC cells.64 Expression of Beclin 1 together with LC3-II and sequestosome 1 (p62) showed an inverse relationship with precancerous stages in the same section of 19 tissues of patients with laryngeal carcinomas (the same section included normal epithelium, hyperplastic epithelium, dysplastic epithelium, and invasive SCCs), whereas there was a parallel relationship in laryngeal carcinomas. The authors reviewed past reports and suggested that nucleocytoplasmic translocation of Beclin 1 was associated with its autophagic function and tumor suppressive roles.65
Beclin 1 Overexpression Promotes Carcinogenesis of Head and Neck Cancers
In oral carcinogenesis, autophagy markers including LC3-II, Beclin 1, ATG7, and P62 were detected by IHC and Western blotting during different stages of 4-nitroquinoline-1-oxide-induced oral carcinogenesis. LC3-II, Beclin 1, ATG7, and P62 expression levels gradually increased with increasing histopathological grade of carcinogenesis (normal, mild, moderate dysplasia, severe dysplasia, and squamous cell carcinoma).66 These results suggested that the level of autophagy was upregulated during the progressive stages of 4QQ-induced tongue carcinogenesis. The authors also found that Beclin 1 expression was associated with the number of regulatory T cells (Tregs) in mice peripheral blood and spleens, while LC3B and p62 were associated with myeloid-derived suppressor cells. These results suggested that autophagy was associated with tumor inflammation during tongue carcinogenesis.66 In esophageal cancer cells, autophagy was suggested to play a cytoprotective role in stressed EC109 cells. Inhibition of Beclin 1 by siRNA suppressed the autophagy level induced by tunicamycin, resulting in the promotion of apoptosis.67
Notably, Obatoclax, a pan-Bcl-2 inhibitor, induced autophagy, and had a cytoprotective role in adenoid cystic carcinoma cell line ACC-M.68 The induced autophagy by Obatoclax was dependent on activation of Beclin 1 and ATG5. Knockdown of Beclin 1 or ATG5 by siRNA significantly inhibited caspase-3 cleavage, resulting in decreased cell death induced by Obatoclax. When apoptosis was inhibited by benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (ZVAD-fmk), Beclin 1 siRNA or ATG5 siRNA still decreased the toxicity of Obatoclax. The possible mechanism may involve Beclin 1 and ATG5 playing dual roles in autophagy and apoptosis.68
Thus, there are multiple mechanisms by which Beclin 1 is involved in carcinogenesis of head and neck cancers: (1) Beclin 1-mediated autophagy can limit DNA damage and chromosomal instability;69 (2) impairment of both autophagy and apoptosis increases necrosis and inflammation, and inflammatory cytokines are correlated with cancer development;70 and (3) chronic bile acid stimuli decrease Beclin 1 expression resulting in promotion of genomic instability and cancer progression.61
Abnormal Expression of Beclin 1 Affects Proliferation, Growth, Invasion, Metastasis, and Prognosis of Head and Neck Cancer Cells
Many Studies Have Demonstrated That Low or Absent Expression of Beclin 1 Is Associated with Cancer Progression and a Poor Prognosis
Laryngeal carcinoma: Using IHC, we found that Beclin 1 expression was lower in 44 head and neck cancer tissues when compared with paracancerous tissues. Beclin 1 expression was negatively correlated with hypoxia-inducible factor-1ɑ (HIF-1ɑ) and Ki67. However, Beclin 1 expression was not significantly associated with clinicopathological features.71 Using IHC, Beclin 1 expression in 82 laryngeal carcinoma tissues (53.66%) was shown to be significantly lower than in paracarcinoma non-tumor tissues (72.5%). Absence of Beclin 1 expression was associated with increased lymph node metastasis, and low expression of Beclin 1 correlated with poor prognoses of laryngeal carcinomas.72
Hypopharyngeal carcinoma: Beclin 1 expression in hypopharyngeal squamous cell carcinomas (HSCCs) was significantly lower (42.7%) in 82 samples from patients with HSCC as detected by IHC when compared with that in adjacent non-cancerous tissues (79.6%, p < 0.001). RT-PCR analysis also showed similar results. Absence of Beclin 1 expression was also associated with poorer overall survival in patients with HSCC, and Beclin 1 was an independent prognostic factor for overall survival.73
Oral carcinoma: In 195 cases of oral cancer, Beclin 1 and Beclin 2 expression was localized in the cytoplasm ranging from a total absence to diffuse strong expression and was occasionally localized in the nuclei. There was a weak correlation between nuclear and cytoplasmic expression levels of Beclin 1.74 Beclin 1 expression was not associated with clinicopathological features. High expression of cytoplasmic Beclin 1 was associated with low disease survival, and absence of nuclear expression of Beclin 1 was associated with recurrence-free survival.74 In vitro, Beclin 1 and Beclin 2 were mainly localized in the cytoplasm of HEK293 oral cancer cells. Overexpression of Beclin 1 or Beclin 2 activated autophagy and promoted cancer cell growth, but the effects were independent of each other.5,74 In 90 primary oral squamous cell carcinomas (OSCCs), Beclin 1 expression was significantly associated with tumor grade and lymph node metastasis, and high expression of Beclin 1 was associated with a lower survival rate.75 Beclin 1 mRNA levels in 10 oral cancers were approximately eight times lower than in normal tissues.76 Beclin 1 expression was increased or decreased in TSCC SCC-9 and SCC-15 cells by transfection of lentivirus Beclin 1 and Beclin 1 short hairpin RNA (shRNA), respectively. Overexpression of Beclin 1 inhibited the proliferation, migration, and invasion of SCC-9 and SCC-15 cells, while decreased Beclin 1 promoted the proliferation, migration, and invasion of SCC-9 and SCC-15 cells. This mechanism may have involved Beclin 1 regulation of the production of VEGF, MMP-2, and MMP-9 in SCC-9 and SCC-15 cells.77 In TSCC cells, Beclin 1 expression in 14 TSCC tissues and five TSCC cell lines detected by RT-PCR and Western blotting was significantly decreased.78 Beclin 1 expression detected by IHC was also decreased in 133 TSCC specimens. The decreased expression of Beclin 1 was associated with poor differentiation, lymph node metastasis, advanced clinical TNM, and a poor five-year overall survival.78 In 74 OSCCs, the positive expression of Beclin 1 was 27.4%, which was significantly associated with lymphatic invasion and differentiation.79 In OSCC KB cells, the expression of Beclin 1 was high. 3-methyladenine (3-MA) significantly inhibited Beclin 1 expression and increased cell death and apoptosis of KB cells under nutrient depletion conditions. The authors suggested that autophagy was dependent on Beclin 1 (canonical autophagy) apoptosis and that Beclin 1-mediated autophagy involved crosstalk in KB cells, which constituted a negative correlation.80 The mechanism might be that Beclin 1 suppressed the transformation of TSCC cells, colony formation, and growth by acting as a tumor suppressor.80 Beclin 1-independent noncanonical activity was found in TSCC TCA-8113 and CAL-27 cells.81 Erlotinib, a small-molecule EGFR tyrosine kinase inhibitor used clinically for many cancers, induced autophagy (increasing the LC3-II/I ratio, which did not affect Beclin 1 expression) in tongue cancer cells.81 Induced autophagy by erlotinib resulted in erlotinib resistance in TCA-8113 and CAL-27 cells. HCQ (an inhibitor of the fusion of autophagosomes with lysosomes) combined with erlotinib treatment significantly decreased cell viability. However, 3-MA (an inhibitor of PI3K, which inhibits by blocking the activity of the Beclin 1-Vps34/class-III-PI3K complex) combined with erlotinib treatment showed less effect when compared with the effect of erlotinib on TCA-8113 and CAL-27 cells.81 These data suggested that erlotinib resistance was independent of the Beclin 1 noncanonical autophagy pathway, but dependent on the Atg5 autophagy pathway.81 InTSCC, Beclin 1 and LC3-II showed lower expression levels using IHC in 50 TSCC samples when compared with adjacent non-cancerous epithelial cells. Low Beclin 1 expression was associated with higher tumor stage, higher clinical stage, and greater differentiation.82 In vitro inhibition of Beclin 1 by 3-MA decreased autophagy levels, resulting in increasing proliferation, migration, and invasion in OTSCC Tca8113 cells.82
Thyroid carcinoma: Beclin 1 and ARHI, which is encoded by an anti-oncogene involved in tumorigenesis and modulation of autophagy activity, were detected by Western blotting in 80 tissues of patients with thyroid cancer. The expression levels of Beclin 1 and ARHI were significantly lower in thyroid cancer than in adjacent tissues. Lower Beclin 1 expression was associated with pathological stage, high pathological differentiation, and lymph node metastasis. Lower expression levels of Beclin 1 and ARHI were associated with poor prognoses. Beclin 1 expression was positively correlated with ARHI expression.83 The mechanism was studied in thyroid cancers. Knockdown of Beclin 1 expression by shRNA in FRO and KTC3 thyroid cancer cells promoted the invasive and migratory activities of cancer cells by upregulating N-cadherin and downregulating E-cadherin to induce the EMT.84 These onco-suppressor functions of Beclin 1 were mediated by posttranscriptional modulation of ZEB1 through the AU-binding factor 1 (AUF1) in thyroid cancer.84 ZEB1 is a crucial regulator of the EMT and AUF1.85 In sporadic medullary thyroid carcinoma (MTC), Beclin 1 expression was associated with the development of residual MTC; however, it was not associated with tumor size, extrathyroidal extension, or nodal metastasis.85 The expression levels of Beclin 1 and LC3-II detected in 50 papillary thyroid carcinomas (PTCs) by IHC and Western blotting were significantly decreased when compared with normal tissues, and Beclin 1 and LC3-II were associated with lymph node metastasis of PTC.86 In 86 PTCs, the expression levels of Beclin 1 in PTC and metastasis were higher than in normal tissues adjacent to tumors, distant normal tissues, and normal lymph nodes. Beclin 1 was significantly correlated with tumorigenesis and lymph node metastasis in human PTCs.87 Beclin 1 and HMGB1 expression levels were high in five thyroid cancer cell lines (K1, KTC-1, TPC-1, FTC-133, and FRO) and thyroid cancer tissues, but not in tissues derived from thyroid benign lesions. HMGB1 knockdown by shRNA downregulated the levels of LC3-II and Beclin 1 and increased p62 levels, which supported a key regulatory role for HMGB1 in autophagy.88
Salivary adenoid cystic carcinoma (ACC): In ACC, Beclin 1 expression was also a prognostic factor.89,90 In 89 ACCs, Beclin 1 expression detected by IHC and RT-PCR was lower in ACC tissues than in normal salivary gland tissue samples.91 Low Beclin 1 expression was associated with distant metastasis and poor prognosis.91 In 79 ACCs, Beclin 1, along with low expression of LC3, was significantly associated with a progressive histological growth pattern, high histological grade, and poor survival.92
Nasopharyngeal carcinoma (NPC): ANXA1 is a calcium-dependent phospholipid binding protein that was initially characterized with phospholipase A2-inhibitory and anti-inflammatory activities, which may regulate BECN1 and LC3-II.93 Upregulated BECN1 and LC3-II along with autophagy-associated protein Sequestosome-1 (SQSTM1), which is an autophagic substrate that can be degraded by autophagy, was detected in ANXAI knockdown 5-8F nasopharyngeal carcinoma cells. Conversely, overexpression of ANXAI decreased BECN1 and LC3-II expression levels along with elevation of SQSTM1 levels. Inhibition of BECN1 by siRNA or 3-MA in ANXA1 knockdown NPC cells led to NPC cell migration, invasion, and metastasis by activating the PI3K/Akt pathway. Pretreatment with angiotensin-(1-7) [Ang-(1-7)] inhibited cell proliferation, migration, and invasion in NPC-TW10 cells.94 The mechanism involved upregulation of Beclin 1 expression and downregulation of Bcl-2 expression, which led to increased autophagy.94
Esophageal carcinoma: Beclin 1 expression detected by IHC in 54 specimens of primary esophageal squamous cell carcinomas (ESCCs) was significantly lower than in normal epithelial cells of the esophagus.95 The decreased Beclin 1 expression was associated with decreased invasion, lymph node metastasis, and clinical stage. ESCC patients with overexpressed Beclin 1 had a good prognosis. In this study, Beclin 1 expression was associated with HIF-1α expression, which activated metabolic and pathogenic pathways leading to tumorigenesis, growth, invasion, and metastasis.95 Weh et al also reported decreased Beclin 1 expression with progression from normal tissues to BE with dysplasia, and in 115 EAC biopsies from 51 patients. They found that Beclin 1 loss in EACs was associated with advanced cancer stage and grade.96 They suggested that Beclin 1 might be a prognostic indicator.96 Further mechanistic studies showed that Beclin 1-dependent and Beclin 1-independent autophagy (noncanonical pathway) involved in suppressing progression of EACs. C-PAC, a cranberry proanthocyanidin extract, reduced Beclin 1 expression and increased LC3-II expression, resulting in a noncanonical autophagy-activated pathway. C-PAC reduced Beclin 1 through dephosphorylation at serine 15, serine 93, and serine 234, resulting in the death of EACcJHAD1 and OE19 cells. Rapamycin increased Beclin 1 levels by phosphorylation of Beclin 1, resulting in long-term cell survival.96 These results showed that Beclin 1 functioned as an onco-suppressor.
High Expression of Beclin 1 Was Associated with Cancer Progression and Poor Prognosis
Another study showed that high expression levels of Beclin 1 and LC3 detected by IHC and Western blotting in 150 samples from patients with II-IVa ESCC were associated with poor survival.97 Elevated Beclin 1 expression detected by IHC and Western blotting in 128 advanced NPC was associated with poor overall survival and progression-free survival. Moreover, Beclin 1 was also closely correlated with HIF-1α expression, and elevated Beclin 1 expression predicted poorer prognoses in a subset of NPC patients with positive HIF-1α expression.98 The authors explained the mechanism of elevated Beclin 1 expression and predicted that opposite prognosis results in different solid cancers might result from different intrinsic properties and the nature of the therapeutic regimen in different cancers.98 In addition, nuclear Beclin 1 had non-autophagic functions in DNA repair according to the results of Havaki et al, who reported that Beclin 1 expression in the nucleus in successive stages of laryngeal carcinogenesis was gradually decreased, which might indicate an impaired DNA repair process that increases genomic instability.65 In 43 ESCCs, the expression levels of Beclin-1, LC3B, and CASP-3 proteins as detected by IHC were not significantly correlated with clinicopathological factors. Beclin 1 expression was also not associated with the prognoses of ESCCs; however, high LC3B expression and low CASP-3 expression were significantly associated with a poor prognosis for ESCC.99 Collectively, these results suggest a role for Beclin 1 in cancer promotion.
Beclin 1 Is Associated with Treatment Resistance
Chemoresistance: In NPC, Beclin 1 and LC3-II/lc3-I were highly expressed in tissue samples of NPC and C666-1 NPC cells after cisplatin treatment, which led to upregulated autophagy.100 The authors suggested that increased autophagy induced resistance to cisplatin-mediated cell death. They also found that inhibition of Beclin 1 by CQ improved the sensitivity to cisplatin by reducing cell viability and promoting cell apoptosis.100 Cisplatin is the main chemotherapeutic treatment for hypopharyngeal carcinoma. CQ, an anti-malarial and anti-rheumatoid drug, is also known as an inhibitor of autophagy.101 CQ suppressed autophagy and promoted apoptosis in cisplatin-treated mice bearing human hypopharyngeal carcinoma FaDu cells. Inhibition of Beclin 1 by shRNA also had a similar effect to CQ on mice.101 In ESCC, O’Donovan et al found that knockdown of Beclin 1 by siRNA only reduced cell viability at the early stage of ESCC KYSE450 cells after 5-FU treatment.102 Combined knockdown of Beclin 1 with ATG7 by siRNA significantly enhanced the anticancer effect of 5-FU. They suggested that Beclin 1 played a cytoprotective role, facilitating recovery at an early stage of autophagy.102 In salivary gland ACC, inhibition of Beclin 1 by siRNA or 3-MA decreased autophagy, which enhanced the cytotoxicity of cisplatin in ACC-M cells.103
MicroRNAs (miRNAs), a class of endogenous RNAs, consisting of 19–25 nucleotides of non-coding RNA, have been shown to regulate autophagy, which leads to chemoresistance. Decreased levels of miR-30d may affect cisplatin in SW 1736 and 8305C human anaplastic thyroid carcinoma (ATC) cell lines mediated by overexpression of Beclin 1 and induction of autophagy.104 miR-30d negatively modulated Beclin 1 expression in ATC cells, and an miR-30d mimic significantly enhanced the sensitivity of ATC cells to cisplatin; however, induced expression of Beclin 1 rescued the downregulation of autophagy induced by the miR-30d mimic.104
In Hep-2 laryngeal carcinoma cells, overexpression of Beclin 1 after transfection with Beclin 1 did not affect cellular proliferation and apoptosis.105 After cisplatin treatment, overexpression of Beclin 1 decreased survival and increased apoptosis, and increased the sensitivity of Hep-2 cells to cisplatin as a result of Bcl-2-regulated autophagy.105 Beclin 1-mediated autophagy might protect the growth of laryngeal carcinoma Hep-2 cells and increase resistance to cisplatin treatment, whereas inhibition of Beclin 1 by siRNA could increase the cytotoxic sensitivity to cisplatin treatment by promoting cisplatin-mediated apoptosis via upregulation of caspase-3 and caspase-9.106
Radioresistance: HMBG1 binding to Beclin 1, as assessed by co-immunoprecipitation, was increased in SCC15 radioresistant oral squamous carcinoma cells. HMGB1 is an important inducer of autophagy. When HMBG1 interacts with Beclin 1, it promotes the formation of a Beclin 1-PI3KIII complex to increase the level of autophagy, which may lead to chemoradioresistance.107 Low power laser irradiation (LPLI) induced Beclin 1-mediated autophagy in OECM-1 and Ca9-22 oral cancer cells, resulting in LPLI resistance. LPLI induced the formation of reactive oxygen species (ROS) and RelA, which is a major member of the canonical NF-κB pathway, and which elicited BECN1 gene expression. Furthermore, ROS might promote the DNA binding activity of RelA-enhancing Beclin 1 expression in oral cancer cells.108
Targeted Beclin 1 as an Adjuvant for Other Anti-Cancer Therapies
Some therapeutic agents and sensitizers may regulate Beclin 1 expression to affect autophagy-inducing apoptosis of head and neck cancer cells.
Taxol: Inhibition of Beclin 1 by siRNA decreased autophagy to improve the effect of Taxol on 5-8F/Taxol Taxol-resistant NPC cells.109 A possible mechanism may be that Beclin 1 siRNA upregulates caspase-1 and IL-1β mRNA levels, as well as the levels of cleaved gasdermin D (GSDMD) and activated caspase-1. GSDMD is a canonical pyroptosis executor. Pyroptosis, a novel programmed cell death process that is initiated by inflammatory caspases, was downregulated by autophagy by suppressing the caspase-1/GSDMD pathway.109 In another study, Taxol induced autophagy in NPC CNE-1, HNE-2, CNE-1/Taxol, and HNE-2 Taxol cells. Inhibition of autophagy by 3-MA and Beclin 1 siRNA increased the effect of Taxol on NPC Taxol-resistance in CNE-1/Taxol and HNE-2/Taxol cell lines. In this study, inhibition of autophagy enhanced the Taxol sensitivity of NPC cells mainly through increased apoptosis via promotion of cleaved caspase-3 expression.110
Brazilin: Brazilin is a purified natural product from sappan wood (Caesalpinia sappan L), which has anticarcinogenic activity in human head and neck squamous cell carcinoma (HNSCC) Cal27 cells. Brazilin might induce apoptosis and autophagy. Beclin 1 siRNA sensitized Ca127 cells to brazilin-induced apoptotic cell death by a mechanism involving activation of the NF-kB p65 pathway, showing that Beclin 1 had a cytoprotective effect.111
Tetrandrine: Tetrandrine is a bisbenzylisoquinoline alkaloid, which induces oral cancer CAL 27 cell death by inducing apoptosis and autophagy. Inhibition of Beclin 1 and Atg5 by siRNA decreased the cytotoxic activity of tetrandrine.112
Luminacin: Luminacin is a metabolite from marine Streptomyces species, which inhibits cell growth and decreases the viability, migration, and invasive activities of HNSCC cell lines (SCCQLL1, SCC15, SCC25, SCC1483, MSKQLL1, and HN6). The results of this study showed that cytotoxicity induced by Tetrandrine was not caused by apoptosis or necrosis, but by autophagy through increased levels of Beclin 1 and LC3BI/II.113
Puquitinib mesylate: Puquitinib mesylate (XC-302) is a molecular-targeted drug, which directly suppresses the activity of PI3K. XC-302 inhibits the viability of and induces autophagy in NPC CNE-2 cells. Beclin 1 siRNA or 3-MA significantly promoted the survival and cloning of CNE-2 cells. The authors suggested that induced autophagy may be a survival disadvantage of NEC-2 cells.114
Areca nut extract (ANE): Although the Areca nut is a common carcinogen, ANE and its 30–100 kDa fraction (ANE 30–100K) significantly induced autophagic cell death in both normal and malignant cells.115 Inhibition of Beclin 1 by an shRNA fragment as well as knockdown of ATG5 improved ANE30–100k-induced autophagy in head and neck cancer OECM-1/CE81T/VGH/Jurkat T and OECM-1/SCC25/SCC-15 cells. Inhibition of Beclin 1 also induced apoptosis, resulting in cell death.116
Erlotinib: Erlotinib is an inhibitor of the EGFR. It decreased cytotoxicity over time in both FaDu and Cal27 HNSCC cells and induced autophagy. CQ, Beclin 1 siRNA, or Atg5 siRNA combined with erlotinib increased the sensitivity to erlotinib in both FaDu and Cal27 HNSCC cells.117
Parthenolide: Parthenolide inhibits growth of MDA-T32 thyroid cancer cells and mouse xenograft tumors in vivo through increased expression of the autophagocytic proteins, LC3-II and Beclin 1.118
Polygonum cuspidatum (Hu Zhang), which is a traditional Chinese medicine, reduces the viability of oral cancer cisplatin-resistant CAR cells by simultaneously inducing autophagy by increasing protein levels of ATGs, Beclin 1, and LC3-II, resulting in apoptosis.119
Buddleja officinalis: Buddleja officinalis (BO) extract, which is a sensitizer to photodynamic therapy, may induce cell death of FaDu hypopharyngeal carcinoma cells.120 The study showed that BO upregulated autophagy by increasing ATG5, Beclin 1, and LC3-II expression levels and decreased mTOR expression and promoted apoptosis by increasing Bax protein expression and decreasing Bcl-2 expression. This study also showed that crosstalk occurred between autophagy and apoptosis, and that activated autophagy occurred prior to activated apoptosis.120
Flavokawain B (FKB): Flavokawain B is a natural kava chalcone, which inhibits growth, migration, and invasion, and induces apoptosis of Tca thyroid cancer cells. During cytotoxicity of FKB, FKB induced autophagy by increasing LC3B-II and Beclin 1 expression and decreasing p62 expression.121 In this study, Beclin 1 was activated by activating AMPK in a similar manner as in other studies. The antitumor effect of FKB was improved by inhibition of autophagy through 3-MA or cotreatment with Beclin 1 siRNA and ATG5 siRNA.121
CZ415: CZ415 is a highly selective oral mTOR kinase inhibitor, which may have an anticancer effect on HNSCC cell lines (SCC-9, AQ20B, and A253) through inhibition of cell survival and proliferation. In this process, CZ415 upregulated Beclin 1, ATG5, ATG7, and LC3B-II, and degraded p62, resulting in autophagy.122 Beclin 1 knockdown by shRNA increased the CZ415-induced anticancer activity. In thyroid carcinomas, inhibition of autophagy by Beclin 1 siRNA or 3-MA increased the sensitivity of TPC-1 cells to CZ415.123
Inhibition of Beclin 1 may increase the antitumor effect obtained by suppressing long non-coding RNA (lncRNA). The lncRNA HOX transcript antisense RNA (lncRNA HOTAIR), which is localized in the endonuclear region, is a functional RNA molecule that contains more than 200 nucleotides and is upregulated in many cancers. HOTAIR siRNA inhibited proliferation, migration, and invasion of oral SCC CAL-27 cells by decreasing the level of autophagosomes and the expression of Beclin 1, ATG5, ATG7, and LC3B-II.124
Conclusions and Future Prospects
The pathogenesis, occurrence, development, and optimal treatment of head and neck cancers are still unclear. It has been shown that Beclin 1 is involved in carcinogenesis of head and neck cancers. There are multiple mechanisms involved in Beclin 1 carcinogenesis of head and neck cancers, including Beclin 1-mediated autophagy, which limits DNA damage and chromosomal instability, while impairment of both autophagy and apoptosis increases necrosis and inflammation. Increased levels of inflammatory cytokines are correlated with cancer development; and chronic bile acid stimuli decrease Beclin 1 expression, resulting in promotion of genomic instability and cancer progression. Abnormal Beclin 1 expression was associated with chemo-radiotherapy resistance in head and neck cancer. In combination with our previous studies,125–128 we will continue to investigate the role of Beclin 1-mediated autophagy in laryngeal carcinoma as well as the role of Beclin1 in glucose transporter-1 (GLUT1)-mediated radioresistance. Furthermore, future studies should be designed to test the effects of Beclin1 activity on GLUT-1 expression as a pathway to improve the radiosensitivity of laryngeal carcinoma.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gewirtz DA. The four faces of autophagy: implications for cancer therapy. Cancer Res. 2014;74(3):647–651. doi:10.1158/0008-5472
2. Li J, Liu G, Li L, Yao Z, Huang J. Research progress on the effect of autophagy-lysosomal pathway on tumor drug resistance. Exp Cell Res. 2020;389(2):111925. doi:10.1016/j.yexcr.2020.111925
3. Hou G, Bai Y, Jia A, et al. Inhibition of autophagy improves resistance and enhances sensitivity of gastric cancer cells to cisplatin. Can J Physiol Pharmacol. 2020. doi:10.1139/cjpp-2019-0477
4. Kang R, Zeh H, Lotze M, Tang D. The multifaceted effects of autophagy on the tumor microenvironment. Adv Exp Med Biol. 2020;1225:99–114. doi:10.1007/978-3-030-35727-6_7
5. Liang XH, Kleeman LK, Jiang HH, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72(11):8586–8596. doi:10.1128/JVI.72.11.8586-8596.1998
6. Aita VM, Liang XH, Murty VV, et al. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59(1):59–65. doi:10.1006/geno.1999.5851
7. Toton E, Lisiak N, Sawicka P, Rybczynska M. Beclin-1 and its role as a target for anticancer therapy. J Physiol Pharmacol. 2014;65(4):459–467.
8. Liang XH, Yu J, Brown K, Levine B. Beclin 1 contains a leucine-rich nuclear export signal that is required for its autophagy and tumor suppressor function. Cancer Res. 2001;61(8):3443–3449.
9. Vega-Rubín-de-Celis S. The role of Beclin 1-dependent autophagy in cancer. Biology (Basel). 2019;9(1):
10. Mei Y, Su M, Soni G, Salem S, Colbert CL, Sinha SC. Intrinsically disordered regions in autophagy proteins. Proteins. 2014;82(4):565–578. doi:10.1002/prot.24424
11. Lee EF, Perugini MA, Pettikiriarachchi A, et al. The BECN1 N-terminal domain is intrinsically disordered. Autophagy. 2016;12(3):460–471. doi:10.1080/15548627.2016.1140292
12. Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: beclin 1 is a novel BH3-only protein. J Biol Chem. 2007;282(17):13123–13132. doi:10.1074/jbc.M700492200
13. Mei Y, Ramanathan A, Glover K, et al. Conformational flexibility enables the function of a BECN1 region essential for starvation-mediated autophagy. Biochemistry. 2016;55(13):1945–1958. doi:10.1021/acs.biochem.5b01264
14. Li X, He L, Che KH, et al. Imperfect interface of Beclin1 coiled-coil domain regulates homodimer and heterodimer formation with Atg14L and UVRAG. Nat Commun. 2012;3:662. doi:10.1038/ncomms1648
15. Noda NN, Kobayashi T, Adachi W, Fujioka Y, Ohsumi Y, Inagaki F. Structure of the novel C-terminal domain of vacuolar protein sorting 30/autophagy-related protein 6 and its specific role in autophagy. J Biol Chem. 2012;287(20):16256–16266. doi:10.1074/jbc.M112.348250
16. Huang W, Choi W, Hu W, et al. Crystal structure and biochemical analyses reveal Beclin 1 as a novel membrane binding protein. Cell Res. 2012;22(3):473–489. doi:10.1038/cr.2012.24.44
17. Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–939. doi:10.1016/j.cell.2005.07.002
18. Takahashi Y, Coppola D, Matsushita N, et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol. 2007;9(10):1142–1151. doi:10.1038/ncb1634
19. Menon MB, Dhamija S. Beclin 1 Phosphorylation - at the center of autophagy regulation. Front Cell Dev Biol. 2018;6:137. doi:10.3389/fcell.2018.00137
20. Yan X, Zhou R, Ma Z. Autophagy-cell survival and death. Adv Exp Med Biol. 2019;1206:667–696. doi:10.1007/978-981-15-0602-4_29
21. Koren I, Kimchi A. Cell biology. Promoting tumorigenesis by suppressing autophagy. Science. 2012;338(6109):889–890. doi:10.1126/science.1230577
22. Luo S, Garcia-Arencibia M, Zhao R, et al. Bim inhibits autophagy by recruiting Beclin 1 to microtubules. Mol Cell. 2012;47(3):359–370. doi:10.1016/j.molcel.2012.05.040
23. Park JM, Seo M, Jung CH, et al. ULK1 phosphorylates Ser30 of BECN1 in association with ATG14 to stimulate autophagy induction. Autophagy. 2018;14(4):584–597. doi:10.1080/15548627.2017.1422851
24. Maejima Y, Kyoi S, Zhai P, et al. Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat Med. 2013;19(11):1478–1488. doi:10.1038/nm.3322
25. Wang RC, Wei Y, An Z, et al. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science. 2012;338(6109):956–959. doi:10.1126/science.1225967
26. Goldsmith J, Levine B, Debnath J. Autophagy and cancer metabolism. Methods Enzymol. 2014;542:25–57. doi:10.1016/B978-0-12-416618-9.00002-9
27. Liang XH, Jackson S, Seaman M, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402(6762):672–676. doi:10.1038/45257
28. Qu X, Yu J, Bhagat G, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112(12):1809–1820. doi:10.1172/JCI20039
29. Yue Z, Jin S, Yang C, et al. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100(25):15077–15082. doi:10.1073/pnas.2436255100
30. Mortensen M, Soilleux EJ, Djordjevic G, et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med. 2011;208(3):455–467. doi:10.1084/jem.20101145
31. Cicchini M, Chakrabarti R, Kongara S, et al. Autophagy regulator BECN1 suppresses mammary tumorigenesis driven by WNT1 activation and following parity. Autophagy. 2014;10(11):2036–2052. doi:10.4161/auto.34398
32. Degenhardt K, Mathew R, Beaudoin B, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10(1):51–64. doi:10.1016/j.ccr.2006.06.001
33. Karantza-Wadsworth V, Patel S, Kravchuk O, et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21(13):1621–1635. doi:10.1101/gad.1565707
34. Cianfanelli V, Fuoco C, Lorente M, et al. AMBRA1 links autophagy to cell proliferation and tumorigenesis by promoting c-Myc dephosphorylation and degradation. Nat Cell Biol. 2015;17(1):20–30. doi:10.1038/ncb3072
35. Xu T, Jiang L, Wang Z. The progression of HMGB1-induced autophagy in cancer biology. Onco Targets Ther. 2018;12:365–377. doi:10.2147/OTT.S185876
36. Xia J, He Y, Meng B, et al. NEK2 induces autophagy-mediated bortezomib resistance by stabilizing Beclin-1 in multiple myeloma. Mol Oncol. 2020;14(4):763–778. doi:10.1002/1878-0261.12641
37. Qian X, Li X, Lu Z. Protein kinase activity of the glycolytic enzyme PGK1 regulates autophagy to promote tumorigenesis. Autophagy. 2017;13(7):1246–1247. doi:10.1080/15548627.2017.1313945
38. Wang L, Yang L, Yang Z, et al. Glycolytic enzyme PKM2 mediates autophagic activation to promote cell survival in NPM1-mutated leukemia. Int J Biol Sci. 2019;15(4):882–894. doi:10.7150/ijbs.30290
39. Chen J, Zhang L, Zhou H, et al. Inhibition of autophagy promotes cisplatin-induced apoptotic cell death through Atg5 and Beclin 1 in A549 human lung cancer cells. Mol Med Rep. 2018;17(5):6859–6865. doi:10.3892/mmr.2018.8686
40. Choudhury S, Kolukula VK, Preet A, Albanese C, Avantaggiati ML. Dissecting the pathways that destabilize mutant p 53: the proteasome or autophagy? Cell Cycle. 2013;12(7):1022–1029. doi:10.4161/cc.24128
41. Yuan G, Yan SF, Xue H, Zhang P, Sun JT, Li G. Cucurbitacin I induces protective autophagy in glioblastoma in vitro and in vivo. J Biol Chem. 2014;289(15):10607–10619. doi:10.1074/jbc.M113.528760
42. Tian M, Chen Y, Tian D, Qiao X, Ma Z, Li J. Beclin1 antagonizes LAPTM4B-mediated EGFR overactivation in gastric cancer cells. Gene. 2017;626:48–53. doi:10.1016/j.gene.2017.05.006
43. Wu CL, Zhang SM, Lin L, et al. BECN1-knockout impairs tumor growth, migration and invasion by suppressing the cell cycle and partially suppressing the epithelial-mesenchymal transition of human triple-negative breast cancer cells. Int J Oncol. 2018;53(3):1301–1312. doi:10.3892/ijo.2018.4472
44. Ahn CH, Jeong EG, Lee JW, et al. Expression of beclin-1, an autophagy-related protein, in gastric and colorectal cancers. APMIS. 2007;115(12):1344–1349. doi:10.1111/j.1600-0463.2007.00858.x
45. Jung YY, Lee YK, Koo JS. The potential of Beclin 1 as a therapeutic target for the treatment of breast cancer. Expert Opin Ther Targets. 2016;20(2):167–178. doi:10.1517/14728222.2016.1085971
46. Thoresen SB, Pedersen NM, Liestol K, et al. A phosphatidylinositol 3-kinase class III sub-complex containing VPS15, VPS34, Beclin 1, UVRAG and BIF-1 regulates cytokinesis and degradative endocytic traffic. Exp Cell Res. 2010;316(20):3368–3378. doi:10.1016/j.yexcr.2010.07.008
47. Liang C, Feng P, Ku B, et al. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol. 2006;8(7):688–699. doi:10.1038/ncb1426
48. Kim MS, Jeong EG, Ahn CH, Kim SS, Lee SH, Yoo NJ. Frameshift mutation of UVRAG, an autophagy-related gene, in gastric carcinomas with microsatellite instability. Hum Pathol. 2008;39(7):1059–1063. doi:10.1016/j.humpath.2007.11.013
49. Zhao Z, Oh S, Li D, et al. A dual role for UVRAG in maintaining chromosomal stability independent of autophagy. Dev Cell. 2012;22(5):1001–1116. doi:10.1016/j.devcel.2011.12.027
50. Oh S, Xiaofei E, Ni D, et al. Downregulation of autophagy by Bcl-2 promotes MCF7 breast cancer cell growth independent of its inhibition of apoptosis. Cell Death Differ. 2011;18(3):452–464. doi:10.1038/cdd.2010.116
51. Payne CM, Crowley-Skillicorn C, Holubec H, et al. Deoxycholate, an endogenous cytotoxin/genotoxin, induces the autophagic stress-survival pathway: implications for colon carcinogenesis. J Toxicol. 2009;2009:785907. doi:10.1155/2009/785907
52. Zhou WH, Tang F, Xu J, et al. Low expression of Beclin 1, associated with high Bcl-xL, predicts a malignant phenotype and poor prognosis of gastric cancer. Autophagy. 2012;8(3):389–400. doi:10.4161/auto.18641
53. Mathew R, Kongara S, Beaudoin B, et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21(11):1367–1381. doi:10.1101/gad.1545107
54. Furuya D, Tsuji N, Yagihashi A, Watanabe N. Beclin 1 augmented cis-diamminedichloroplatinum induced apoptosis via enhancing caspase-9 activity. Exp Cell Res. 2005;307(1):26–40. doi:10.1016/j.yexcr.2005.02.023
55. Kim SY, Song X, Zhang L, Bartlett DL, Lee YJ. Role of Bcl-xL/Beclin-1 in interplay between apoptosis and autophagy in oxaliplatin and bortezomib-induced cell death. Biochem Pharmacol. 2014;88(2):178–188. doi:10.1016/j.bcp.2014.01.027
56. Wang Y, Xie J, Wang H, Huang H, Xie P. Beclin-1 suppresses gastric cancer progression by promoting apoptosis and reducing cell migration. Oncol Lett. 2017;14(6):6857–6862. doi:10.3892/ol.2017.7046
57. Shi YH, Ding ZB, Zhou J, Qiu SJ, Fan J. Prognostic significance of Beclin 1-dependent apoptotic activity in hepatocellular carcinoma. Autophagy. 2009;5(3):380–382. doi:10.4161/auto.5.3.7658
58. Li Z, Li Q, Lv W, et al. The interaction of Atg4B and Bcl-2 plays an important role in Cd-induced between apoptosis and autophagy through disassociation of Bcl-2-Beclin1 in A549 cells. Free Radic Biol Med. 2019;130:576–591. doi:10.1016/j.freeradbiomed.2018.11.020
59. Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18(4):571–580. doi:10.1038/cdd.2010.191
60. Wang ZH, Xu L, Duan ZL, Zeng LQ, Yan NH, Peng ZL. Beclin 1-mediated macroautophagy involves regulation of caspase-9 expression in cervical cancer HeLa cells. Gynecol Oncol. 2007;107(1):107–113. doi:10.1016/j.ygyno.2007.05.034
61. Roesly HB, Khan MR, Chen HD, et al. The decreased expression of Beclin-1 correlates with progression to esophageal adenocarcinoma: the role of deoxycholic acid. Am J Physiol Gastrointest Liver Physiol. 2012;302(8):G864–G872. doi:10.1152/ajpgi.00340.2011
62. Zhang HY, Du ZX, Meng X, Zong ZH, Wang HQ. Beclin 1 enhances proteasome inhibition-mediated cytotoxicity of thyroid cancer cells in macroautophagy-independent manner. J Clin Endocrinol Metab. 2013;98(2):E217–E226. doi:10.1210/jc.2012-2679
63. Yeşil C, Kandemir O, Haksever H, Dabakoğlu T. Is BECLIN-1 immunoreactivity more effective than HBME-1 in diagnosis of papillary thyroid cancer? Acta Chir Belg. 2015;115(4):299–305. doi:10.1080/00015458.2015.11681116
64. Liao JK, Zhou B, Zhuang XM, Zhuang PL, Zhang DM, Chen WL. Cancer-associated fibroblasts confer cisplatin resistance of tongue cancer via autophagy activation. Biomed Pharmacother. 2018;97:1341–1348. doi:10.1016/j.biopha.2017.11.024
65. Havaki S, Vlachou V, Zampetidis CP, et al. Monitoring autophagy immunohistochemically and ultrastructurally during human head and neck carcinogenesis. Relationship with the DNA damage response pathway. Int J Mol Sci. 2017;18(9):
66. Wu JS, Li L, Wang SS, et al. Autophagy is positively associated with the accumulation of myeloid-derived suppressor cells in 4-nitroquinoline-1-oxide-induced oral cancer. Oncol Rep. 2018;40(6):3381–3391. doi:10.3892/or.2018.6747
67. Pang XL, He G, Liu YB, Wang Y, Zhang B. Endoplasmic reticulum stress sensitizes human esophageal cancer cell to radiation. World J Gastroenterol. 2013;19(11):1736–1748. doi:10.3748/wjg.v19.i11.1736
68. Liang LZ, Ma B, Liang YJ, et al. Obatoclax induces Beclin1- and ATG5-dependent apoptosis and autophagy in adenoid cystic carcinoma cells. Oral Dis. 2015;21(4):470–477. doi:10.1111/odi.12305
69. Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7(12):961–967. doi:10.1038/nrc2254
70. White E, Karp C, Strohecker AM, Guo Y, Mathew R. Role of autophagy in suppression of inflammation and cancer. Curr Opin Cell Biol. 2010;22(2):212–217. doi:10.1016/j.ceb.2009.12.008
71. Lin W, Yin CY, Yu Q, et al. Expression of glucose transporter-1, hypoxia inducible factor-1α and beclin-1 in head and neck cancer and their implication. Int J Clin Exp Pathol. 2018;11(7):3708–3717.
72. Huang L, Wang S, Li SS, Yang XM. Prognostic significance of Beclin-1 expression in laryngeal squamous cell carcinoma. Pathol Oncol Res. 2013;19(4):771–777. doi:10.1007/s12253-013-9642-0
73. Wang J, Pan XL, Ding LJ, Liu DY, Da-Peng Lei JT. Aberrant expression of Beclin-1 and LC3 correlates with poor prognosis of human hypopharyngeal squamous cell carcinoma. PLoS One. 2013;8(7):e69038. doi:10.1371/journal.pone.0069038
74. Liu JL, Chen FF, Chang SF, et al. Expression of beclin family proteins is associated with tumor progression in oral cancer. PLoS One. 2015;10(10):e0141308. doi:10.1371/journal.pone.0141308
75. Tang JY, Fang YY, Hsi E, et al. Immunopositivity of Beclin-1 and ATG5 as indicators of survival and disease recurrence in oral squamous cell carcinoma. Anticancer Res. 2013;33(12):5611–5616.
76. Kapoor V, Paliwal D, Baskar Singh S, Mohanti BK, Das SN. Deregulation of Beclin 1 in patients with tobacco-related oral squamous cell carcinoma. Biochem Biophys Res Commun. 2012;422(4):764–769. doi:10.1016/j.bbrc.2012.05.079
77. Weng J, Wang C, Wang Y, et al. Beclin1 inhibits proliferation, migration and invasion in tongue squamous cell carcinoma cell lines. Oral Oncol. 2014;50(10):983–990. doi:10.1016/j.oraloncology.2014.06.020
78. Hu Z, Zhong Z, S H, et al. Decreased expression of Beclin‑1 is significantly associated with a poor prognosis in oral tongue squamous cell carcinoma. Mol Med Rep. 2016;14(2):1567–1573. doi:10.3892/mmr.2016.5437
79. Sakakura K, Takahashi H, Kaira K, Toyoda M, Oyama T, Chikamatsu K. Immunological significance of the accumulation of autophagy components in oral squamous cell carcinoma. Cancer Sci. 2015;106(1):1–8. doi:10.1111/cas.12559
80. Jiang LC, Xin ZY, Deborah B, et al. Inhibition of autophagy augments apoptosis in human oral squamous cell carcinoma under nutrient depletion. J Oral Pathol Med. 2015;44(5):361–366. doi:10.1111/jop.12250.
81. Huang K, Liu D. Targeting non-canonical autophagy overcomes erlotinib resistance in tongue cancer. Tumour Biol. 2016;37(7):9625–9633. doi:10.1007/s13277-015-4689-z
82. Wang Y, Wang C, Tang H, et al. Decrease of autophagy activity promotes malignant progression of tongue squamous cell carcinoma. J Oral Pathol Med. 2013;42(7):557–564. doi:10.1111/jop.12049
83. Zhu H, Qu Y. Expression levels of ARHI and Beclin1 in thyroid cancer and their relationship with clinical pathology and prognosis. Oncol Lett. 2020;19(2):1241–1246. doi:10.3892/ol.2019.11223
84. Li S, Zhang HY, Du ZX, et al. Induction of epithelial-mesenchymal transition (EMT) by Beclin 1 knockdown via posttranscriptional upregulation of ZEB1 in thyroid cancer cells. Oncotarget. 2016;7(43):70364–70377. doi:10.18632/oncotarget.12217
85. Gundara JS, Zhao J, Gill AJ, et al. Noncoding RNA blockade of autophagy is therapeutic in medullary thyroid cancer. Cancer Med. 2015;4(2):174–182. doi:10.1002/cam4.355
86. Yang M, Bai L, Yu W, et al. Expression of autophagy-associated proteins in papillary thyroid carcinoma. Oncol Lett. 2017;14(1):411–415. doi:10.3892/ol.2017.6101
87. Li X, Xu H, Ma H. Beclin 1 is highly expressed in papillary thyroid carcinoma and correlates with lymph node metastasis. Acta Chir Belg. 2013;113(3):175–181. doi:10.1080/00015458.2013.11680907
88. Chai W, Ye F, Zeng L, Li Y, Yang L. HMGB1-mediated autophagy regulates sodium/iodide symporter protein degradation in thyroid cancer cells. J Exp Clin Cancer Res. 2019;38(1):325. doi:10.1186/s13046-019-1328-3
89. Li C, Chen Q, Tian Z, et al. Expression of MIF, Beclin1, and LC3 in human salivary gland adenoid cystic carcinoma and its prognostic value. Medicine (Baltimore). 2019;98(20):e15402. doi:10.1097/MD.0000000000015402
90. Jiang LC, Huang SY, Zhang DS, et al. Expression of beclin 1 in primary salivary adenoid cystic carcinoma and its relation to Bcl-2 and p53 and prognosis. Braz J Med Biol Res. 2014;47(3):252–258. doi:10.1590/1414-431X20133231
91. Liang LZ, Ma B, Liang YJ, et al. High expression of the autophagy gene Beclin-1 is associated with favorable prognosis for salivary gland adenoid cystic carcinoma. J Oral Pathol Med. 2012;41(8):621–629. doi:10.1111/j.1600-0714.2012.01171.x
92. Jiang L, Huang S, Li W, et al. Expression of autophagy and ER stress-related proteins in primary salivary adenoid cystic carcinoma. Pathol Res Pract. 2012;208(11):635–641. doi:10.1016/j.prp.2012.07.008
93. Zhu JF, Huang W, Yi HM, et al. Annexin A1-suppressed autophagy promotes nasopharyngeal carcinoma cell invasion and metastasis by PI3K/AKT signaling activation. Cell Death Dis. 2018;9(12):1154. doi:10.1038/s41419-018-1204-7
94. Lin YT, Wang HC, Chuang HC, Hsu YC, Yang MY, Chien CY. Pre-treatment with angiotensin-(1-7) inhibits tumor growth via autophagy by downregulating PI3K/Akt/mTOR signaling in human nasopharyngeal carcinoma xenografts. J Mol Med (Berl). 2018;96(12):1407–1418. doi:10.1007/s00109-018-1704-z
95. Chen Y, Lu Y, Lu C, Zhang L. Beclin-1 expression is a predictor of clinical outcome in patients with esophageal squamous cell carcinoma and correlated to hypoxia-inducible factor (HIF)-1alpha expression. Pathol Oncol Res. 2009;15(3):487–493. doi:10.1007/s12253-008-9143-8
96. Weh KM, Howell AB, Kresty LA. Expression, modulation, and clinical correlates of the autophagy protein Beclin-1 in esophageal adenocarcinoma. Mol Carcinog. 2016;55(11):1876–1885. doi:10.1002/mc.22432
97. Chen Y, Li X, Wu X, et al. Autophagy-related proteins LC3 and Beclin-1 impact the efficacy of chemoradiation on esophageal squamous cell carcinoma. Pathol Res Pract. 2013;209(9):562–567. doi:10.1016/j.prp.2013.06.006
98. Wan XB, Fan XJ, Chen MY, et al. Elevated Beclin 1 expression is correlated with HIF-1alpha in predicting poor prognosis of nasopharyngeal carcinoma. Autophagy. 2010;6(3):395–404. doi:10.4161/auto.6.3.11303
99. Chen HI, Tsai HP, Chen YT, Tsao SC, Chai CY. Autophagy and apoptosis play opposing roles in overall survival of esophageal squamous cell carcinoma. Pathol Oncol Res. 2016;22(4):699–705. doi:10.1007/s12253-016-0051-z
100. Aga T, Endo K, Tsuji A, et al. Inhibition of autophagy by chloroquine makes chemotherapy in nasopharyngeal carcinoma more efficient. Auris Nasus Larynx. 2019;46(3):443–450. doi:10.1016/j.anl.2018.10.013
101. Zhao XG, Sun RJ, Yang XY, et al. Chloroquine-enhanced efficacy of cisplatin in the treatment of hypopharyngeal carcinoma in xenograft mice. PLoS One. 2015;10(4):e0126147. doi:10.1371/journal.pone.0126147
102. O’Donovan TR, O’Sullivan GC, McKenna SL. Induction of autophagy by drug-resistant esophageal cancer cells promotes their survival and recovery following treatment with chemotherapeutics. Autophagy. 2011;7(5):509–524. doi:10.4161/auto.7.5.15066
103. Ma B, Liang LZ, Liao GQ, et al. Inhibition of autophagy enhances cisplatin cytotoxicity in human adenoid cystic carcinoma cells of salivary glands. J Oral Pathol Med. 2013;42(10):774–780. doi:10.1111/jop.12066
104. Zhang Y, Yang WQ, Zhu H, et al. Regulation of autophagy by miR-30d impacts sensitivity of anaplastic thyroid carcinoma to cisplatin. Biochem Pharmacol. 2014;87(4):562–570. doi:10.1016/j.bcp.2013.12.004
105. Yang M, Yang XM, Yin DH, et al. Beclin1 enhances cisplatin-induced apoptosis via Bcl-2-modulated autophagy in laryngeal carcinoma cells Hep-2. Neoplasma. 2018;65(1):42–48. doi:10.4149/neo_2018_161102N528
106. Kang R, Wang ZH, Wang BQ, et al. Inhibition of autophagy-potentiated chemosensitivity to cisplatin in laryngeal cancer Hep-2 cells. Am J Otolaryngol. 2012;33(6):678–684. doi:10.1016/j.amjoto.2012.05.005
107. Min HJ, Suh KD, Lee YH, Kim KS, Mun SK, Lee SY. Cytoplasmic HMGB1 and HMGB1-Beclin1 complex are increased in radioresistant oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2019;57(3):219–225. doi:10.1016/j.bjoms.2019.01.011
108. Shu C-W, Chang H-T, Wu C-S, et al. RelA-mediated BECN1 expression is required for reactive oxygen species-induced autophagy in oral cancer cells exposed to low-power laser irradiation. PLoS One. 2016;11(9):e0160586. doi:10.1371/journal.pone.0160586
109. Wang X, Li H, Li W, et al. The role of Caspase-1/GSDMD-mediated pyroptosis in Taxol-induced cell death and a Taxol-resistant phenotype in nasopharyngeal carcinoma regulated by autophagy. Cell Biol Toxicol. 2020. doi:10.1007/s10565-020-09514-8
110. Song Y, Li W, Peng X, Xie J, Li H, Tan G. Inhibition of autophagy results in a reversal of taxol resistance in nasopharyngeal carcinoma by enhancing taxol-induced caspase-dependent apoptosis. Am J Transl Res. 2017;9(4):1934–1942.
111. He ZJ, Zhu FY, Li SS, Zhong L, Tan HY, Wang K. Inhibiting ROS-NF-κB-dependent autophagy enhanced brazilin-induced apoptosis in head and neck squamous cell carcinoma. Food Chem Toxicol. 2017;101:55–66. doi:10.1016/j.fct.2017.01.002
112. Lien JC, Lin MW, Chang SJ, et al. Tetrandrine induces programmed cell death in human oral cancer CAL 27 cells through the reactive oxygen species production and caspase-dependent pathways and associated with beclin-1-induced cell autophagy. Environ Toxicol. 2017;32(1):329–343. doi:10.1002/tox.22238
113. Shin YS, Cha HY, Lee BS, et al. Anti-cancer effect of luminacin, a marine microbial extract, in head and neck squamous cell carcinoma progression via autophagic cell death. Cancer Res Treat. 2016;48(2):738–752. doi:10.4143/crt.2015.102
114. Wang KF, Yang H, Jiang WQ, Li S, Cai YC. Puquitinib mesylate (XC-302) induces autophagy via inhibiting the PI3K/AKT/mTOR signaling pathway in nasopharyngeal cancer cells. Int J Mol Med. 2015;36(6):1556–1562. doi:10.3892/ijmm.2015.2378
115. Liu SY, Lin MH, Hsu YR, et al. Arecoline and the 30–100 kDa fraction of areca nut extract differentially regulate mTOR and respectively induce apoptosis and autophagy: a pilot study. J Biomed Sci. 2008;15(6):823–831. doi:10.1007/s11373-008-9273-8
116. Yen CY, Chiang WF, Liu SY, et al. Impacts of autophagy-inducing ingredient of areca nut on tumor cells. PLoS One. 2015;10(5):e0128011. doi:10.1371/journal.pone.0128011
117. Sobhakumari A, Schickling BM, Love-Homan L, et al. NOX4 mediates cytoprotective autophagy induced by the EGFR inhibitor erlotinib in head and neck cancer cells. Toxicol Appl Pharmacol. 2013;272(3):736–745. doi:10.1016/j.taap.2013.07.013
118. Li C, Zhou Y, Cai Y, et al. Parthenolide inhibits the proliferation of MDA-T32 papillary thyroid carcinoma cells in vitro and in mouse tumor xenografts and activates autophagy and apoptosis by downregulation of the mammalian target of rapamycin (mTOR)/PI3K/AKT signaling pathway. Med Sci Monit. 2019;25:5054–5061. doi:10.12659/MSM.915387
119. Wang YL, Horng CT, Hsieh MT, et al. Autophagy and apoptotic machinery caused by Polygonum cuspidatum extract in cisplatin-resistant human oral cancer CAR cells. Oncol Rep. 2019;41(4):2549–2557. doi:10.3892/or.2019.6985
120. Cho H, Zheng H, Sun Q, et al. Development of novel photosensitizer using the buddleja officinalis extract for head and neck cancer. Evid Based Complement Alternat Med. 2018;2018:6917590. doi:10.1155/2018/6917590
121. He Q, Liu W, Sha S, et al. Adenosine 5ʹ-monophosphate-activated protein kinase-dependent mTOR pathway is involved in flavokawain B-induced autophagy in thyroid cancer cells. Cancer Sci. 2018;109(8):2576–2589. doi:10.1111/cas.13699
122. Xie J, Li Q, Ding X, Gao Y. Targeting mTOR by CZ415 inhibits head and neck squamous cell carcinoma cells. Cell Physiol Biochem. 2018;46(2):676–686. doi:10.1159/000488724
123. Li X, Li Z, Song Y, Liu W, Liu Z. The mTOR kinase inhibitor CZ415 inhibits human papillary thyroid carcinoma cell growth. Cell Physiol Biochem. 2018;46(2):579–590. doi:10.1159/000488625
124. Wang X, Liu W, Wang P, Li S. RNA interference of long noncoding RNA HOTAIR suppresses autophagy and promotes apoptosis and sensitivity to cisplatin in oral squamous cell carcinoma. J Oral Pathol Med. 2018;47(10):930–937. doi:10.1111/jop.12769
125. Dai LB, Yu Q, Zhou SH, et al. Effect of combination of curcumin and GLUT-1 AS-ODN on radiosensitivity of laryngeal carcinoma through regulating autophagy. Head Neck. 2020. doi:10.1002/hed.26180
126. Zhong JT, Yu Q, Zhou SH, et al. GLUT-1 siRNA enhances radiosensitization of laryngeal cancer stem cells via enhanced DNA damage, cell cycle redistribution, and promotion of apoptosis in vitro and in vivo. Onco Targets Ther. 2019;12:9129–9142. doi:10.2147/OTT.S221423
127. Lu ZJ, Yu Q, Zhou SH, et al. Construction of a GLUT-1 and HIF-1α gene knockout cell model in HEp-2 cells using the CRISPR/Cas9 technique. Cancer Manag Res. 2019;11:2087–2096. doi:10.2147/CMAR.S183859
128. Zhou SH, Fan J, Chen XM, Cheng KJ, Wang SQ. Inhibition of cell proliferation and glucose uptake in human laryngeal carcinoma cells by antisense oligonucleotides against glucose transporter-1. Head Neck. 2009;31:1624–1633. doi:10.1002/hed.21137
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.