Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 11
Autologous non-cultured melanocyte–keratinocyte transplantation in the treatment of vitiligo: patient selection and perspectives
Authors Bassiouny D, Esmat S
Received 14 May 2018
Accepted for publication 16 July 2018
Published 26 October 2018 Volume 2018:11 Pages 521—540
DOI https://doi.org/10.2147/CCID.S151503
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Dalia Bassiouny, Samia Esmat
Department of Dermatology, Kasr El-Ainy Teaching Hospital, Faculty of Medicine, Cairo University, Cairo, Egypt
Abstract: Autologous non-cultured melanocyte–keratinocyte transplantation procedure (MKTP) is one of the simplest cellular grafting techniques. Various modifications were done over the years to make the technique easier and more economical which led to its great popularity among dermatologists. Proper patient selection and good technical skills are essential for achieving success with this technique. In this review, different patient-related and procedure-related factors that affect the outcome are discussed. This review may guide dermatologists to select suitable candidates, and explains what to expect in each case and indicates different techniques which can be used. The expected complications and stability of acquired pigmentation, which are an essential part of the pretreatment patient counseling, are also discussed.
Keywords: cellular grafting, vitiligo surgery, patient variables, procedure variables, repigmentation
Introduction
Surgical treatment of vitiligo is the final resort to regain the pigmentation in lesions failing to repigment despite various medical and light therapies. Multiple cellular and tissue graft techniques are used successfully to introduce melanocytes and/or their stem cells to vitiligo lesions devoid of them.1
Autologous non-cultured melanocyte–keratinocyte transplantation procedure (MKTP) is one of the simplest cellular grafting techniques and is currently the most popular among dermatologists. It offers 50%–100% repigmentation rates with 1:3 up to 1:10 donor-to-recipient ratio and very good color matching in most of the treated cases.2–6 Since its first description by Gauthier and Surleve-Bazeille in 1992,7 the technique of cellular grafting evolved over the years with various modifications simplifying it and improving the results.
The response to MKTP in general is affected by several factors. As with other surgical techniques, proper patient selection is a crucial point as well as good technical skills. Different patient-related and procedure-related factors that affect the outcome are discussed in this review. This review may guide dermatologists to select suitable candidates, and explains what to expect in each case and indicates different techniques which can be used. The expected complications and stability of acquired pigmentation, which are an essential part of the pretreatment patient counseling, are also discussed.
Technique evolution
In 1992, Gauthier and Surleve-Bazeille7 described the MKTP as a 2-day procedure. On the first day, a shave biopsy was harvested from the scalp and incubated overnight in 0.25% trypsin at 4°C. The recipient site was also prepared by liquid nitrogen. On the second day, the trypsinized epidermis was placed in EDTA for 15 minutes, after which it was transferred to a calculated volume of saline. The basal layer was rubbed, the skin was agitated to dislodge the cells and finally the suspension was aspirated by an insulin syringe and injected into cryoblebs.
Several modifications aiming at simplifying the technique and improving the outcome were done over the years. Tissue was harvested from the gluteal area8,9 which allowed for harvesting a larger area and was also less vascular. Shave biopsy is simple and fast but may lead to development of textural change or scar in the donor site which prompted the researchers to search for a solution. In vivo preparation of epidermal cell suspension was introduced by Gupta et al10 with excellent response in five treated cases. MKTP was performed entirely in sterile blisters on the patient’s body with no donor site scarring and very high cell viability (99%). Roofs of suction blisters were used in another study for suspension preparation; however, no clear data were given in the study about repigmentation rate.11 Nevertheless, suction blister formation is a time-consuming process.
Since the hair follicles are the main reservoir and the source of melanocytes that repopulate the epidermis in non-glabrous skin, it was only a matter of time before dermatologists thought of harvesting this rich source of melanocytes for cellular transplantation. In 2009, Vanscheidt and Hunziker12 used plucked hair follicles for preparation of cell suspension which produced >90% repigmentation in 3/5 cases of vitiligo. Mohanty et al13 used follicular unit extraction technique instead of plucking to harvest anagen hair follicles which was a more tedious process but provided a significantly higher number of stem cells, as well as ten times more cell yield per hair follicle.14 Although this suspension was rich in highly proliferative melanocytes and stem cells, it lacked an abundance of healthy keratinocytes which are essential for supplying melanocytes with growth factors needed for their proliferation. In a trial to get the best of both worlds, recent publications used a mixture of epidermal and follicular suspensions with better results than epidermal suspension alone.15,16
Preparation of the suspension underwent major changes too. Olsson and Juhlin8 incubated the skin at 37°C for 50 minutes in a CO2 incubator, used trypsin inhibitor to stop tissue digestion, centrifuged the cells to obtain a cell pellet and added supplements such as antimicrobials and growth factors to the suspension medium. These additional steps increased the cost of the procedure. Mulekar made the procedure easier and more economical by using an ordinary incubator and DMEM/F12 without any additives for suspending the cell pellet.3 He later replaced trypsin inhibitor by washing the epidermis several times in DMEM/F12 before separating it from the dermis.17 Later, PBS was used during suspension preparation to further cut the cost.18 Kumar et al19 introduced a four-compartment technique in 2014 in which pipettes, autoclaved tips, centrifuge tube and centrifuge machine were no longer needed. The dermatologists estimated and used the exact amount of PBS to prepare the suspension according to the size of the lesion to be treated which was then aspirated by a syringe and spread evenly onto the denuded recipient surface.
Finally, in order to complete the procedure in 1 day, preparation of the recipient area using dermabrasion8 or laser resurfacing9 as opposed to cryotherapy7 was done. Not only did this save time, but it produced better cosmetic outcome as the cells were evenly spread over the whole lesion. Less invasive methods such as dermaroller20 or fractional CO221 were recently used to introduce the cells into the skin to minimize the downtime. To improve cell handling, collagen sheets8 or hyaluronic acid (HA) was used to create a paste.9 This allowed using MKTP even on curved surfaces with ease. Details of these changes over the years are given in Table 1. The effect of these changes on the response is discussed in detail later in this review.
Effect of patient-related factors on response to MKTP
Duration of disease stability
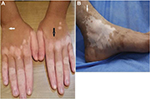
A strict selection of patients with stable vitiligo is the most important factor for successful outcome. Disease activity is defined as the appearance of new lesions or enlargement of old ones observed in the past year and/or the presence of Koebner phenomenon.5 Our only available activity score, the vitiligo index of disease activity score,22 depends on clinical history given by the patient. However, certain clinical features can help the dermatologist to identify disease activity, including hypomelanotic color of vitiligo lesions and poorly defined borders as opposed to amelanotic lesions with sharply demarcated borders which denote stability.23 Other signs of activity include confetti lesions and trichrome vitiligo (Figure 1).24
On reviewing the literature, earlier studies using MKTP in the treatment of vitiligo chose different durations of disease stability as an inclusion criterion. Some authors considered 6 months of disease stability to be sufficient,2,4,17,25,26 while others required 1 year of disease stability.8,9 In the early reports by Mulekar,2,4 in which cases with a minimum of 6 months of disease stability were included, ≥95% repigmentation occurred in 65/122, 13/19 and 36/43 cases of generalized vitiligo (GV), focal vitiligo and segmental vitiligo (SV), respectively. However, a high relapse rate was noted in 15 of those responders. Huggins et al26 achieved ≥95% repigmentation in only 4/23 treated cases with 6-month duration of stability. In a retrospective long-term follow-up study of cases treated over the past 6 years, 6 months of stability was sufficient as an inclusion criterion. More than 75% repigmentation was noted in 71% and 54% of SV and non-segmental vitiligo (NSV) cases, respectively.27 On the contrary, a higher percentage of repigmentation was noted in one study where all three SV and 13/17 NSV cases with 1-year stability showed 95%–100% repigmentation8 and in another study where all four cases achieved 84%–100% repigmentation.9
In a retrospective study by Olsson and Juhlin,28 cases that showed stable improvement of their vitiligo had a 78% repigmentation in response to different melanocyte transplantation techniques including MKTP after 4.8 years compared to cases with unstable vitiligo that showed a 33% repigmentation after 6.5 years. The authors recommended that patients with extensive GV and those who have not had completely stable, non-progressive vitiligo for at least 2 years should not be chosen for transplantation.
Active disease and the presence of Koebner phenomenon were found to negatively influence treatment results.5,13 Activity resulted in failure of MKTP suspension in one study5 with a median area percentage of repigmentation of zero compared to 93% in stable cases at 12-month posttreatment follow-up. Similarly, 79% repigmentation occurred in cases with ≥1-year stability vs only 18% in cases with <1-year duration of stability (P=0.02) in another study.13 A significant positive correlation between duration of stability and percentage repigmentation of the lesions was also found in a recent study.29
Based on the above data, since 2004 the majority of authors including Mulekar3 consider 1 year as the minimum duration of stability needed for a favorable outcome of surgery.30–41
When uncertain about stability, a longer pretreatment observation documented by photography or a minigrafting test42 may be indicated to avoid unfavorable outcome of surgery. Disease stability should be considered in both SV and NSV. SV can respond to medical treatment during the first 6 months.
Type of vitiligo
When it was first described by Gauthier and Surleve-Bazeille in 1992,7 MKTP was used in the treatment of localized vitiligo areas of ≤50 cm2 in three SV and eight focal vitiligo cases. The response was better in SV cases with an average repigmentation rate of 92% vs 41% in focal vitiligo cases with 4/7 cases failing to repigment. However, comparable excellent response (≥95% repigmentation) was found in a series of 25 children and adolescents with SV and focal vitiligo.25
SV cases showed a significantly better response than NSV with 85% vs 70% repigmentation (P=0.011) in one study6 and >50% repigmentation in 88% vs 71% of cases in another study (P=0.007).40 Other studies reported a better response in SV cases, although statistical analysis was not performed2,3,8,26,28,43 or was not significant.13,38 Immunological disturbances probably interfere with the outcome of transplantation in GV.28
No difference in improvement according to the type of vitiligo was noted in a recent study using outer root sheath hair follicle suspension (ORSHFS) in the treatment of 25 cases of stable vitiligo (nine SV, eleven acrofacial vitiligo, five GV).44
Mixed vitiligo cases responded less favorably than SV and GV cases to MKTP.38,40
Acrofacial type in general is less responsive to surgical therapy. Lesions on the fingertips were even considered an exclusion criterion by some authors.2,27 Interestingly, the presence of vitiligo on lips and fingertips (lip-tip type) was associated with a poor response, even when MKTP was performed at other sites in the same patient.26
Extent of vitiligo and size of treated lesion
Surgical therapy in general is indicated in stable cases with limited areas of vitiligo which are nonresponsive to medical therapy. Several authors excluded cases with widespread vitiligo involving >30% of the body surface area.2,37,43 The probability of a successful transplantation outcome to non-cultured epidermal suspension (NCES), ultrathin sheet transplantation and cultured epidermal suspension (CES) was found to be 20 times higher (OR) in patients with <100 cm2 white areas, three times higher in the 101–500 cm2 group and two times higher in the 501–1,500 cm2 group, compared with patients with >1,500 cm2 white area.28 Similarly, a negative correlation was found between Vitiligo Area Scoring Index, and Vitiligo European Task Force area and stage scores and percentage repigmentation in a more recent study.28
A significant negative relation was found by the authors of a study between the total treated surface area and the treatment outcome (P=0.0086).6 No similar correlation was found by other authors.29 The majority of authors used MKTP in treatment of lesions <100 cm2 with a favorable outcome.5,7,13,15,31,34,36,38,41,45 A few achieved favorable outcome for lesions up to 250 cm2.3,8,9,18,25,26,29,30,33
Skin type
Most of the reports do not comment on the skin type of cases treated; however, when analyzed statistically, similar repigmentation rates were reported in different ethnic groups26 and in different skin phototypes.6,27
Age
Age did not affect the percentage of repigmentation in several studies.6,27 Many studies included children and adolescents in the treated cases.2,5,6,8,17,25,27,30,31,34,36,38,41,43,46 Two studies focused solely on the treatment outcome in this age group. Mulekar et al25 treated 25 children and adolescents using general anesthesia with ≥95% repigmentation in 8/13 SV and 7/12 focal vitiligo cases. New lesions developed during follow-up in 5/12 focal vitiligo cases which could be attributed to the short duration of disease stability (6 months) applied in this study. The second study involved 13 cases of vitiligo (six SV, 1 focal vitiligo and six GV) with 1-year disease stability. Topical anesthesia followed by local infiltration was applied in 15/19 lesions achieving >90% repigmentation.18 In both studies, the procedure was well tolerated and accepted by both children and their parents. The main concern in children was pain intolerability and fidgeting during the procedure. Increasing the concentration of topically applied creams can be a good option in cooperative children, but general anesthesia may be still needed in selected cases.
Gender
No significant difference was found in repigmentation between males and females.6,27,28,41
Disease duration
The effect of this variable was assessed in a few studies with no correlation found in two6,41 and a negative correlation where patients with shorter disease duration got better treatment results in another.27
Site of lesion
On reviewing the literature, the head and neck lesions usually showed the best response, lesions on the limbs (excluding the elbows, knees and ankles) and trunk showed an intermediate response and lesions over the joints and acral skin tended to respond less favorably.6,7,26,37,46 Two exceptions were the study by Olsson and Juhlin28 where the neck was found to show the poorest response to transplantation and the study by Mulekar4 where the response over the face was worse than other sites and was explained by the traumatizing action of UV sunrays. Other authors did not find a significant effect of the site of treated lesion on the rate of repigmentation.27,34,36,38,40,41,44
Over the head and neck, response rates ranged from 70% to 100% with over half of the lesions achieving ≥95% repigmentation.7–9,18,25,40,46
Acral lesions are usually resistant to medical therapy, and hence, MKTP may be one of the few available effective modalities of therapy. It is important to note that lesions over the dorsum of the hands and feet respond better than those over the fingers or toes. Some authors do not recommend treating fingertips (distal fingers) owing to the poor response.2,27 Mulekar4 reported an excellent response in 62.5% of lesions over the dorsum of hands and feet and 66% of those over the fingers in a series of 142 cases of NSV. Fingers and toes also responded well with 42% of lesions achieving ≥95% repigmentation in another report by the same authors.17 However, it was not clear in both studies if any of those lesions were over the fingertips (distal fingers). Holla et al35 achieved >75% repigmentation in 78% of lesions over the dorsum of hands and feet and 42% of lesions over fingers and toes with only 6/80 lesions over acral skin showing <50% response (two over the ankle and four in distal fingers). Fingertips were found to show full repigmentation in one case in which cryoblebbing was used for recipient site preparation29 raising the possibility that perhaps poor response was due to the difficulty in performing dermabrasion or laser resurfacing at this site. Cryoblebs produce separation at the dermoepidermal junction regardless of the skin thickness.
In a recent study, 35% of acral lesions (excluding those on fingertips and toes) demonstrated excellent repigmentation with no difference in outcome found between difficult-to-treat sites and other sites.27 Others however showed less favorable outcome with 25%,13 15%26,28 or less5,18,29,30,38,46 of the lesions over the hands and feet showing ≥95% repigmentation.
Similarly, lesions over the joints including elbows, knees and ankles tend to respond less favorably with <30% of the lesions showing excellent repigmentation.5,7,26,28–30,43 Lesions over the joints showed a significantly lower response compared with presternal lesions and those on the trunk and extremities.6 On the contrary, some authors reported an excellent response of lesions overlying joints with ≥95% repigmentation achieved in 54% of lesions in one study4 and in 100% of lesions in others.18,27 Inadequate depth of dermabrasion due to heavily cornified skin as well as the high mobility at these sites may explain the poor response.46 Holla et al35 used strict immobilization up to the extent of using plaster casts when needed and achieved >75% repigmentation in 21/33 (64%) treated lesions. In another study, a diamond fraise wheel at a high speed of 12,000–15,000 rpm was used to assure proper dermabrasion of the thick cornified skin with an excellent response achieved in 15/43 (35%) lesions over joints without applying strict immobilization.17
Certain sites are considered difficult to manage due to the delicacy of the skin and/or the difficulty in immobilization such as the eyelids, nipples and genital skin. Manual dermabrasion or diamond fraise wheel at a low speed of 5,000 rpm was used with an excellent response achieved in 67% of eyelid lesions and 25% of genital lesions.17
Effect of procedure-related factors on response to MKTP
Donor tissue
Type of tissue used
Currently, there are two types of suspension used in MKTP, NCES8 where the epidermis is the source of cells and ORSHFS13 where the anagen hair follicle is the source.
Two studies compared cellular grafting in the form of NCES to tissue grafting in the form of suction blister epidermal grafting (SBEG). The first involved two groups of cases with comparable results regarding very good repigmentation (≥75% repigmentation), color matching and side-effect profile. However more cases achieved excellent repigmentation (≥90%) in the NCES group (P=0.002). Patient satisfaction and dermatological quality-of-life score reduction were also significantly higher in the NCES group.34 On the contrary, NCES, CES and SBEG were compared in the same patient in another study with significantly better response in SBEG lesions while NCES and CES lesions were comparable.39
In another study, no significant difference was found between NCES and CES with >70% response achieved in 62% and 52% of cases, respectively. Although CES could cover large areas using a small donor sample, it required expensive equipment and reagents in addition to highly trained personnel.37
A few studies compared NCES and ORSHFS with no significant difference found29,36 despite the significantly higher total cell count yielded by NCES.29 This may be due to the higher variety of cell populations including melanocyte stem cells in ORSHFS.49,50 On the other hand, inferior results were found in ORSHFS group compared to NCES group (43% vs 90% of cases with >75% repigmentation) in another study.41 The authors explained these poor results in ORSHFS group by the higher number of elderly people and the lack of proper surgical skills in follicle unit extraction which led to the use of insufficient numbers and transected hair follicles for preparation of the cellular suspension. These comments highlight the importance of proper choice of patients with abundant dark anagen hairs as well as higher level of experience required for performing this technique. It should be noted that long-term stability of pigmentation from hair follicle-derived melanocytes has not been established yet. Hair graying is known to occur with aging.48
Finally, combined NCES and ORSHFS produced superior repigmentation when compared to NCES in lesions over the joints and acral skin,15,16 while similar repigmentation rates were seen over the face15 in two recent studies.
Anatomical site used
The density of melanocytes varies at different body sites from around 900 melanocytes/mm2 on the back to around 1,500 melanocytes/mm2 in the genital region.50 The scalp was originally chosen for skin harvesting.7 This site has pros and cons. Being covered by hair, textural or color changes that may occur are unapparent, and being rich in hair follicles, the upper part of the outer root sheath is probably included in the sample. However, harvesting of skin necessitates shaving part of the scalp which may be inconvenient to the patient; also, the procedure is a bit more difficult as the scalp is curved and more vascular. The gluteal region3,4,8,9 or thigh18,35 is usually chosen as the donor area in MKTP. We found no significant difference in melanocytic cell count between both sites (unpublished data, Bassiouny et al, 2017). However, it is better to avoid the front of the thigh as a scar, color or textural change may occur.
Donor-to-recipient area ratio
Despite the fact that NCES can be prepared using donor tissue which is one-tenth the recipient area treated, a closer look at literature reveals that a donor area of one-third to one-fifth the recipient site was used in the majority of cases, with 1:10 ratio reserved for cases with a relatively large recipient area.3,4,8,9,17,18,33,40,45
The ratio of donor to recipient areas will be reflected on the cell count and number of transplanted cells/cm2. Olsson and Jhulin8 suggested that a melanocytes count of 190/mm2 was the lower limit capable of producing repigmentation. In an interesting study of effect of donor-to-recipient area ratio on repigmentation, a significant difference in total and melanocytes cell counts as well as extent of repigmentation was found between cases with a 1:3 and those with a 1:5 donor-to-recipient ratio. The authors concluded that the minimum number of melanocytes in epidermal cell suspension required to produce satisfactory repigmentation (>75%) was probably in the range of 210–250 cells/mm2.47
Cell viability is another important factor. As expected, a positive correlation was found between the percentage of repigmentation and the total number of all viable cells and viable melanocytes transplanted.45
Regarding ORSHFS, a lower mean number of melanocytes of 119 cells/mm2 was associated with optimum pigmentation which may be due to the different morphology and ultrastructural characteristics of hair follicle melanocytes. A count of 76 cells/mm2 CD200+ stem cells was present in cases achieving >75% repigmentation with a significant positive correlation between repigmentation rate at 6 months and both melanocyte and hair follicle stem cell counts.38 No similar correlation between percentage of CD200+ cells and clinical repigmentation was found in a more recent study.44
Recipient site preparation
Recipient vitiligo skin may be prepared by different methods including cryoblebbing,7,29,30,47 dermabrasion2–4,8,15–18,25–28,33–38,41,43,44,46,51 or laser resurfacing.5,9,29,40,45,52,53 The ideal method should be simple to perform, safe with minimal side effects and efficient reaching the dermoepidermal junction to avoid scarring or loss of the grafted melanocytes. Dermabrasion is more economical and relatively safe but requires technical skills. Pinpoint bleeding denotes reaching the ideal level. CO2 laser resurfacing produces uniform resurfacing but is more expensive. However, pinpoint bleeding does not appear. Two studies were done comparing different ablative CO2 laser settings with similar repigmentation rates achieved using less invasive resurfacing. A depth of 209 vs 300 µm was used in one study,52 while 144 vs 209 µm was used in the second.53 As expected, less invasive resurfacing resulted in faster healing and less persistent erythema at 6-month follow-up.53 In the same study, fractional laser resurfacing failed to produce an efficient response when used for recipient site preparation.53 In a pilot study, dermabrasion using a high-speed dermabrader fitted with a diamond fraise wheel produced better repigmentation than fractional CO2 laser resurfacing,21 but the latter was faster and simpler to perform. In our experience, laser resurfacing surpassed manual dermabrasion in improving repigmentation following MKTP in acral and non-acral lesions (unpublished data, Esmat et al, 2014).
Cryoblebbing must be done 24 hours before MKTP, and therefore, the procedure is done over 2 days. It also requires longer healing time; however, it may have a role in certain sites like the fingertips.30 Only one study compared cryoblebbing to laser resurfacing. Cryoblebbing produced ≥75% repigmentation in significantly more lesions (38 vs 10 lesions) (P=0.001) mainly due to excellent response achieved over the distal fingers.29
Less invasive methods of cellular suspension delivery were recently described. Benzekri and Gauthier20 delivered cellular suspension using a dermaroller equipped with 0.2 mm microneedles with >75% repigmentation in 3/5 cases with lesions over the face. Successful migration of viable melanocytes to the basal epidermal layer was demonstrated using this minimally invasive technique. However, larger case series are needed to assess this innovative method. Intralesional injection of NCES was also attempted in a recent study involving a large number of cases (300) where >50% repigmentation was obtained in 32.2% of treated patches (1,060) 9 months after therapy.54
Postoperative dressing and wound care
The type of dressing used postoperatively was found to affect the outcome of repigmentation at 12 months. In one study, 83% of cases where collagen dressing was used vs 63% of those where HA was used achieved ≥50% response (P=0.017).40 Collagen dressing was compared to petrolatum-impregnated gauze in the same patient in a pilot study with no significant difference in repigmentation. However, the gauze was more difficult to remove after 1 week.21 This encouraging finding is useful when collagen sheet is unavailable or too expensive to use in certain developing countries.
Factors enhancing repigmentation after MKTP
A few solar exposures lead to coalescence of pigmented areas in the earliest description of MKTP.7 Over the years, several studies used post-transplantation phototherapy5,20,29,30,40,44 or sun exposure18,43 to enhance repigmentation. No comparative studies were done to confirm this enhancing role; in fact, targeted phototherapy (UVB + UVA) post-grafting did not significantly improve the rate or the final repigmentation outcome at 12-month follow-up when compared to cases where it was not used.40 Interestingly, hyperpigmentation was linked to sun exposure recommended by the dermatologist postoperatively in one study in an Indian population,2 while sun exposure had a significant beneficial effect on color mismatch in another study performed in Belgium.6 This is probably related to skin type as darker skin types have a higher tendency of tanning. More studies are needed to explore the impact of post-MKTP phototherapy in different skin types.
Complications
MKTP is a safe technique with minimal complications. These include short-term complications in the form of infection or erythema and long-term complications, the most important of which are scars and color mismatch.
Short-term complications
Infection
A low incidence of infection in 5%–16% of cases was reported by some authors.18,31,37,44,46 A slightly higher incidence of 29% was reported in cases where cryoblebbing was used at the recipient site. This was attributed to the moist nature of the lesion and longer healing time.29 In all cases, infection was well controlled by broad-spectrum systemic antibiotics.
Persistent erythema
A bright pink color or mild erythema which lasts for a few weeks is expected after MKTP.2,5 Sometimes, erythema persisted for a few months, especially in cases where CO2 laser resurfacing was used for recipient site preparation. This was reported less frequently when more superficial full ablation was performed (50% of lesions at 144 µm vs 70% of lesions at 209 µm depth of ablation).53b
Long-term complications
Color mismatch
This is probably the commonest long-term complication. Treated lesions may appear slightly darker or slightly lighter than the surrounding skin. It was reported in several studies in varying percentages ranging from 5%–20%3–5,15,18,26,33,34,43 to >50% of cases treated.2,6,9,27,36,40,47
This mismatch improved after 6–8 months in some studies.2,5,9 A degree of mismatch persisted in 64% of lesions (36% darker, 28% lighter) 16.5 months after MKTP in one study, but this did not bother most of the cases (79%).6 Sun exposure can have an effect on improving6 or worsening2 the color mismatch as mentioned earlier. In another study, hyperpigmentation was more frequent over the joints which led the authors to suggest it may be due to frictional melanosis.35
Paul33 linked color mismatch to donor-to-recipient tissue ratio. He noticed that hyperpigmentation occurred in cases where a larger donor area (<1:5 ratio) was harvested while hypopigmentation affected cases in which the donor-to-recipient ratio was more than 1:10. A similar observation was reported by Sahni et al18 where hypopigmentation was found in 1/13 treated cases in whom a large donor-to-recipient ratio of 1:10 was used.
Hypopigmented halo
A rim of hypopigmentation at the edges of the lesion was reported in 6%–25% of cases in several studies.3,27,33,41 Extending the dermabrasion 2–3 mm into normal skin decreased the occurrence of this complication.33
Scars or textural change
Textural changes or scar may occur at the donor site,6,29,45,52 and therefore, tissue should be harvested from a relatively concealed area over the thigh or buttocks.
Stability of acquired pigmentation
A few long-term follow-up studies have emerged which gave a clear idea about stability of repigmentation achieved following MKTP. Excellent retention of acquired repigmentation was found in cases of SV on long-term follow-up.3,6 Repigmentation was maintained in 19/23 (83%) treated cases after 5 years of follow-up in one study.40 In GV, the majority of cases retain acquired repigmentation. However, some GV cases may lose some of this repigmentation especially if disease activity occurs. This was reported in 4%,4 16%,6 11%40 and 21% of cases27 in long-term follow-up studies. It is therefore essential to inform these patients clearly that MKTP is not a cure for vitiligo and does not prevent new lesions from appearing in the future in order to avoid patient frustration.
Improvement of repigmentation continued for a mean period of 10 months in one study.6 More pigmentation was reported in 67% and 62% of treated areas in SV and NSV cases, respectively, during 12–24 months of follow-up.27 This increase was significant in SV cases. These data are very useful since patients sometimes require a second session of MKTP to achieve full repigmentation. It would be therefore wise to wait for several months for full response to be judged.
Finally, patients are sometimes concerned about associated leucotrichia. An interesting retrospective study found that >90% repigmentation of hair occurred in 58% (10/17), 28% (2/7) and 12% (2/16) of lesions over the trunk, scalp and face, respectively, after MKTP.55 The authors noticed that the skin repigmented first followed by hair repigmentation after a lag period which was attributed to retrograde migration of melanocytes from the repigmented epidermis.56 Similar improvement was reported by other authors.18,27
Details about patients’ data and technique used in cited papers are included in Table S1.
Recommendation for a successful MKTP
Success in MKTP is achieved by fulfilling certain criteria with 12-month stability being its cornerstone. In our opinion, a minigrafting test is essential to predict the response to MKTP and should be routinely done in all cases because a clear-cut objective method of ensuring disease stability is still unavailable. The site and size of the lesions are influential factors too. Some sites such as the face, trunk and limbs are easier to resurface with ability to secure the dressings used postoperatively. Technique adjustments are needed at other sites such as delicate skin of eyelids or genitals where gentle resurfacing is needed as opposed to thick acral skin or skin overlying the joints where aggressive resurfacing is mandatory. Lesions over the fingers remain a challenge; the authors are dedicating research focusing on the best option especially in periangual lesions; cryoblebbing seems to give hope and splints using a tongue depressor are a simple way of limiting movement. The procedure is more suited to patients with limited extent of vitiligo, while cases with extensive lesions involving >30% of the body surface area are less likely to respond.
The tissue harvested for suspension preparation is another variable to consider. Keeping the ratio of donor to recipient areas around 1:3 or 1:5 when feasible increases the cell count/mm2 improving the response. Exposing the donor area to ultraviolet rays a few weeks before grafting was found to significantly increase the melanocytic count in NSV cases (unpublished data, Bassiouny et al, 2017) which can further boost the response. Finally, the use of mixed suspension (NCES and ORSHFS) may be beneficial in acral skin. Postoperative wound dressing and phototherapy are also important and are still fresh fields for exploring.
Most of the SV cases achieve an excellent response to MKTP because they possess many good prognostic factors, namely long periods of disease stability and small-sized lesions located over the face. Paying special attention to details of the technique can improve the response in NSV even in difficult-to-treat areas. Knowing what to predict and how to handle each case allows for successful outcome and realistic expectations (Figure 2). MKTP is an effective method of treatment in stable cases of vitiligo which produces long-lasting repigmentation with very good color matching offering a beam of hope for vitiligo patients.
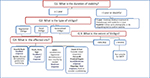  | Figure 2 How to proceed in a case of vitiligo resistant to medical therapy in whom MKTP is considered? Abbreviations: BSA, body surface area; MKTP, melanocyte–keratinocyte transplantation procedure. |
Disclosure
The authors report no conflicts of interest in this work.
References
Mulekar SV, Isedeh P. Surgical interventions for vitiligo: an evidence-based review. Br J Dermatol. 2013;169(Suppl 3):S57–S66. | ||
Mulekar SV. Melanocyte-keratinocyte cell transplantation for stable vitiligo. Int J Dermatol. 2003;42(2):132–136. | ||
Mulekar SV. Long-term follow-up study of segmental and focal vitiligo treated by autologous, noncultured melanocyte-keratinocyte cell transplantation. Arch Dermatol. 2004;140(10):1211–1215. | ||
Mulekar SV. Long-term follow-up study of 142 patients with vitiligo vulgaris treated by autologous, non-cultured melanocyte-keratinocyte cell transplantation. Int J Dermatol. 2005;44(10):841–845. | ||
van Geel N, Ongenae K, de Mil M, Haeghen YV, Vervaet C, Naeyaert JM. Double-blind placebo-controlled study of autologous transplanted epidermal cell suspensions for repigmenting vitiligo. Arch Dermatol. 2004;140(10):1203–1208. | ||
van Geel N, Wallaeys E, Goh BK, de Mil M, Lambert J. Long-term results of noncultured epidermal cellular grafting in vitiligo, halo naevi, piebaldism and naevus depigmentosus. Br J Dermatol. 2010;163(6):1186–1193. | ||
Gauthier Y, Surleve-Bazeille JE. Autologous grafting with noncultured melanocytes: a simplified method for treatment of depigmented lesions. J Am Acad Dermatol. 1992;26(2 Pt 1):191–194. | ||
Olsson MJ, Juhlin L. Leucoderma treated by transplantation of a basal cell layer enriched suspension. Br J Dermatol. 1998;138(4):644–648. | ||
van Geel N, Ongenae K, de Mil M, Naeyaert JM. Modified technique of autologous noncultured epidermal cell transplantation for repigmenting vitiligo: a pilot study. Dermatol Surg. 2001;27(10):873–876. | ||
Gupta S, Sahni K, Tembhre MK, Mathur S, Sharma VK. A novel point-of-care in vivo technique for preparation of epidermal cell suspension for transplantation in vitiligo. J Am Acad Dermatol. 2015;72(2):e65–e66. | ||
Jeong HS, Vandergriff T, Pandya AG. Use of Suction Blisters for Noncultured Epidermal Suspension Grafting in Patients With Vitiligo. Dermatol Surg. 2016;42(5):688–691. | ||
Vanscheidt W, Hunziker T. Repigmentation by outer-root-sheath-derived melanocytes: proof of concept in vitiligo and leucoderma. Dermatology. 2009;218(4):342–343. | ||
Mohanty S, Kumar A, Dhawan J, Sreenivas V, Gupta S. Noncultured extracted hair follicle outer root sheath cell suspension for transplantation in vitiligo. Br J Dermatol. 2011;164(6):1241–1246. | ||
Kumar A, Gupta S, Mohanty S, Bhargava B, Airan B. Stem Cell Niche is Partially Lost during Follicular Plucking: A Preliminary Pilot Study. Int J Trichology. 2013;5(2):97–100. | ||
Razmi TM, Parsad D, Kumaran SM. Combined epidermal and follicular cell suspension as a novel surgical approach for acral vitiligo. J Am Acad Dermatol. 2017;76(3):564–567. | ||
Razmi TM, Kumar R, Rani S, Kumaran SM, Tanwar S, Parsad D. Combination of Follicular and Epidermal Cell Suspension as a Novel Surgical Approach in Difficult-to-Treat Vitiligo: A Randomized Clinical Trial. JAMA Dermatol. 2018;154(3):301. | ||
Mulekar SV, Al Issa A, Al Eisa A. Treatment of vitiligo on difficult-to-treat sites using autologous noncultured cellular grafting. Dermatol Surg. 2009;35(1):66–71. | ||
Sahni K, Parsad D, Kanwar AJ. Noncultured epidermal suspension transplantation for the treatment of stable vitiligo in children and adolescents. Clin Exp Dermatol. 2011;36(6):607–612. | ||
Kumar R, Parsad D, Singh C, Yadav S. Four compartment method: a simplified and cost-effective method of noncultured epidermal cell suspension for the treatment of vitiligo. Br J Dermatol. 2014;170(3):581–585. | ||
Benzekri L, Gauthier Y. The first transepidermal transplantation of non-cultured epidermal suspension using a dermarolling system in vitiligo: Asequential histological and clinical study. Pigment Cell Melanoma Res. 2017;30(5):493–497. | ||
Silpa - Archa N, Griffith JL, Williams MS, Lim HW, Hamzavi IH. Prospective comparison of recipient-site preparation with fractional carbon dioxide laser vs. dermabrasion and recipient-site dressing composition in melanocyte– keratinocyte transplantation procedure in vitiligo: a preliminary study. Br J Dermatol. 2016;174:895–89. | ||
Njoo MD, das PK, Bos JD, Westerhof W. Association of the Köbner phenomenon with disease activity and therapeutic responsiveness in vitiligo vulgaris. Arch Dermatol. 1999;135(4):407–413. | ||
Benzekri L, Gauthier Y, Hamada S, Hassam B. Clinical features and histological findings are potential indicators of activity in lesions of common vitiligo. Br J Dermatol. 2013;168(2):265–271. | ||
Aboul-Fettouh N, Hinojosa J, Tovar-Garza A, Pandya AG. The majority of patients presenting with vitiligo have a clinical sign of activity. J Am Acad Dermatol. 2017;77(4):774–775. | ||
Mulekar SV, Al Eisa A, Delvi MB, Al Issa A, Al Saeed AH. Childhood vitiligo: a long-term study of localized vitiligo treated by noncultured cellular grafting. Pediatr Dermatol. 2010;27(2):132–136. | ||
Huggins RH, Henderson MD, Mulekar SV, et al. Melanocyte-keratinocyte transplantation procedure in the treatment of vitiligo: the experience of an academic medical center in the United States. J Am Acad Dermatol. 2012;66(5):785–793. | ||
Silpa-Archa N, Griffith JL, Huggins RH, et al. Long-term follow-up of patients undergoing autologous noncultured melanocyte-keratinocyte transplantation for vitiligo and other leukodermas. J Am Acad Dermatol. 2017;77(2):318–327. | ||
Olsson MJ, Juhlin L. Long-term follow-up of leucoderma patients treated with transplants of autologous cultured melanocytes, ultrathin epidermal sheets and basal cell layer suspension. Br J Dermatol. 2002;147(5):893–904. | ||
El-Zawahry BM, Esmat S, Bassiouny D, et al. Effect of Procedural-Related Variables on Melanocyte-Keratinocyte Suspension Transplantation in Nonsegmental Stable Vitiligo: A Clinical and Immunocytochemical Study. Dermatol Surg. 2017;43(2):226–235. | ||
El-Zawahry BM, Zaki NS, Bassiouny DA, et al. Autologous melanocyte-keratinocyte suspension in the treatment of vitiligo. J Eur Acad Dermatol Venereol. 2011;25(2):215–220. | ||
Toossi P, Shahidi-Dadras M, Mahmoudi Rad M, Fesharaki RJ. Non-cultured melanocyte-keratinocyte transplantation for the treatment of vitiligo: a clinical trial in an Iranian population. J Eur Acad Dermatol Venereol. 2011;25(10):1182–1186. | ||
Vázquez-Martínez OT, Martínez-Rodríguez HG, Velásquez-Arenas L, et al. Treatment of vitiligo with a melanocyte-keratinocyte cell suspension versus dermabrasion only: a pilot study with a 12-month follow up. J Drugs Dermatol. 2011;10(9):1032–1036. | ||
Paul M. Autologous Non-cultured Basal Cell-Enriched Epidermal Cell Suspension Transplantation in Vitiligo: Indian Experience. J Cutan Aesthet Surg. 2011;4(1):23–28. | ||
Budania A, Parsad D, Kanwar AJ, Dogra S. Comparison between autologous noncultured epidermal cell suspension and suction blister epidermal grafting in stable vitiligo: a randomized study. Br J Dermatol. 2012;167(6):1295–1301. | ||
Holla AP, Sahni K, Kumar R, Parsad D, Kanwar A, Mehta SD. Acral vitiligo and lesions over joints treated with non-cultured epidermal cell suspension transplantation. Clin Exp Dermatol. 2013;38(4):332–337. | ||
Singh C, Parsad D, Kanwar AJ, Dogra S, Kumar R. Comparison between autologous noncultured extracted hair follicle outer root sheath cell suspension and autologous noncultured epidermal cell suspension in the treatment of stable vitiligo: a randomized study. Br J Dermatol. 2013;169(2):287–293. | ||
Verma R, Grewal RS, Chatterjee M, Pragasam V, Vasudevan B, Mitra D. A comparative study of efficacy of cultured versus non cultured melanocyte transfer in the management of stable vitiligo. Med J Armed Forces India. 2014;70(1):26–31. | ||
Vinay K, Dogra S, Parsad D, et al. Clinical and treatment characteristics determining therapeutic outcome in patients undergoing autologous non-cultured outer root sheath hair follicle cell suspension for treatment of stable vitiligo. J Eur Acad Dermatol Venereol. 2015;29(1):31–37. | ||
Bao H, Hong W, Fu L, Wei X, Qian G, Xu A. Blister roof grafting, cultured melanocytes transplantation and non-cultured epidermal cell suspension transplantation in treating stable vitiligo: A mutual self-control study. J Dermatolog Treat. 2015;26(6):571–574. | ||
Gan EY, Kong YL, Tan WD, Thng ST, Goh BK. Twelve-month and sixty-month outcomes of noncultured cellular grafting for vitiligo. J Am Acad Dermatol. 2016;75(3):564–571. | ||
Donaparthi N, Chopra A. Comparative Study of Efficacy of Epidermal Melanocyte Transfer Versus Hair Follicular Melanocyte Transfer in Stable Vitiligo. Indian J Dermatol. 2016;61(6):640–644. | ||
Falabella R, Arrunategui A, Barona MI, Alzate A. The minigrafting test for vitiligo: detection of stable lesions for melanocyte transplantation. J Am Acad Dermatol. 1995;32(2 Pt 1):228–232. | ||
Ramos MG, Ramos DG, Ramos CG. Evaluation of treatment response to autologous transplantation of noncultured melanocyte/keratinocyte cell suspension in patients with stable vitiligo. An Bras Dermatol. 2017;92(3):312–318. | ||
Kumar P, Bhari N, Tembhre MK, et al. Study of efficacy and safety of noncultured, extracted follicular outer root sheath cell suspension transplantation in the management of stable vitiligo. Int J Dermatol. 2018;57(2):245–249. | ||
Komen L, Vrijman C, Tjin EP, et al. Autologous cell suspension transplantation using a cell extraction device in segmental vitiligo and piebaldism patients: A randomized controlled pilot study. J Am Acad Dermatol. 2015;73(1):170–172. | ||
Pandya V, Parmar KS, Shah BJ, Bilimoria FE. A study of autologous melanocyte transfer in treatment of stable vitiligo. Indian J Dermatol Venereol Leprol. 2005;71(6):393–397. | ||
Tegta GR, Parsad D, Majumdar S, Kumar B. Efficacy of autologous transplantation of noncultured epidermal suspension in two different dilutions in the treatment of vitiligo. Int J Dermatol. 2006;45(2):106–110. | ||
Tobin DJ, Paus R. Graying: gerontobiology of the hair follicle pigmentary unit. Exp Gerontol. 2001;36(1):29–54. | ||
Gho CG, Braun JE, Tilli CM, Neumann HA, Ramaekers FC. Human follicular stem cells: their presence in plucked hair and follicular cell culture. Br J Dermatol. 2004;150(5):860–868. | ||
Bolognia JL, Jorizzo JL, Schaffer JV. Melanocyte biology. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. 3rd ed. UK: Elsevier Health Sciences; 2012:1011–1022. | ||
Shah AN, Marfatia RK, Saikia SS. A Study of Noncultured Extracted Hair Follicle Outer Root Sheath Cell Suspension for Transplantation in Vitiligo. Int J Trichology. 2016;8(2):67–72. | ||
Komen L, Vrijman C, Wietze van der Veen JP, de Rie MA, Wolkerstorfer A. Observations on CO2 Laser Preparation of Recipient Site for Noncultured Cell Suspension Transplantation in Vitiligo. J Cutan Aesthet Surg. 2016;9(2):133–135. | ||
Lommerts JE, Meesters AA, Komen L, et al. Autologous cell suspension grafting in segmental vitiligo and piebaldism: a randomized controlled trial comparing full surface and fractional CO2 laser recipient-site preparations. Br J Dermatol. 2017;177(5):1293–1298. | ||
Orouji Z, Bajouri A, Ghasemi M, et al. A single-arm open-label clinical trial of autologous epidermal cell transplantation for stable vitiligo: A 30-month follow-up. J Dermatol Sci. 2018;89(1):52–59. | ||
Holla AP, Sahni K, Kumar R, Kanwar A, Mehta S, Parsad D. Repigmentation of leukotrichia due to retrograde migration of melanocytes after noncultured epidermal suspension transplantation. Dermatol Surg. 2014;40(2):169–175. | ||
Agrawal K, Agrawal A. Vitiligo: surgical repigmentation of leukotrichia. Dermatol Surg. 1995;21(8):711–715. |
Supplementary material
References
Gauthier Y, Surleve-Bazeille JE. Autologous grafting with noncultured melanocytes: a simplified method for treatment of depigmented lesions. J Am Acad Dermatol. 1992;26(2 Pt 1):191–194. | ||
Olsson MJ, Juhlin L. Leucoderma treated by transplantation of a basal cell layer enriched suspension. Br J Dermatol. 1998;138(4):644–648. | ||
van Geel N, Ongenae K, de Mil M, Naeyaert JM. Modified technique of autologous noncultured epidermal cell transplantation for repigmenting vitiligo: a pilot study. Dermatol Surg. 2001;27(10):873–876. | ||
Olsson MJ, Juhlin L. Long-term follow-up of leucoderma patients treated with transplants of autologous cultured melanocytes, ultrathin epidermal sheets and basal cell layer suspension. Br J Dermatol. 2002;147(5):893–904. | ||
Mulekar SV. Melanocyte-keratinocyte cell transplantation for stable vitiligo. Int J Dermatol. 2003;42(2):132–136. | ||
van Geel N, Ongenae K, de Mil M, Haeghen YV, Vervaet C, Naeyaert JM. Double-blind placebo-controlled study of autologous transplanted epidermal cell suspensions for repigmenting vitiligo. Arch Dermatol. 2004;140(10):1203–1208. | ||
Mulekar SV. Long-term follow-up study of segmental and focal vitiligo treated by autologous, noncultured melanocyte-keratinocyte cell transplantation. Arch Dermatol. 2004;140(10):1211–1215. | ||
Mulekar SV. Long-term follow-up study of 142 patients with vitiligo vulgaris treated by autologous, non-cultured melanocyte-keratinocyte cell transplantation. Int J Dermatol. 2005;44(10):841–845. | ||
Pandya V, Parmar KS, Shah BJ, Bilimoria FE. A study of autologous melanocyte transfer in treatment of stable vitiligo. Indian J Dermatol Venereol Leprol. 2005;71(6):393–397. | ||
Tegta GR, Parsad D, Majumdar S, Kumar B. Efficacy of autologous transplantation of noncultured epidermal suspension in two different dilutions in the treatment of vitiligo. Int J Dermatol. 2006;45(2):106–110. | ||
Mulekar SV, Al Issa A, Al Eisa A. Treatment of vitiligo on difficult-to-treat sites using autologous noncultured cellular grafting. Dermatol Surg. 2009;35(1):66–71. | ||
Mulekar SV, Al Eisa A, Delvi MB, Al Issa A, Al Saeed AH. Childhood vitiligo: a long-term study of localized vitiligo treated by noncultured cellular grafting. Pediatr Dermatol. 2010;27(2):132–136. | ||
van Geel N, Wallaeys E, Goh BK, de Mil M, Lambert J. Long-term results of noncultured epidermal cellular grafting in vitiligo, halo naevi, piebaldism and naevus depigmentosus. Br J Dermatol. 2010;163(6):1186–1193. | ||
El-Zawahry BM, Zaki NS, Bassiouny DA, et al. Autologous melanocyte-keratinocyte suspension in the treatment of vitiligo. J Eur Acad Dermatol Venereol. 2011;25(2):215–220. | ||
Toossi P, Shahidi-Dadras M, Mahmoudi Rad M, Fesharaki RJ. Non-cultured melanocyte-keratinocyte transplantation for the treatment of vitiligo: a clinical trial in an Iranian population. J Eur Acad Dermatol Venereol. 2011;25(10):1182–1186. | ||
Sahni K, Parsad D, Kanwar AJ. Noncultured epidermal suspension transplantation for the treatment of stable vitiligo in children and adolescents. Clin Exp Dermatol. 2011;36(6):607–612. | ||
Vázquez-Martínez OT, Martínez-Rodríguez HG, Velásquez-Arenas L, et al. Treatment of vitiligo with a melanocyte-keratinocyte cell suspension versus dermabrasion only: a pilot study with a 12-month follow up. J Drugs Dermatol. 2011;10(9):1032–1036. | ||
Paul M. Autologous Non-cultured Basal Cell-Enriched Epidermal Cell Suspension Transplantation in Vitiligo: Indian Experience. J Cutan Aesthet Surg. 2011;4(1):23–28. | ||
Mohanty S, Kumar A, Dhawan J, Sreenivas V, Gupta S. Noncultured extracted hair follicle outer root sheath cell suspension for transplantation in vitiligo. Br J Dermatol. 2011;164(6):1241–1246. | ||
Huggins RH, Henderson MD, Mulekar SV, et al. Melanocyte-keratinocyte transplantation procedure in the treatment of vitiligo: the experience of an academic medical center in the United States. J Am Acad Dermatol. 2012;66(5):785–793. | ||
Budania A, Parsad D, Kanwar AJ, Dogra S. Comparison between autologous noncultured epidermal cell suspension and suction blister epidermal grafting in stable vitiligo: a randomized study. Br J Dermatol. 2012;167(6):1295–1301. | ||
Holla AP, Sahni K, Kumar R, Parsad D, Kanwar A, Mehta SD. Acral vitiligo and lesions over joints treated with non-cultured epidermal cell suspension transplantation. Clin Exp Dermatol. 2013;38(4):332–337. | ||
Singh C, Parsad D, Kanwar AJ, Dogra S, Kumar R. Comparison between autologous noncultured extracted hair follicle outer root sheath cell suspension and autologous noncultured epidermal cell suspension in the treatment of stable vitiligo: a randomized study. Br J Dermatol. 2013;169(2):287–293. | ||
Holla AP, Sahni K, Kumar R, Kanwar A, Mehta S, Parsad D. Repigmentation of leukotrichia due to retrograde migration of melanocytes after noncultured epidermal suspension transplantation. Dermatol Surg. 2014;40(2):169–175. | ||
Verma R, Grewal RS, Chatterjee M, Pragasam V, Vasudevan B, Mitra D. A comparative study of efficacy of cultured versus non cultured melanocyte transfer in the management of stable vitiligo. Med J Armed Forces India. 2014;70(1):26–31. | ||
Vinay K, Dogra S, Parsad D, et al. Clinical and treatment characteristics determining therapeutic outcome in patients undergoing autologous non-cultured outer root sheath hair follicle cell suspension for treatment of stable vitiligo. J Eur Acad Dermatol Venereol. 2015;29(1):31–37. | ||
Komen L, Vrijman C, Tjin EP, et al. Autologous cell suspension transplantation using a cell extraction device in segmental vitiligo and piebaldism patients: A randomized controlled pilot study. J Am Acad Dermatol. 2015;73(1):170–172. | ||
Bao H, Hong W, Fu L, Wei X, Qian G, Xu A. Blister roof grafting, cultured melanocytes transplantation and non-cultured epidermal cell suspension transplantation in treating stable vitiligo: A mutual self-control study. J Dermatolog Treat. 2015;26(6):571–574. | ||
Gan EY, Kong YL, Tan WD, Thng ST, Goh BK. Twelve-month and sixty-month outcomes of noncultured cellular grafting for vitiligo. J Am Acad Dermatol. 2016;75(3):564–571. | ||
Donaparthi N, Chopra A. Comparative Study of Efficacy of Epidermal Melanocyte Transfer Versus Hair Follicular Melanocyte Transfer in Stable Vitiligo. Indian J Dermatol. 2016;61(6):640–644. | ||
Shah AN, Marfatia RK, Saikia SS. A Study of Noncultured Extracted Hair Follicle Outer Root Sheath Cell Suspension for Transplantation in Vitiligo. Int J Trichology. 2016;8(2):67–72. | ||
Silpa-Archa N, Griffith JL, Huggins RH, et al. Long-term follow-up of patients undergoing autologous noncultured melanocyte-keratinocyte transplantation for vitiligo and other leukodermas. J Am Acad Dermatol. 2017;77(2):318–327. | ||
El-Zawahry BM, Esmat S, Bassiouny D, et al. Effect of Procedural-Related Variables on Melanocyte-Keratinocyte Suspension Transplantation in Nonsegmental Stable Vitiligo: A Clinical and Immunocytochemical Study. Dermatol Surg. 2017;43(2):226–235. | ||
Benzekri L, Gauthier Y. The first transepidermal transplantation of non-cultured epidermal suspension using a dermarolling system in vitiligo: Asequential histological and clinical study. Pigment Cell Melanoma Res. 2017;30(5):493–497. | ||
Ramos MG, Ramos DG, Ramos CG. Evaluation of treatment response to autologous transplantation of noncultured melanocyte/keratinocyte cell suspension in patients with stable vitiligo. An Bras Dermatol. 2017;92(3):312–318. | ||
Lommerts JE, Meesters AA, Komen L, et al. Autologous cell suspension grafting in segmental vitiligo and piebaldism: a randomized controlled trial comparing full surface and fractional CO2 laser recipient-site preparations. Br J Dermatol. 2017;177(5):1293–1298. | ||
Silpa - Archa N, Griffith J L, Williams M S, Lim H W, Hamzavi IH. Prospective comparison of recipient-site preparation with fractional carbon dioxide laser vs. dermabrasion and recipient-site dressing composition in melanocyte– keratinocyte transplantation procedure in vitiligo: a preliminary study. Br J Dermatol. 2016;174:895–89. | ||
Kumar P, Bhari N, Tembhre MK, et al. Study of efficacy and safety of noncultured, extracted follicular outer root sheath cell suspension transplantation in the management of stable vitiligo. Int J Dermatol. 2018;57(2):245–249. | ||
Orouji Z, Bajouri A, Ghasemi M, et al. A single-arm open-label clinical trial of autologous epidermal cell transplantation for stable vitiligo: A 30-month follow-up. J Dermatol Sci. 2018;89(1):52–59. | ||
Razmi TM, Parsad D, Kumaran SM. Combined epidermal and follicular cell suspension as a novel surgical approach for acral vitiligo. J Am Acad Dermatol. 2017;76(3):564–567. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.