Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Autologous Concentrated Growth Factor Used to Treat Linear Scleroderma En Coup de Sabre: A Case Report
Authors Wang L, Lv S, Lin W, Yang D
Received 4 January 2022
Accepted for publication 1 April 2022
Published 14 April 2022 Volume 2022:15 Pages 675—679
DOI https://doi.org/10.2147/CCID.S356972
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Lei Wang,1 Shuying Lv,1,2 Wenjun Lin,1,2 Dingquan Yang1
1Department of Dermatology, China-Japan Friendship Hospital, Beijing, People’s Republic of China; 2School of Clinical Medicine, Beijing University of Chinese Medicine, Beijing, People’s Republic of China
Correspondence: Dingquan Yang, Department of Dermatology, China-Japan Friendship Hospital, Beijing, People’s Republic of China, Tel +86 13901218671, Email [email protected]
Abstract: Linear scleroderma en coup de sabre (LSCS) is a variant of localized scleroderma associated with band-like fibrotic lesions in the frontoparietal area. We report a case of LSCS in a woman who presented with progressive mild hyperchromia on the right side of her forehead, with dermal atrophy and hair and eyebrow loss. After the failure of conservative treatments, the patient responded dramatically to injection of autologous localized concentrated growth factor. After three treatments, the atrophy, stiffness, and angiotelectasis on the affected area had improved. No recurrence was detected 24 months after the last treatment. This is the first study describing the use of autologous concentrated growth factor injection to alleviate clinical symptoms of LSCS. This suggests that concentrated growth factor may be a treatment for LSCS in the clinic.
Keywords: concentrated growth factor, linear scleroderma en coup de sabre
Introduction
Linear scleroderma is a localized scleroderma of unknown etiology characterized by band-like sclerotic lesions of the skin and underlying tissues. When it occurs as alopecia of the scalp, eyebrow, eyelashes, or forehead, it is termed linear scleroderma en coup de sabre (LSCS).1 LSCS can cause significant functional disability and cosmetic problems. The variety of extracutaneous features include hair follicle atrophy and hair loss. In long-term cases, the follicle disappears and a permanent bald spot forms with obvious boundaries, longitudinal in shape. In severe cases, the scalp is atrophied, depressed, and adhered to the bone surface to varying degrees. This usually causes serious psychological distress in young female patients.
The histopathological features of LSCS are vacuolar degeneration at the dermoepidermal junction, perivascular or periappendageal lymphocytic infiltrate (or both), and vacuolar degeneration of the follicular epithelium.2 Worldwide, no effective systematic treatment is available that can cure localized scleroderma. Therefore, focus should be directed to slowing disease progression and preventing the development of irreversible sequalae, such as cutaneous and subcutaneous atrophy. Most widely used for this purpose are topical corticosteroids, tacrolimus ointment, calcipotriol ointment, ultraviolet phototherapy, systemic corticosteroids, oral methotrexate, mycophenolate mofetil, and biological agent.3–5 However, in a setting of disease progression and recurrence, attempting other therapeutic regimens is warranted.
Concentrated growth factor (CGF) was first isolated from venous blood by Sacco in 2006, using a special centrifuge with controlled speeds.6 CGF is now the latest generation of platelet concentrate. Its component growth factors include VEGF (vascular endothelial growth factor), TGF-β (transforming growth factor beta 1), PDGF (platelet-derived growth factor), and IGF (insulin-like growth factor). CGF also includes platelets, cytokines, and abundant CD34-positive cells, all of which facilitate healing and tissue regeneration.6,7 The unique composition of CGF, and its ease of preparation and cost-effectiveness, has led to wide application in the fields of dentistry and cosmetic surgery.8 It has been suggested that CGF may be a promising candidate for peripheral nerve regeneration with an excellent safety profile.7
We speculated that CGF might improve the prognosis of LSCS. As far as we know, the effect of autologous CGF applied in LSCS has not been reported. This case report describes the use of autologous CGF to treat LSCS in a 31-year-old woman. The patient provided informed consent for the report of her clinical course.
Case Presentation
A 31-year-old woman presented with a 4-year history of mild hyperchromia, which began on the right side of her forehead and evolved into localized dermal atrophy accompanied by abnormal loss of local hair and eyebrows on the right side. The patient had no history of autoimmune disorders, smoking, or drinking. She had no known allergies. There was no family history of autoimmune or rheumatological disease, or scleroderma.
On examination, she was anxious and in mild discomfort. There was a band of non-tender, non-erythematous sclerotic skin over the right eyebrow; abnormal loss of eyebrows, and a linear band of sclerotic skin accompanied by patch of alopecia on the right corner of the forehead (Figure 1A–C). The patient reported no fever, dry eyes, dry mouth, myalgia, arthralgia, Raynaud’s phenomenon, joint swelling, rash, or oral lesions. The remainder of the physical examination was normal.
Blood levels of glucose, electrolytes, and vitamin B12 were normal, as was complete blood count, erythrocyte-sedimentation rate, and kidney, liver, and thyroid functions. The following screening tests were negative: antinuclear antibodies, anti-double-stranded DNA, anticentromere, anti-RNP, and anti-Scl-70 antibodies. Other laboratory test results were normal.
Dermoscopy showed pili torti, and linear and branching vessels on whitish patches (Figure 1D) that is consistent with LSCS disease.9
The patient began treatment with 0.05% halometasone cream on the affected scalp, and asiaticoside ointment on the right eyebrow twice a day. However, 6 months after the initiation of treatment there was no regrowth of hair or eyebrow, and there was an aggravating contraction and stiffness of the right forehead. Given the clinically significant progression of the disease, we recommended GCF, because she refused to take methotrexate and mycophenolate orally due to side effects such as gastrointestinal reactions.
Autologous CGF Preparation
Two 10-mL non‑anticoagulant negative pressure tubes and a centrifuge device (Medifuge MF200, Silfradent srl, Forlì, Italy) were used during the procedure. Nine milliliters of intravenous blood, collected from the antecubital fossa of the patient, were placed in each negative pressure tube. Proper centrifugation resulted in 3 layers in each tube, specifically a top, middle, and bottom layer corresponding to platelet-poor plasma, CGF, and red blood cells, respectively. After removing the top layer with a sterile syringe, the middle layers were extracted for application.
CGF Treatment Procedure
With the patient supine, a local anesthesia of 1% lidocaine was given via infiltration. Liquid phase CGF was injected into the areas of dermal atrophy and alopecia, at a dose of 0.1 mL/cm2, using a disposable 32G needle. After removal of the needle, the dermatologist gently pressed the needled skin area using a dry sterilized cotton ball. The treatment was performed once per month for a total of 3 treatments in 3 consecutive months.
After the initial treatment, the atrophy, stiffness, and angiotelectasis of the localized affected area showed a definite improvement (Figure 1E–H). The patient was completely asymptomatic at all the subsequent treatment dates, suggesting a successful treatment outcome. During the subsequent 6 months of follow-up, the patient’s sclerotic skin gradually improved and no further hair loss was noted. There was no injection-related nodule, induration, swelling, or other adverse event throughout the treatment period. No recurrence was detected 24 months after the last treatment.
After three treatments, the atrophy, stiffness, and angiotelectasis on the affected area had improved (Figure 1E–H), particularly in the right forehead, with lessening of the skin hyperpigmentation and atrophy (Figure 2A–D).
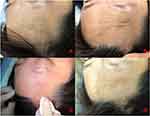 |
Figure 2 Before treatment and after immediate effect. (A and B) Initial clinical presentation. The right forehead at rest and lifting the eyebrow. (C and D) Immediate effect after treatment. |
Discussion
To our best knowledge, this is the first study to describe treatment with autologous CGF injection to relieve clinical symptoms of LSCS. This report should raise awareness among dermatologists of the potential benefit of CGF for treating LSCS.
Very little is known about the pathogenesis of LSCS. Evidence suggests that linear scleroderma is an autoimmune disease, in which localized skin inflammation triggers excessive collagen synthesis and deposition, leading to skin hardening and atrophy of normal structures. In some individuals with LSCS, symptoms may be improved by conservative treatments such as topical or intralesional or systemic corticosteroids, topical tacrolimus ointment, phototherapy, or systemic methotrexate.4 Yet, there is no therapy that will effectively cure or completely resolve the risk of unpredictable progress and recurrence.
Our present patient with LSCS experienced improved hyperpigmentation, dermal atrophy, and subcutaneous atrophy of lesions after receiving autologous CGF injection treatments. There were no adverse effects. The mechanisms underlying the benefits of CGF remain unknown. However, platelet-rich plasma has been used for facial rejuvenation and nonscarring alopecia in several studies.10–12 Platelet-rich plasma may induce hair growth and follicle survival by stimulating the stem cells located in the bulge region, which in turn can activate the proliferative phase of the hair cycle.10,12,13 CGF is the latest generation of platelet concentrate and includes various growth factors (eg, VEGF, TGF-β, PDGF and IGF) and abundant CD34-positive cells. The participation of these growth factors in regulating cell proliferation, migration, matrix remodeling, differentiation, and angiogenesis has been demonstrated,6 as well as the effect of circulating CD34-positive cells to improve vascular maintenance, neovascularization, and angiogenesis.6 Therefore, we hypothesize that CGF, secreted by platelets, may be a crucially important component of platelet-rich plasma that can improve the symptoms of LSCS. A primary attribute of CGF is that it is autologous and thus free from the risk of cross-contamination. In addition, it is simple and inexpensive to make.
The results of the current case study are limited by its open-label and non-comparative nature. In addition, the 6-month follow-up may be insufficient, since LSCS is prone to reoccur within the first 2 years after discontinuation of treatment. We will continue to follow this patient to evaluate her long-term outcome. Also, before treatment a skin biopsy to assess the pathological changes in the skin lesions was not performed before treatment. Follow-up studies might include, as an important indicator, comparisons of histopathological changes at the skin lesions before and after CGF treatment.
Conclusion
In this study, we observed that 3 monthly localized injections with autologous CGF to treat a patient with LSCS appeared to significantly accelerate clinical improvement, without notable injection-site reaction. This regimen may be a new treatment option for patients with LSCS. Further reports are needed, and prospective studies, to determine whether CGF may be recommended to patients with LSCS as a better option to traditional choices.
Data Sharing Statement
All data obtained or analyzed during the current study are available from the corresponding author upon reasonable request.
Consent for Publication
The patient in this manuscript gave a clear statement that written informed consent to the publication of her case details. In addition, this is a case report and no institutional approval is required to publish case details.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Kister I, Inglese M, Laxer RM, Herbert J. Neurologic manifestations of localized scleroderma: a case report and literature review. Neurology. 2008;71(19):1538–1545.
2. Taniguchi T, Asano Y, Tamaki Z, et al. Histological features of localized scleroderma ‘en coup de sabre’: a study of 16 cases. J Eur Acad Dermatol Venereol. 2014;28(12):1805–1810.
3. Kreuter A, Krieg T, Worm M, et al. German guidelines for the diagnosis and therapy of localized scleroderma. J German Soc Dermatol JDDG. 2016;14(2):199–216.
4. Mertens JS, Seyger MMB, Thurlings RM, et al. Morphea and Eosinophilic Fasciitis: an Update. Am J Clin Dermatol. 2017;18(4):491–512.
5. Florez-Pollack S, Kunzler E, Jacobe HT. Morphea: current concepts. Clin Dermatol. 2018;36(4):475–486.
6. Rodella LF, Favero G, Boninsegna R, et al. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc Res Tech. 2011;74:772–777.
7. Qin J, Wang L, Sun Y, et al. Concentrated growth factor increases Schwann cell proliferation and neurotrophic factor secretion and promotes functional nerve recovery in vivo. Int J Mol Med. 2016;37:493–500.
8. Man D, Plosker H, Winland-Brown JE. The use of autologous platelet- rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107:229–237.
9. Saceda-Corralo DT. Antonella. Trichoscopic features of linear morphea on the scalp. Skin Appendage Disorders. 2018;4:31–33.
10. Niţă AC, Jianu DM, Florescu IP, et al. The synergy between lasers and adipose tissues surgery in cervicofacial rejuvenation: histopatholgical aspects. Rom J Morphol Embryol. 2013;54(4):1039–1043.
11. Giordano S, Romeo M, Lankinen P. Platelet-rich plasma for androgenetic alopecia: does it work? Evidence from meta analysis. J Cosmet Dermatol. 2017;16(3):374–381.
12. Darwin E, Hirt PA, Fertig R, et al. Alopecia Areata: review of epidemiology, clinical features, pathogenesis, and new treatment options. Int J Trichology. 2018;10(2):51–60.
13. Gupta AK, Versteeg SG, Rapaport J, Hausauer AK, Shear NH, Piguet V. The efficacy of platelet-rich plasma in the field of hair restoration and facial aesthetics-a systematic review and meta- analysis. J Cutan Med Surg. 2019;23(2):185–203.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

